Corrosion-Resistant Organic Superamphiphobic Coatings
Abstract
1. Introduction
2. Principles of Superamphiphobicity
2.1. Basic Principles
2.1.1. Young’s Equation

2.1.2. Wenzel Model and Cassie–Baxter Model
2.2. Principle of Corrosion Resistance in Superamphiphobic Coatings
3. Regulation Strategies of Corrosion-Resistant Superamphiphobic Coating Performance
3.1. Liquid-Repellency Performance
3.2. Wear Resistance Performance
3.3. Adhesion Performance
3.4. Antibacterial Performance
3.5. Self-Repairing Performance
4. Summary and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. NACE International Impact Report: International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Cui, J.; Bao, Y.; Sun, Y.; Wang, H.; Jing, L.I. Critical factors on corrosion protective waterborne coatings containing functionalized graphene oxide: A review. Compos. Part A 2023, 174, 107729. [Google Scholar] [CrossRef]
- Kulyk, B.; Freitas, M.A.; Santos, N.F.; Mohseni, F.; Carvalho, A.F.; Yasakau, K.; Fernandes, A.J.S.; Bernardes, A.; Figueiredo, B.; Silva, R.; et al. A critical review on the production and application of graphene and graphene-based materials in anti-corrosion coatings. Crit. Rev. Solid State Mater. Sci. 2021, 47, 309–355. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Yu, H. Bioinspired strategies for making superior graphene composite coatings. Chem. Eng. J. 2022, 435, 134808. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, W.; Zhao, H.; Wang, L. Non-covalent assembly of a super-tough, highly stretchable and environmentally adaptable self-healing material inspired by nacre. J. Mater. Chem. A 2021, 9, 20737–20747. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Sanjid, A.; Banerjee, P.C.; Arya, A.K.; Parmar, R.; Amati, M.; Gregoratti, L. Remarkably Corrosion Resistant Graphene Coating on Steel Enabled Through Metallurgical Tailoring. Small 2023, e2302498. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Caldona, E.B.; Leng, W.; Street, J.; Wang, G.; Zhang, Z. Anticorrosive Epoxy Coatings Containing Ultrafine Bamboo Char and Zinc Particles. J. Environ. Chem. Eng. 2021, 9, 105707. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Zhou, M.; Liu, P.; Yu, H. Super-anticorrosive inverse nacre-like graphene-epoxy composite coating. Carbon 2021, 181, 204–211. [Google Scholar] [CrossRef]
