Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Zeolites
2.2. Cation Exchange
2.3. Characterization of Materials
2.4. CO2 Adsorption Isotherms and CO2/N2 Selectivity Calculation
3. Results and Discussion
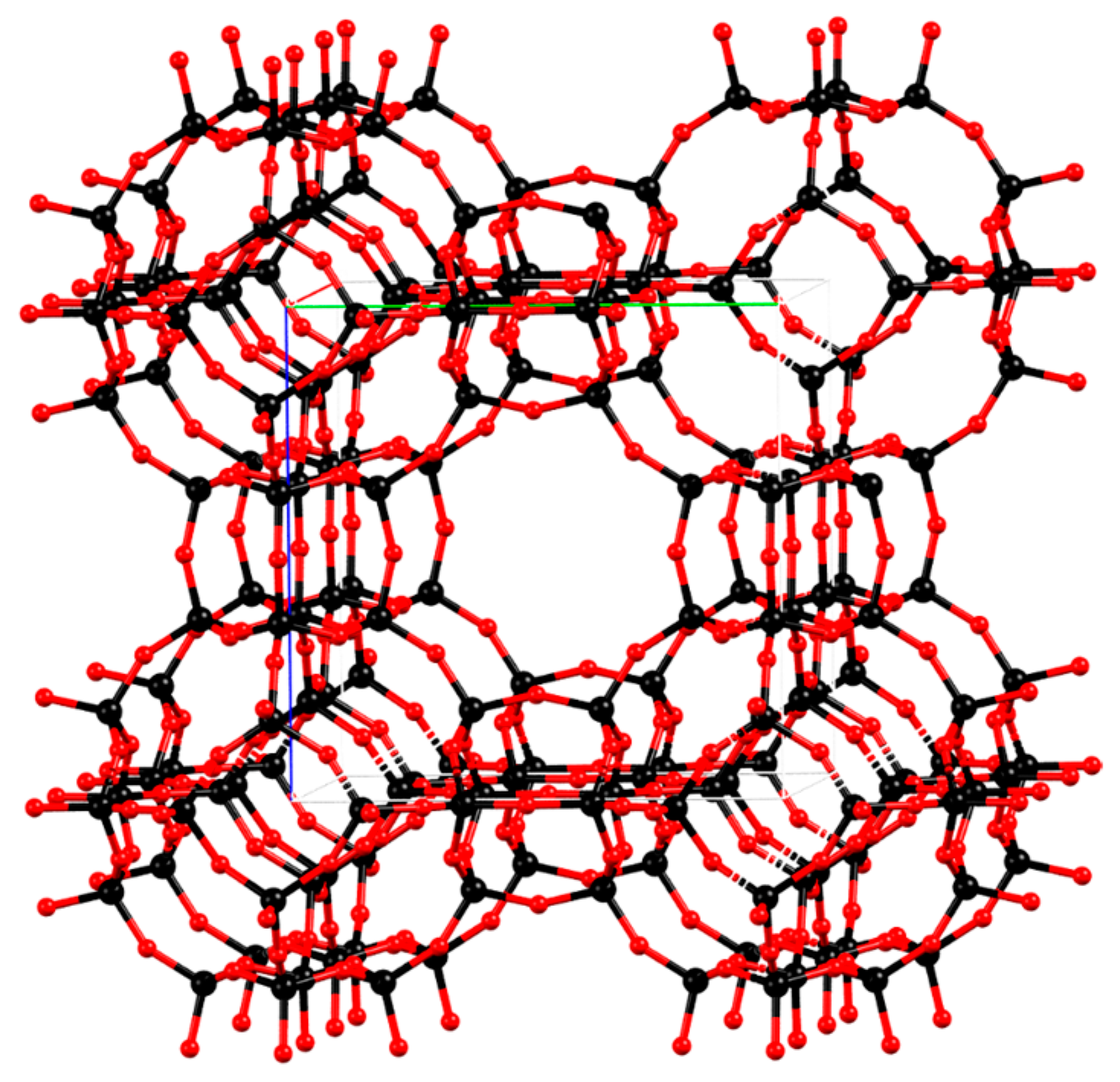
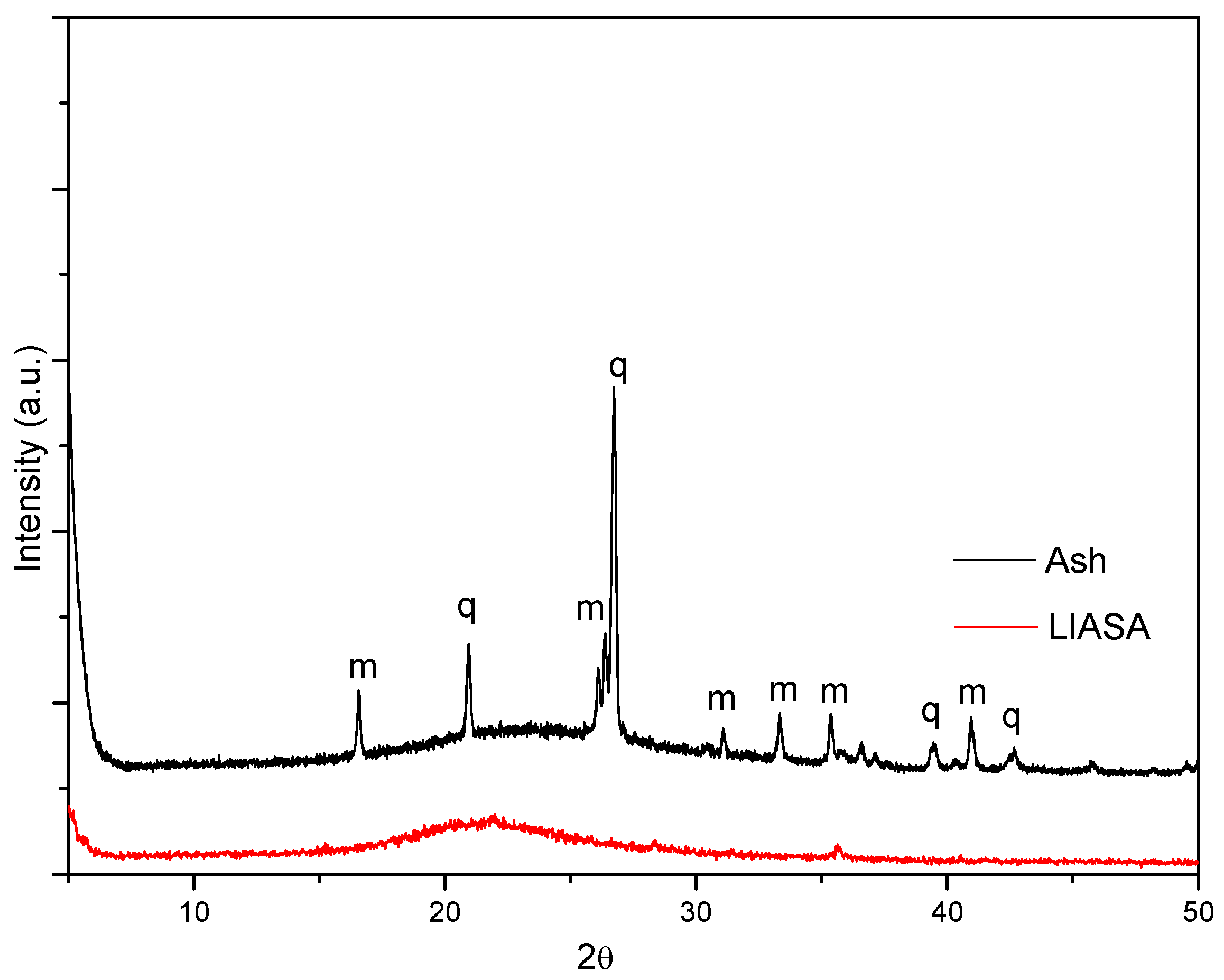
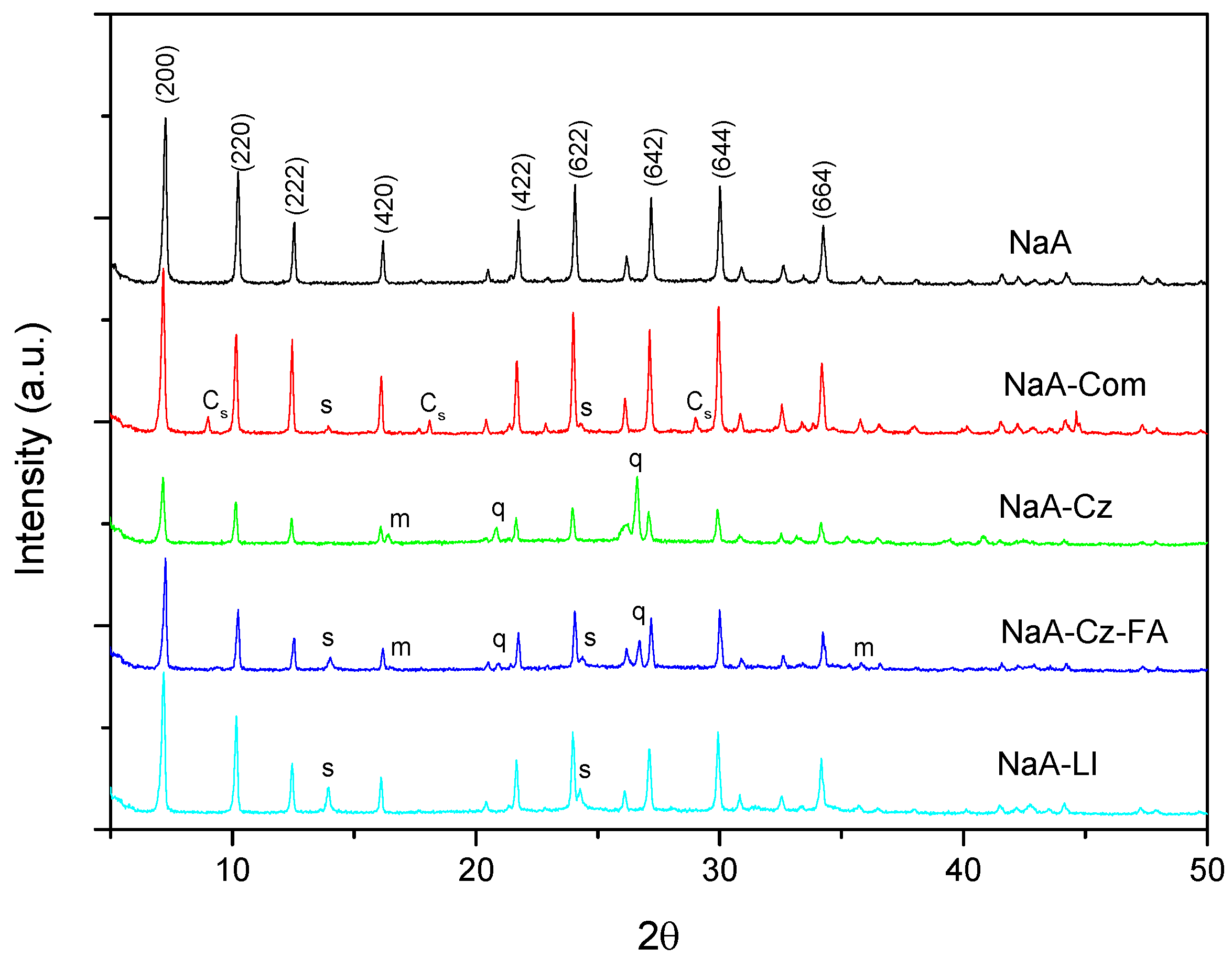
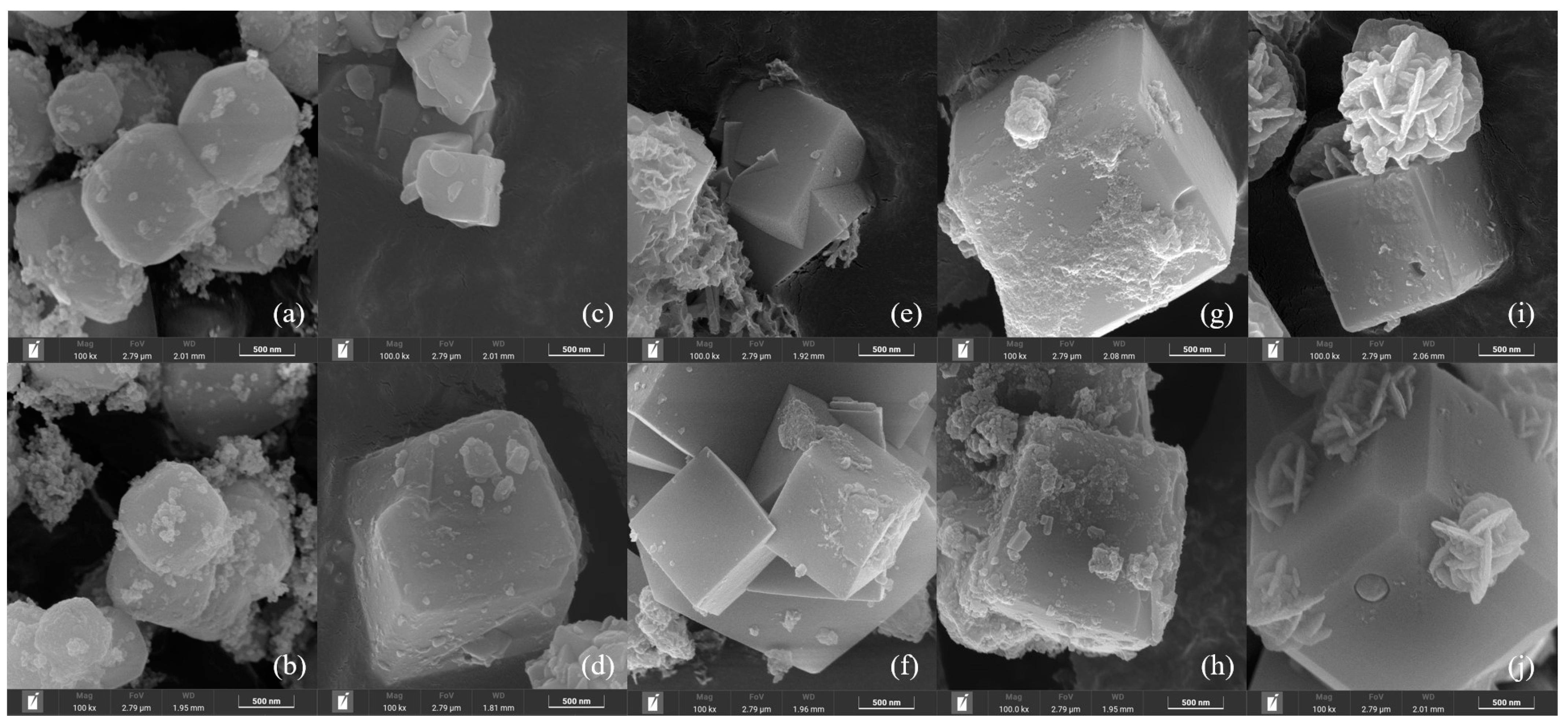
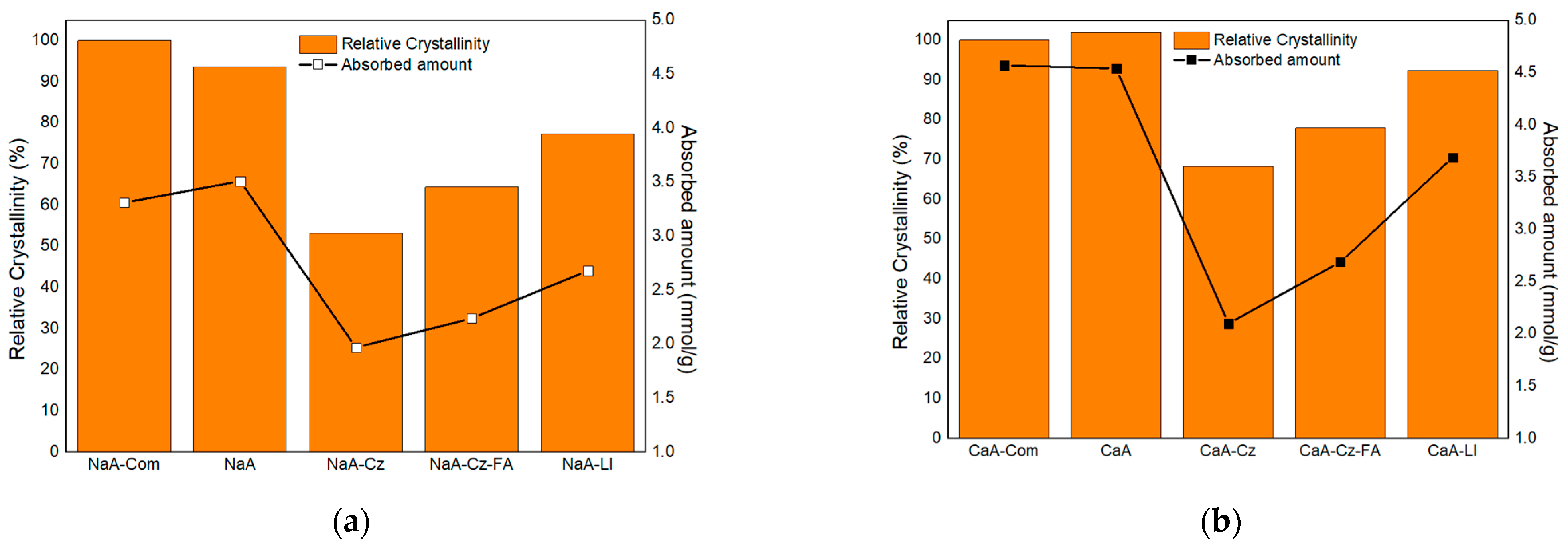
3.1. Characterization of the Materials Obtained
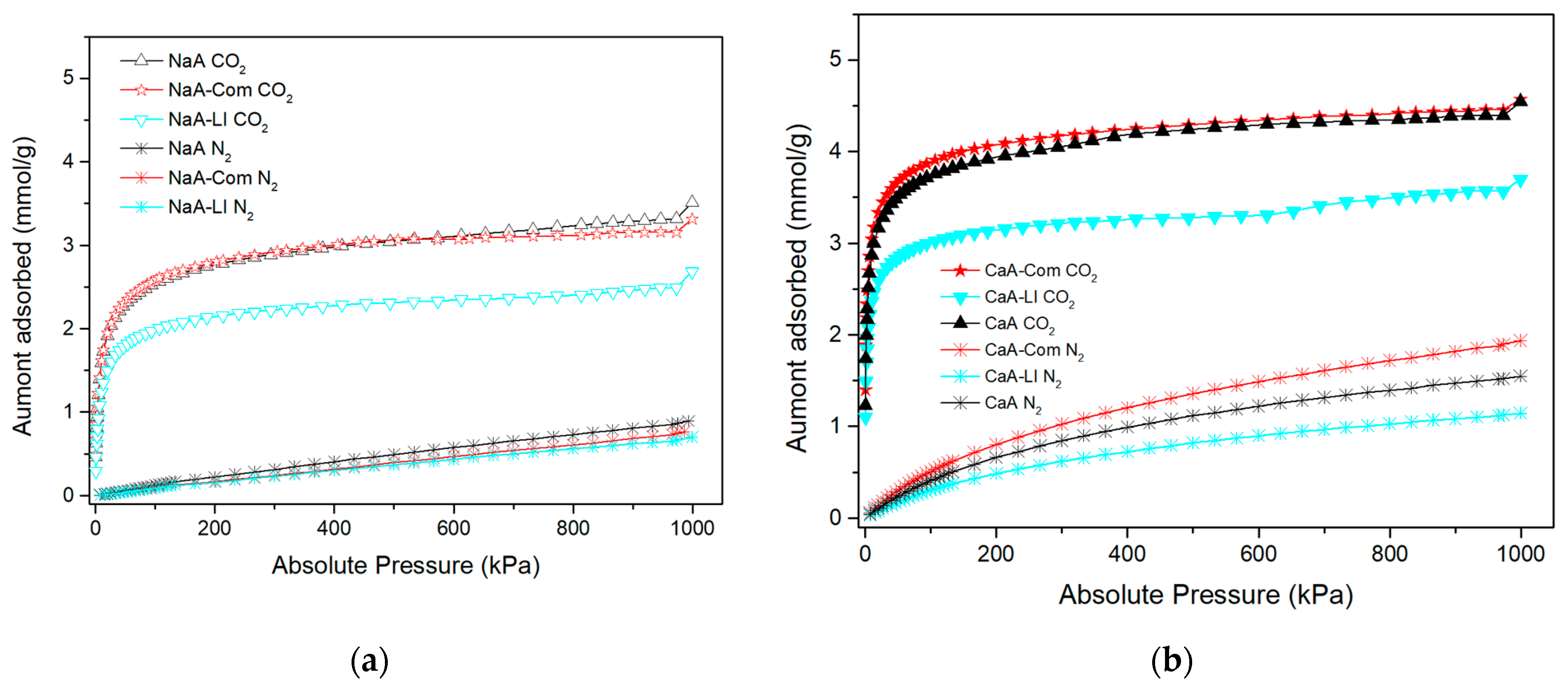
3.2. CO2 Adsorption
3.3. CO2/N2 Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, R.C.; Bieseki, L.; Melguizo, P.V.; Pergher, S.B.C. Environmentally Friendly Zeolites; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-19969-2. [Google Scholar]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Campoverde, J.; Guaya, D. From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials 2023, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Mayorov, V.Y.; et al. A Novel Approach for Rice Straw Agricultural Waste Utilization: Synthesis of Solid Aluminosilicate Matrices for Cesium Immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Yoldi, M.; Fuentes-Ordoñez, E.G.; Korili, S.A.; Gil, A. Zeolite Synthesis from Industrial Wastes. Microporous Mesoporous Mater. 2019, 287, 183–191. [Google Scholar] [CrossRef]
