Advances in Organic Multiferroic Junctions
Abstract
:1. Introduction
2. Characteristics of Organic Spin Valves and Organic Ferroelectric Junctions
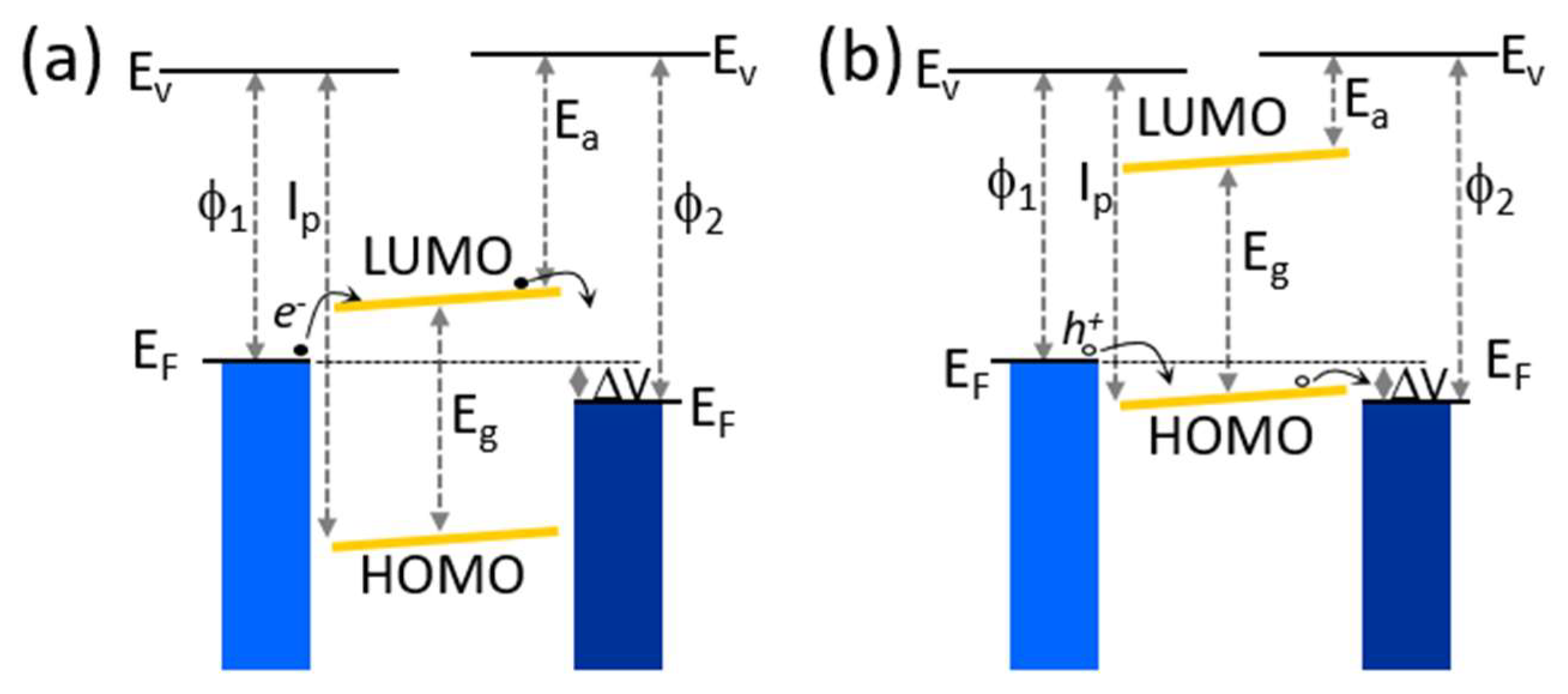
2.1. Magnetic Junctions and Organic Spin Valves
- The spin polarization of the ferromagnetic electrodes;
- Tunneling/Spin injection from the ferromagnetic electrode into the organic layer;
- Spin transport through the organic layer;
- Spin detection at the other electrode.