- Song, S.; Yan, H.; Cai, M.; Huang, Y.; Fan, X.; Zhu, M. Multilayer structural epoxy composite coating towards long-term corrosion/wear protection. Carbon 2021, 183, 42–52. [Google Scholar] [CrossRef]
- Prabakaran, E.; Vasanth Kumar, D.; Jaganathan, A.; Ashok Kumar, P.; Veeerapathran, M. Analysis on Fiber Reinforced Epoxy Concrete Composite for Industrial Flooring—A Review. J. Phys. Conf. Ser. 2022, 2272, 012026. [Google Scholar] [CrossRef]
- Luo, H.; Wei, H.; Wang, L.; Gao, Q.; Chen, Y.; Xiang, J.; Fan, H. Anti-smudge and self-cleaning characteristics of waterborne polyurethane coating and its construction. J. Colloid Interface Sci. 2022, 628, 1070–1081. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings—A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Li, W.; Wang, W.; Zhao, X.; Song, L. Multi-level self-healing ability of shape memory polyurethane coating with microcapsules by induction heating. Chem. Eng. J. 2019, 368, 1033–1044. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, J.; Jian, S.; Tan, H.; Lv, Y.; Hu, H.; Wang, W.; Yang, R.; Manuka, M.; Yin, Y.; et al. Photocurable resin as rapid in-situ protective coating for slag concrete against dry shrinkage. Constr. Build. Mater. 2023, 396, 132171. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Rao, Y.; Shen, X.; Tang, Z.; Chen, H. Facile fabrication of robust and universal UV-curable polyurethane composite coatings with antibacterial properties. Polym. Eng. Sci. 2023, 63, 3371–3381. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.-X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Meng, G.-H.; Chen, L.; Li, G.-R.; Liu, M.-J.; Zhang, W.-X.; Zhao, L.-N.; Zhang, Q.; Zhang, X.-D.; Wan, C.-L.; et al. Progress in ceramic materials and structure design toward advanced thermal barrier coatings. J. Adv. Ceram. 2022, 11, 985–1068. [Google Scholar] [CrossRef]
- Azarian, N.; Mousavi Khoei, S.M. Characteristics of a multi-component MgO-based bioceramic coating synthesized in-situ by plasma electrolytic oxidation. J. Magnes. Alloys 2021, 9, 1595–1608. [Google Scholar] [CrossRef]
- Yuan, Q.; Yan, L.; Tian, J.; Ding, W.; Heng, Z.; Liang, M.; Chen, Y.; Zou, H. In Situ Ceramization of Nanoscale Interface Enables Aerogel with Thermal Protection at 1950 °C. ACS Nano 2024, 18, 3520–3530. [Google Scholar] [CrossRef]