- García-Soto, A.R.; Rodríguez-Niño, G.; Trujillo, C.A. Zeolite LTA Synthesis: Optimising Synthesis Conditions by Using the Modified Sequential Simplex Method. Ing. Investig. 2013, 33, 22–27. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Chen, C.; Pan, S.; Zhang, W.; Meng, X.; Maurer, S.; Feyen, M.; Müller, U.; Xiao, F.S. Atom-Economical Synthesis of a High Silica CHA Zeolite Using a Solvent-Free Route. Chem. Commun. 2015, 51, 16920–16923. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Q. Synthesis and Characterisation of Zeolite LTA with Sheet Structure. Micro Nano Lett. 2020, 15, 433–436. [Google Scholar] [CrossRef]
- de Andrade Bessa, R.; de Sousa Costa, L.; Oliveira, C.P.; Bohn, F.; do Nascimento, R.F.; Sasaki, J.M.; Loiola, A.R. Kaolin-Based Magnetic Zeolites A and P as Water Softeners. Microporous Mesoporous Mater. 2017, 245, 64–72. [Google Scholar] [CrossRef]
- Palomino, M.; Corma, A.; Jordà, J.L.; Rey, F.; Valencia, S. Zeolite Rho: A Highly Selective Adsorbent for CO2/CH4 Separation Induced by a Structural Phase Modification. Chem. Commun. 2012, 48, 215–217. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, S.; Ding, B.; Jia, H.; Chen, P.; Du, T.; Wang, Y. Optimization of Preparation of NaA Zeolite from Fly Ash for CO2 Capture. Environ. Sci. Pollut. Res. 2023, 30, 102803–102817. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Wang, Y.P.; Cui, X.M.; He, Y.; Mao, J. Influence of Synthesis Parameters on NaA Zeolite Crystals. Powder Technol. 2013, 243, 184–193. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. Int. J. Mol. Sci. 2023, 24, 14040. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Yu, S.; Zeng, C.; Yu, L.; Wang, C.; Zhang, L. Binderless Zeolite NaX Microspheres with Enhanced CO2 Adsorption Selectivity. Microporous Mesoporous Mater. 2019, 278, 267–274. [Google Scholar] [CrossRef]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of Zeolites Crystal Growth: New Findings and Open Questions. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A Critical Review of Waste Resources, Synthesis, and Applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Wang, Y.; Du, T.; Jia, H.; Qiu, Z.; Song, Y. Synthesis, Characterization and CO2 Adsorption of NaA, NaX and NaZSM-5 from Rice Husk Ash. Solid State Sci. 2018, 86, 24–33. [Google Scholar] [CrossRef]
- Cruz, T.J.T.; Melo, M.I.S.; Pergher, S. Optimization of Parameters and Methodology for the Synthesis of LTA-Type Zeolite Using Light Coal Ash. Appl. Sci. 2020, 10, 7332. [Google Scholar] [CrossRef]
- Antúnez-garcía, J.; Galv, D.H.; Petranovskii, V.; Murrieta-rico, F.N.; Shelyapina, M.G.; Fuentes-moyado, S. The Effect of Chemical Composition on the Properties of LTA Zeolite: A Theoretical Study. Comput. Mater. Sci. 2021, 196, 110557. [Google Scholar] [CrossRef]
- Wenten, I.G.; Dharmawijaya, P.T.; Aryanti, P.T.P.; Mukti, R.R. Khoiruddin LTA Zeolite Membranes: Current Progress and Challenges in Pervaporation. RSC Adv. 2017, 7, 29520–29539. [Google Scholar] [CrossRef]
- Rigo, R.T.; Pergher, S.B.C.; Petkowicz, D.I.; Dos Santos, J.H.Z. A New Procedure for a Zeolite Synthesis from Natural Clays. Quim. Nova 2009, 32, 21–25. [Google Scholar] [CrossRef]