- Shi, Z.-A.; Wu, J.-M.; Fang, Z.-Q.; Tian, C.; Wang, Q.-W.; Mao, C.; Fu, L.-X.; Shi, Y.-S. Investigation of curing behavior and mechanical properties of SiC ceramics prepared by vat photopolymerization combined with pressureless liquid-phase sintering using Al2O3-coated SiC powder. Addit. Manuf. 2024, 79, 103942. [Google Scholar] [CrossRef]
- Tombesi, A.; Li, S.; Sathasivam, S.; Page, K.; Heale, F.L.; Pettinari, C.; Carmalt, C.J.; Parkin, I.P. Aerosol-assisted chemical vapour deposition of transparent superhydrophobic film by using mixed functional alkoxysilanes. Sci. Rep. 2019, 9, 7549. [Google Scholar] [CrossRef]
- Adarraga, O.; Agustín-Sáenz, C.; Bustero, I.; Brusciotti, F. Superhydrophobic and oleophobic microtextured aluminum surface with long durability under corrosive environment. Sci. Rep. 2023, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Zhang, Y.; Hu, N.; Zhang, J. Superamphiphobic triple-scale micro-/nanostructured aluminum surfaces with self-cleaning and anti-icing properties. J. Mater. Sci. 2021, 56, 15463–15480. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, S.; Geng, H.; Zhang, X.; Zhang, M.; Xu, F.; Lin, D.; Gao, Y.; Wang, H. Robust and multifunctional superamphiphobic coating toward effective anti-adhesion. Chem. Eng. J. 2022, 428, 131162. [Google Scholar] [CrossRef]
- Sattari, M.; Olad, A.; Maryami, F.; Ahadzadeh, I.; Nofouzi, K. Facile fabrication of durable and fluorine-free liquid infused surfaces on aluminum substrates with excellent anti-icing, anticorrosion, and antibiofouling properties. Surf. Interfaces 2023, 38, 102860. [Google Scholar] [CrossRef]
- Si, W.; Guo, Z. Enhancing the lifespan and durability of superamphiphobic surfaces for potential industrial applications: A review. Adv. Colloid Interface Sci. 2022, 310, 102797. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, J.; Xiao, F.; Yuan, S.; Lu, H.; Liang, B. Preparation and Antiscaling Application of Superhydrophobic Anodized CuO Nanowire Surfaces. Ind. Eng. Chem. Res. 2015, 54, 6874–6883. [Google Scholar] [CrossRef]