- Dyballa, M.; Obenaus, U.; Lang, S.; Gehring, B.; Traa, Y.; Koller, H.; Hunger, M. Brønsted Sites and Structural Stabilization Effect of Acidic Low-Silica Zeolite A Prepared by Partial Ammonium Exchange. Microporous Mesoporous Mater. 2015, 212, 110–116. [Google Scholar] [CrossRef]
- Hashemi, S.E.; Sarker, S.; Lien, K.M.; Schnell, S.K.; Austbø, B. Cryogenic vs. Absorption Biogas Upgrading in Liquefied Biomethane Production—An Energy Efficiency Analysis. Fuel 2019, 245, 294–304. [Google Scholar] [CrossRef]
- Baxter, L.; Baxter, A.; Burt, S. Cryogenic CO2 Capture as a Cost-Effective CO2 Capture Process. In Proceedings of the 26th Annual International Pittsburgh Coal Conference 2009, PCC 2009, Pittsburgh, PA, USA, 20–23 September 2009; Volume 1, pp. 762–775. [Google Scholar]
- Kyotani, T.; Richter, H. Zeolite Membrane: From Microstructure to Separation Performance. Membranes 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Valencia, S.; Irabien, Á. Estimating CO2/N2 Permselectivity through Si/Al = 5 Small-Pore Zeolites/PTMSP Mixed Matrix Membranes: Influence of Temperature and Topology. Membranes 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Yogo, K.; Goto, K.; Kai, T.; Yamada, H. Advanced CO2 Capture Technologies: Absorption, Adsorption, and Membrane Separation Methods; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-18857-3. [Google Scholar]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon Capture by Physical Adsorption: Materials, Experimental Investigations and Numerical Modeling and Simulations—A Review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Ferella, F.; Innocenzi, V.; Ippolito, N.M.; De Michelis, I. Adsorption of CO2 by Synthetic Zeolites; EDP Sciences: Les Ulis, France, 2020. [Google Scholar]
- Sai Bhargava Reddy, M.; Ponnamma, D.; Sadasivuni, K.K.; Kumar, B.; Abdullah, A.M. Carbon Dioxide Adsorption Based on Porous Materials. RSC Adv. 2021, 11, 12658–12681. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; McDonald, T.M.; Bae, T.H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a High-Throughput Analyzer in Evaluating Solid Adsorbents for Post-Combustion Carbon Capture via Multicomponent Adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Fujioka, Y.; Yogo, K. Pure Silica CHA Type Zeolite for CO2 Separation Using Pressure Swing Adsorption at High Pressure. J. Mater. Chem. 2012, 22, 20186–20189. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, W.S. CO2 Adsorption on LTA Zeolites: Effect of Mesoporosity. Appl. Surf. Sci. 2014, 311, 107–109. [Google Scholar] [CrossRef]
- Cheung, O.; Wardecki, D.; Bacsik, Z.; Vasiliev, P.; McCusker, L.B.; Hedin, N. Highly Selective Uptake of Carbon Dioxide on the Zeolite |na10.2KCs0.8|-LTA—A Possible Sorbent for Biogas Upgrading. Phys. Chem. Chem. Phys. 2016, 18, 16080–16083. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Liu, Q.; Lobo, R.F. Carbon Dioxide and Nitrogen Adsorption on Cation-Exchanged SSZ-13 Zeolites. Langmuir 2013, 29, 832–839. [Google Scholar] [CrossRef]