- Peng, J.; Geng, H.; Xu, F.; Zhang, M.; Ye, P.; Jiang, Y.; Wang, H. Endowing versatility and superamphiphobicity to composite coating via a bioinspired strategy. Chem. Eng. J. 2022, 455, 140772. [Google Scholar]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Liu, W. Special Superwetting Materials from Bioinspired to Intelligent Surface for On-Demand Oil/Water Separation: A Comprehensive Review. Small 2022, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, S.; Zhao, X.; Shao, L.; Pan, Y. Durable Superoleophobic Janus Fabric with Oil Repellence and Anisotropic Water-Transport Integration toward Energetic-Efficient Oil–Water Separation. ACS Appl. Mater. Interfaces 2022, 32, 37170–37181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Z.; Luo, X.; Chen, C.; Jiang, G.; Hu, X.; Peng, R.; Zhang, H.; Zhong, M. Superomniphobic surfaces for easy-removals of environmental-related liquids after icing and melting. Nano Res. 2022, 16, 3267–3277. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Huang, J.; Guo, Z.; Liu, W. Advance in Structural Classification and Stability Study of Superamphiphobic Surfaces. J. Bionic Eng. 2022, 20, 366–389. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Jin, R.; Lin, Z.; Qiu, H.; Xu, Y. Mechanism analysis and durability evaluation of anti-icing property of superhydrophobic surface. Int. J. Heat Mass Transfer 2020, 156, 119768. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, H.; Gu, X.; Yang, T.; Tian, C. Thermal-hydraulic performance of super-amphiphobic louver-fin flat-tube heat exchanger under fouled condition. Appl. Therm. Eng. 2023, 233, 121142. [Google Scholar] [CrossRef]
- Yin, X.; Liu, L.; Yan, Y.; Yang, K.; Pi, P.; Peng, X.; Wen, X. Superamphiphobic surface with high aperture ratio interconnected pore structures for anti–condensation and repelling hot fluids. Mater. Today Nano 2023, 24, 100417. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes—A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Hejazi, V. Why Superhydrophobic Surfaces Are Not Always Icephobic. ACS Nano 2012, 6, 8488–8491. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1832, 1, 171–172. [Google Scholar]
- Wang, B.; Nian, J.-Y.; Tie, L.; Zhang, Y.-B.; Guo, Z.-G. Theoretical progress in designs of stable superhydrophobic surfaces. Acta Phys. Sin. 2013, 62, 146801. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Rodriguez, E.; Roberts, M.R.; Yu, H.; Huh, C.; Bryant, S.L. Enhanced Migration of Surface-Treated Nanoparticles in Sedimentary Rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Ha, M.Y.; Yoon, H.S.; Ambrosia, M. Dynamic Behavior of Water Droplets on Solid Surfaces with Pillar-Type Nanostructures. Langmuir 2012, 28, 5360–5371. [Google Scholar] [CrossRef]