- Lozinska, M.M.; Mowat, J.P.S.; Wright, P.A.; Thompson, S.P.; Jorda, J.L.; Palomino, M.; Valencia, S.; Rey, F. Cation Gating and Relocation during the Highly Selective “Trapdoor” Adsorption of CO2 on Univalent Cation Forms of Zeolite Rho. Chem. Mater. 2014, 26, 2052–2061. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Raybaud, P.; Belmabkhout, Y.; Čejka, J.; Zukal, A. The Role of the Extra-Framework Cations in the Adsorption of CO2 on Faujasite y. Phys. Chem. Chem. Phys. 2010, 12, 13534–13546. [Google Scholar] [CrossRef] [PubMed]
- Dusselier, M.; Kang, J.H.; Xie, D.; Davis, M.E. CIT-9: A Fault-Free Gmelinite Zeolite. Angew. Chem. 2017, 129, 13660–13663. [Google Scholar] [CrossRef]
- Davis, M.E. The Quest for Extra-Large Pore, Crystalline Molecular Sieves. Chem.-A Eur. J. 1997, 3, 1745–1750. [Google Scholar] [CrossRef]
- Thompson, R.W.; Huber, M.J. Analysis of the Growth of Molecular Sieve Zeolite NaA in a Batch Precipitation System. J. Cryst. Growth 1982, 56, 711–722. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bae, T.H.; Hudson, M.R.; Mason, J.A.; Queen, W.L.; Dutton, J.J.; Sumida, K.; Micklash, K.J.; Kaye, S.S.; Brown, C.M.; Long, J.R. Evaluation of Cation-Exchanged Zeolite Adsorbents for Post-Combustion Carbon Dioxide Capture. Energy Env. Sci. 2013, 6, 128–138. [Google Scholar] [CrossRef]
- Palomino, M.; Corma, A.; Rey, F.; Valencia, S. New Insights on CO2-Methane Separation Using LTA Zeolites with Different Si/Al Ratios and a First Comparison with MOFs. Langmuir 2010, 26, 1910–1917. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Zgureva, D. Carbon Dioxide Adsorption Studies on Fly Ash Zeolites. Coal Combust. Gasif. Prod. 2016, 8, 54–59. [Google Scholar] [CrossRef]



| Zeolites | Cation Na+ | Cation Ca2+ |
|---|---|---|
| Standard zeolite | NaA | CaA |
| Commercial zeolite | NaA-Com | CaA-Com |
| Zeolite from light coal ash without pre-treatment | NaA-Cz | CaA-Cz |
| Zeolite from coal fly ash with alkaline melt pre-treatment | NaA-Cz-FA | CaA-Cz-FA |
| Zeolite from silica fume | NaA-LI | CaA-LI |
| Component | Coal Ash Light (%) | Silica Fume (%) |
|---|---|---|
| SiO2 | 54.25 | 93.36 |
| Al2O3 | 24.09 | 1.90 |
| Na2O | 1.50 | - |
| CaO | 2.60 | 0.36 |
| K2O | 4.03 | 1.65 |
| MgO | 2.00 | 1.6 |
| Cl | - | 0.5 |
| Fe2O3 | 8.12 | 0.26 |
| TiO2 | 1.75 | - |
| Others | 4.26 | 0.37 |
| Samples | BET | t-Plot | Total Volume | Horvath–Kawazoe | ||
|---|---|---|---|---|---|---|
| SBET | Se | Sm | Vm | Vtotal | Dpm | |
| NaA | 19 | 18 | 1 | 0.001 | 0.028 | 0.50 |
| NaA-Com | 6 | 4 | 2 | 0.001 | 0.008 | 0.50 |
| NaA-Cz | 8 | 7 | 1 | 0.001 | 0.024 | 0.50 |
| NaA-Cz-FA | 14 | 13 | 1 | 0.001 | 0.023 | 0.50 |
| NaA-LI | 6 | 5 | 1 | 0.001 | 0.012 | 0.51 |
| CaA | 468 | 54 | 414 | 0.181 | 0.227 | 0.55 |
| CaA-Com | 537 | 45 | 492 | 0.212 | 0.248 | 0.54 |
| CaA-Cz | 234 | 25 | 209 | 0.090 | 0.125 | 0.54 |
| CaA-Cz-FA | 316 | 38 | 278 | 0.121 | 0.163 | 0.55 |
| CaA-LI | 434 | 37 | 397 | 0.170 | 0.202 | 0.56 |
| Samples | Amount Adsorbed in 15 kPa (mmol/g) | Amount Adsorbed in 101.33 kPa (mmol/g) | Amount Adsorbed in 1000 kPa (mmol/g) |