- Quéré, D. Wetting and Roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic surfaces. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Yi, W.; Kai, Y.; Guilin, X.; Chenguang, Y.; Dong, W. Facile preparation of super-oleophobic TiO2/SiO2 composite coatings by spraying method. Prog. Org. Coat. 2021, 159, 106411. [Google Scholar]
- Liu, P.; Liu, S.Q.; Yu, X.Q.; Zhang, Y.F. Silane-triggered fabrication of stable waterborne superamphiphobic coatings. Chem. Eng. J. 2021, 406, 127153. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Li, X.; Hou, B. Self-cleaning and corrosion-resistant superamphiphobic coating with super-repellency towards low-surface-tension liquids. J. Mater. Res. Technol. 2023, 23, 1094–1104. [Google Scholar] [CrossRef]
- Chu, D.; Singh, S.C.; Yong, J.; Zhan, Z.; Sun, X.; Duan, J.A.; Guo, C. Superamphiphobic Surfaces with Controllable Adhesion Fabricated by Femtosecond Laser Bessel Beam on PTFE. Adv. Mater. Interfaces 2019, 6, 14. [Google Scholar] [CrossRef]
- Song, W.; Major, Z.; Guo, Y.; Karsch, S.; Guo, H.; Ferenc, K.; Fukumoto, M.; Dingwell, D.B. Biomimetic Super “Silicate” Phobicity and Superhydrophobicity of Ceramic Material. Adv. Mater. Interfaces 2022, 9, 2201267. [Google Scholar] [CrossRef]
- Ye, Z.; Li, S.; Zhao, S.; Deng, L.; Zhang, J.; Dong, A. Textile coatings configured by double-nanoparticles to optimally couple superhydrophobic and antibacterial properties. Chem. Eng. J. 2021, 420, 127680. [Google Scholar] [CrossRef]
- Xia, Y.; Gu, W.; Shao, L.; Jiao, X.; Ji, Y.; Deng, W.; Yu, X.; Zhang, Y.; Zhang, Y. Flexibility and abrasion tolerance of superamphiphobic coatings with rigid core–shell particles. Chem. Eng. J. 2023, 476, 146746. [Google Scholar] [CrossRef]
- Jiao, X.; Li, M.; Yu, X.; Wong, W.S.Y.; Zhang, Y. Oil-immersion stable superamphiphobic coatings for long-term super liquid-repellency. Chem. Eng. J. 2021, 420, 127606. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, J.; Fu, Y.; Zhao, P.; Zhang, Y.; He, B.; He, P. Underwater superoleophobic APTES-SiO2/PVA organohydrogel for low-temperature tolerant, self-healing, recoverable oil/water separation mesh. Chem. Eng. J. 2020, 382, 122925. [Google Scholar] [CrossRef]