|---|---|---|---|
| NaA | 1.79 | 2.54 | 3.51 |
| NaA-Com | 1.76 | 2.58 | 3.31 |
| NaA-Cz | 0.85 | 1.22 | 1.97 |
| NaA-Cz-FA | 1.18 | 1.70 | 2.24 |
| NaA-LI | 1.40 | 1.98 | 2.68 |
| CaA | 3.02 | 3.73 | 4.54 |
| CaA-Com | 3.19 | 3.88 | 4.57 |
| CaA-Cz | 1.36 | 1.71 | 2.10 |
| CaA-Cz-FA | 1.72 | 2.19 | 2.69 |
| CaA-LI | 2.49 | 3.01 | 3.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.; Elias, E.B.C.D.; Cruz, T.J.T.; Pinto, F.G.H.S.; de Mello, M.I.S.; Bieseki, L.; Pergher, S.B.C. Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption. Coatings 2024, 14, 680. https://doi.org/10.3390/coatings14060680
da Silva A, Elias EBCD, Cruz TJT, Pinto FGHS, de Mello MIS, Bieseki L, Pergher SBC. Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption. Coatings. 2024; 14(6):680. https://doi.org/10.3390/coatings14060680
Chicago/Turabian Styleda Silva, Aryandson, Emanuel Bruno Costa Dantas Elias, Thiago Jackson Torres Cruz, Francisco Gustavo Hayala Silveira Pinto, Mariele Iara Souza de Mello, Lindiane Bieseki, and Sibele Berenice Castellã Pergher. 2024. "Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption" Coatings 14, no. 6: 680. https://doi.org/10.3390/coatings14060680
APA Styleda Silva, A., Elias, E. B. C. D., Cruz, T. J. T., Pinto, F. G. H. S., de Mello, M. I. S., Bieseki, L., & Pergher, S. B. C. (2024). Synthesis and Cation Exchange of LTA Zeolites Synthesized from Different Silicon Sources Applied in CO2 Adsorption. Coatings, 14(6), 680. https://doi.org/10.3390/coatings14060680