- Xu, H.; Miao, C.; Wang, L.; Zhang, L.; Feng, H.; Qiu, J. A Robust Superhydrophobic Perfluoropolysiloxane and Self-doped Polyaniline/Epoxy Resin Composite Coating with Excellent Performance. Chem. Lett. 2021, 50, 1818–1821. [Google Scholar] [CrossRef]
- Miao, C.; Xun, X.; Dodd, L.J.; Niu, S.; Wang, H.; Yan, P.; Wang, X.-C.; Li, J.; Wu, X.; Hasell, T.; et al. Inverse Vulcanization with SiO2-Embedded Elemental Sulfur for Superhydrophobic, Anticorrosion, and Antibacterial Coatings. ACS Appl. Polym. Mater. 2022, 4, 4901–4911. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, B.; Ye, T.; Gao, W.; Pei, G.; Luo, J.; Deng, J.; Yuan, W. One-Step Fabrication of Flexible Bioinspired Superomniphobic Surfaces. ACS Appl. Mater. Interfaces 2022, 34, 39665–39672. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, J.; Tian, N.; Liang, W.; Zhang, J. Facile Preparation of Robust Superamphiphobic Coatings on Complex Substrates via Nonsolvent-Induced Phase Separation. ACS Appl. Mater. Interfaces 2022, 14, 49047–49058. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2016, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Zhang, B.; Zhang, J. Waterborne robust superamphiphobic coatings based on palygorskite for self-cleaning and anti-fouling. Colloids Surf. A 2023, 672, 131759. [Google Scholar] [CrossRef]
- Song, S.; Yan, H.; Cai, M.; Huang, Y.; Fan, X.; Zhu, M. Constructing Mechanochemical Durable Superhydrophobic Composite Coating towards Superior Anticorrosion. Adv. Mater. Technol. 2022, 7, 2101223. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, M.; Wen, J.; Yuan, J.; Zhu, L.; Yu, H. Robust, Transparent, and Superhydrophobic Coating Fabricated with Waterborne Polyurethane and Inorganic Nanoparticle Composites. Ind. Eng. Chem. Res. 2019, 19, 8050–8060. [Google Scholar] [CrossRef]
- Qiao, Z.; Ren, G.; Chen, X.; Gao, Y.; Tuo, Y.; Lu, C. Fabrication of Robust Waterborne Superamphiphobic Coatings with Antifouling, Heat Insulation, and Anticorrosion. ACS Omega 2023, 8, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Yanping, D. Improve the mechanical durability of superhydrophobic/superamphiphobic coating through multiple cross-linked mesh structure. Colloids Surf. A 2022, 642, 5. [Google Scholar]
- Yu, D.; Huang, J.; Zhang, Z.; Weng, J.; Xu, X.; Zhang, G.; Zhang, J.; Wu, X.; Johnson, M.; Lyu, J.; et al. Simultaneous Realization of Superoleophobicity and Strong Substrate Adhesion in Water via a Unique Segment Orientation Mechanism. Adv. Mater. 2021, 34, 2106908. [Google Scholar] [CrossRef]
- Meena, M.K.; Tudu, B.K.; Kumar, A.; Bhushan, B. Development of polyurethane-based superhydrophobic coatings on steel surfaces. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190446. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xu, J.; Zhao, Q. Biomimetic Superhydrophobic Biobased Polyurethane-Coated Fertilizer with Atmosphere “Outerwear”. ACS Appl. Mater. Interfaces 2017, 18, 15868–15879. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Tian, K.; Li, Y.; Su, C.; Duan, F.; Xu, Y. Super-hydrophobic PTFE hollow fiber membrane fabricated by electrospinning of Pullulan/PTFE emulsion for membrane deamination. Sep. Purif. Technol. 2020, 274, 118186. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Liu, W.; Steffen, W.; Butt, H.-J. Fabrication of Stretchable Superamphiphobic Surfaces with Deformation-Induced Rearrangeable Structures. Adv. Mater. 2022, 34, 2107901. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, S.; Amirfazli, A.; Rahim Siddiqui, A.; Li, W. Recent Advances in Antibacterial Superhydrophobic Coatings. Adv. Eng. Mater. 2022, 24, 2101053. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Y.; Li, Q.; Liu, Y.; She, Z.; Chen, F.; Li, L.; Zhang, X.; Zhang, P. Researching a highly anti-corrosion superhydrophobic film fabricated on AZ91D magnesium alloy and its anti-bacteria adhesion effect. Mater. Charact. 2015, 99, 200–209. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Wang, Q.; Zhang, W.; Gao, N.; Li, J.; Okonkwo, P.C.; Liu, F.; Han, E.-H. Anti-bacterial, icephobic, and corrosion protection potentials of superhydrophobic nanodiamond composite coating. Colloids Surf. A 2021, 630, 127532. [Google Scholar] [CrossRef]
- Bruzaud, J.; Tarrade, J.; Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Guittard, F.; Herry, J.-M.; Guilbaud, M.; Bellon-Fontaine, M.-N. The design of superhydrophobic stainless steel surfaces by controlling nanostructures: A key parameter to reduce the implantation of pathogenic bacteria. Mater. Sci. Eng. C 2017, 73, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Li, C.; Huang, X.; Yang, T.; Wang, Y.; Mao, J.; Wang, Y.; Cui, X.; Xu, H.; Wu, X. A robust anticorrosive coating derived from superhydrophobic, superoleophobic, and antibacterial SiO2@POS/N+ composite materials. Mater. Today Commun. 2023, 35, 105566. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Z. Recent advances in self-healing superhydrophobic coatings. Nano Today 2023, 51, 101933. [Google Scholar] [CrossRef]
- Qin, L.; Chen, N.; Zhou, X.; Pan, Q. A superhydrophobic aerogel with robust self-healability. J. Mater. Chem. A 2018, 6, 4424–4431. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, J.; He, Y.; Sun, L.; Zhao, Y.; Zhang, X. Heating repairable superamphiphobic coatings for long-lasting antifouling application. Colloids Surf. A 2023, 678, 132517. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Self-Healing Underwater Superoleophobic and Antibiofouling Coatings Based on the Assembly of Hierarchical Microgel Spheres. ACS Nano 2015, 10, 1386–1394. [Google Scholar] [CrossRef] [PubMed]

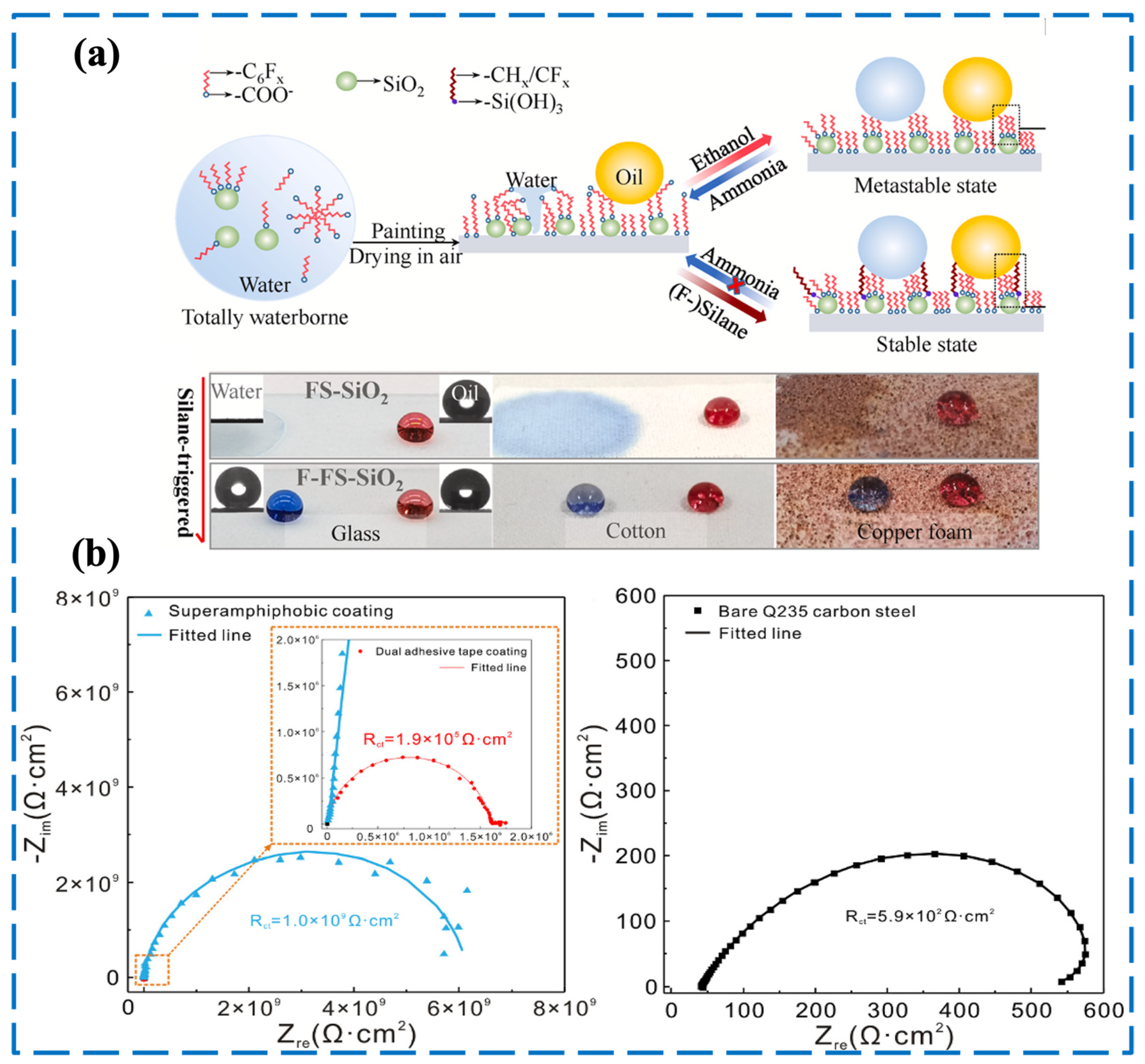

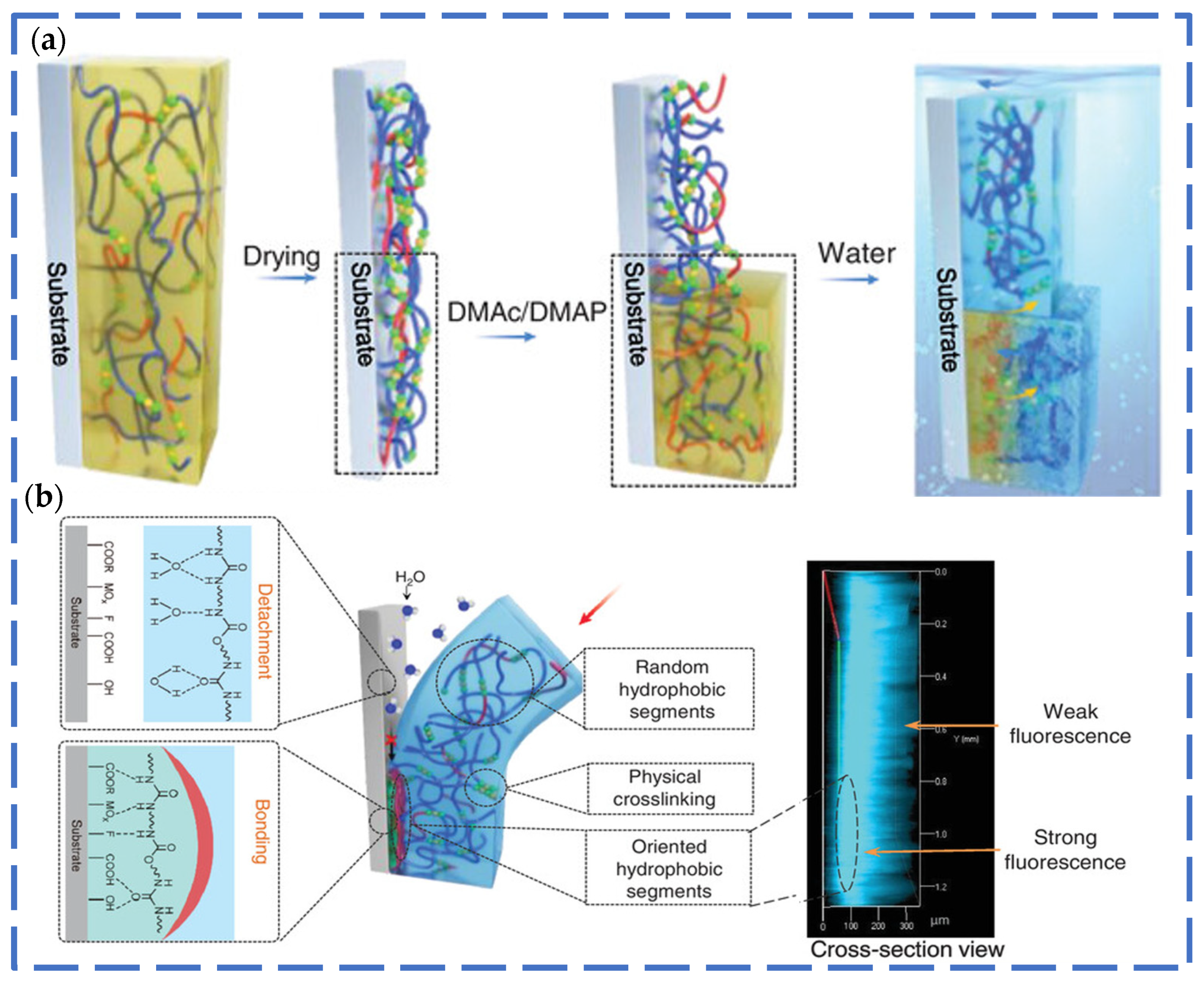
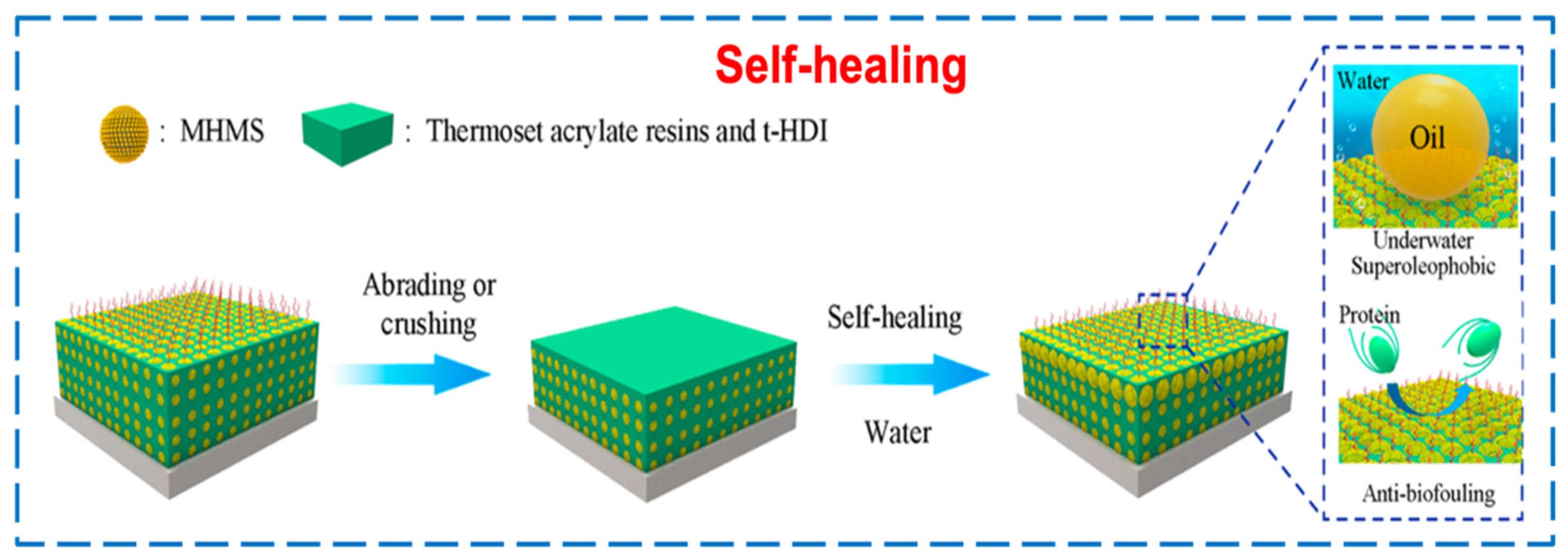
| Dsilica (nm) | 10 | 15 | 50 | 100 |
|---|---|---|---|---|
| CA(deg) | 162 ± 1.3 | 163.9 ± 2.3 | 154.1 ± 1.5 | 151.4 ± 2.2 |
| SA (deg) | 4.0 ± 1.0 | 2.0 ± 0.6 | 17.3 ± 2.1 |
| Step | Low-Molecular-Weight Biocides | Polymeric Biocides | Dendritic Biocides |
|---|---|---|---|
| Initial adsorption | Low | High | High |
| Binding to the membrane | Low | Medium | High |
| Diffusion past the cell wall | High | Low | Medium |
| Disruption of the membrane | Low | Medium | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wei, R.; Zhang, Q.; Fu, A.; Lv, N.; Yuan, J. Corrosion-Resistant Organic Superamphiphobic Coatings. Coatings 2024, 14, 678. https://doi.org/10.3390/coatings14060678
Qi Y, Wei R, Zhang Q, Fu A, Lv N, Yuan J. Corrosion-Resistant Organic Superamphiphobic Coatings. Coatings. 2024; 14(6):678. https://doi.org/10.3390/coatings14060678
Chicago/Turabian StyleQi, Yixing, Rong Wei, Qiuli Zhang, Anqing Fu, Naixin Lv, and Juntao Yuan. 2024. "Corrosion-Resistant Organic Superamphiphobic Coatings" Coatings 14, no. 6: 678. https://doi.org/10.3390/coatings14060678
APA StyleQi, Y., Wei, R., Zhang, Q., Fu, A., Lv, N., & Yuan, J. (2024). Corrosion-Resistant Organic Superamphiphobic Coatings. Coatings, 14(6), 678. https://doi.org/10.3390/coatings14060678