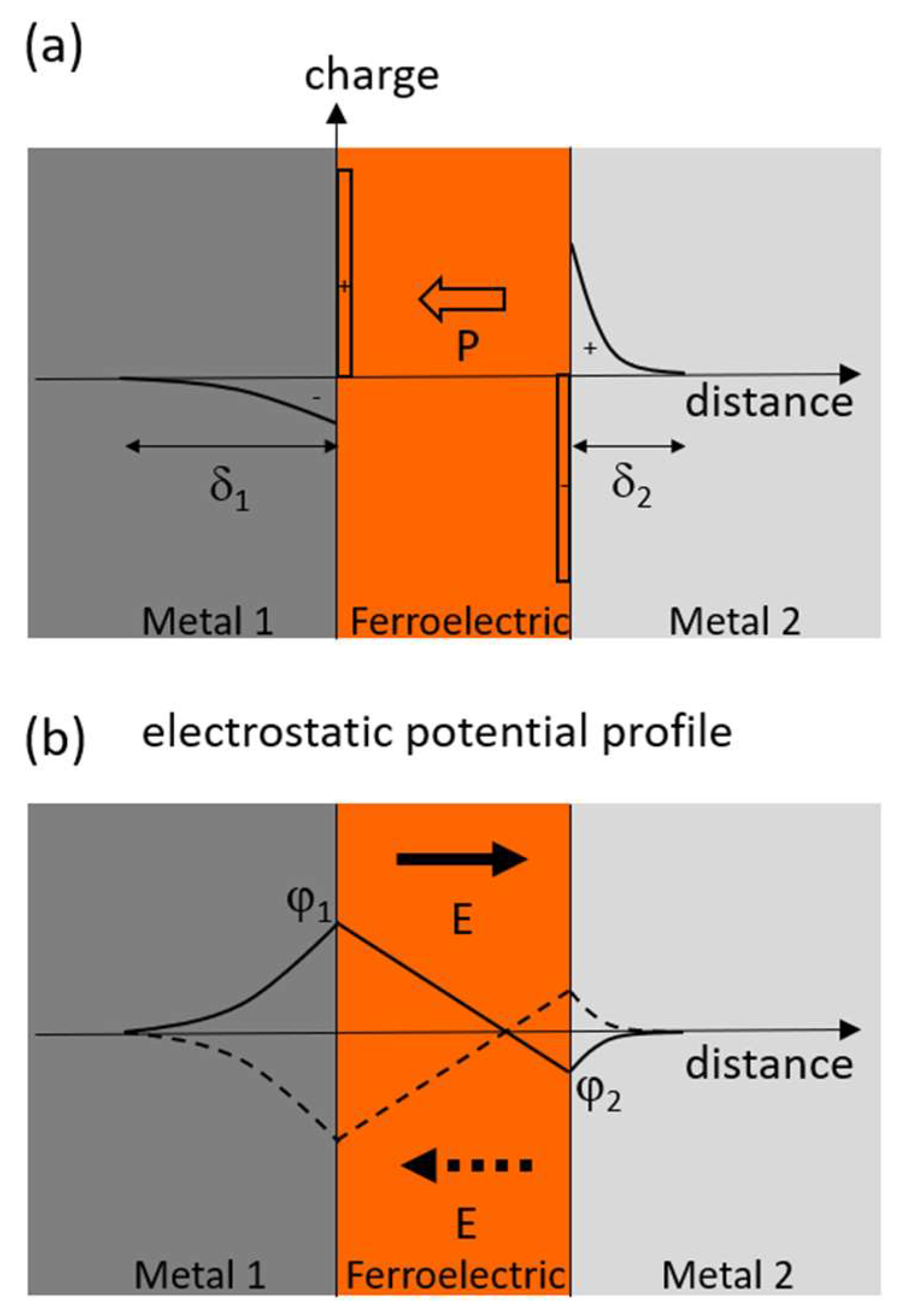
2.2. Ferroelectric Junctions and Organic Ferroelectrics
- the presence of electrical dipoles;
- the arrangement of the dipoles in non-centrosymmetric structures to avoid canceling each other through dipole–dipole interactions; and
- the compliance of properties with electrical polarization switching transitions that should have a sufficiently low energy-switching barrier affordable by the organic system.
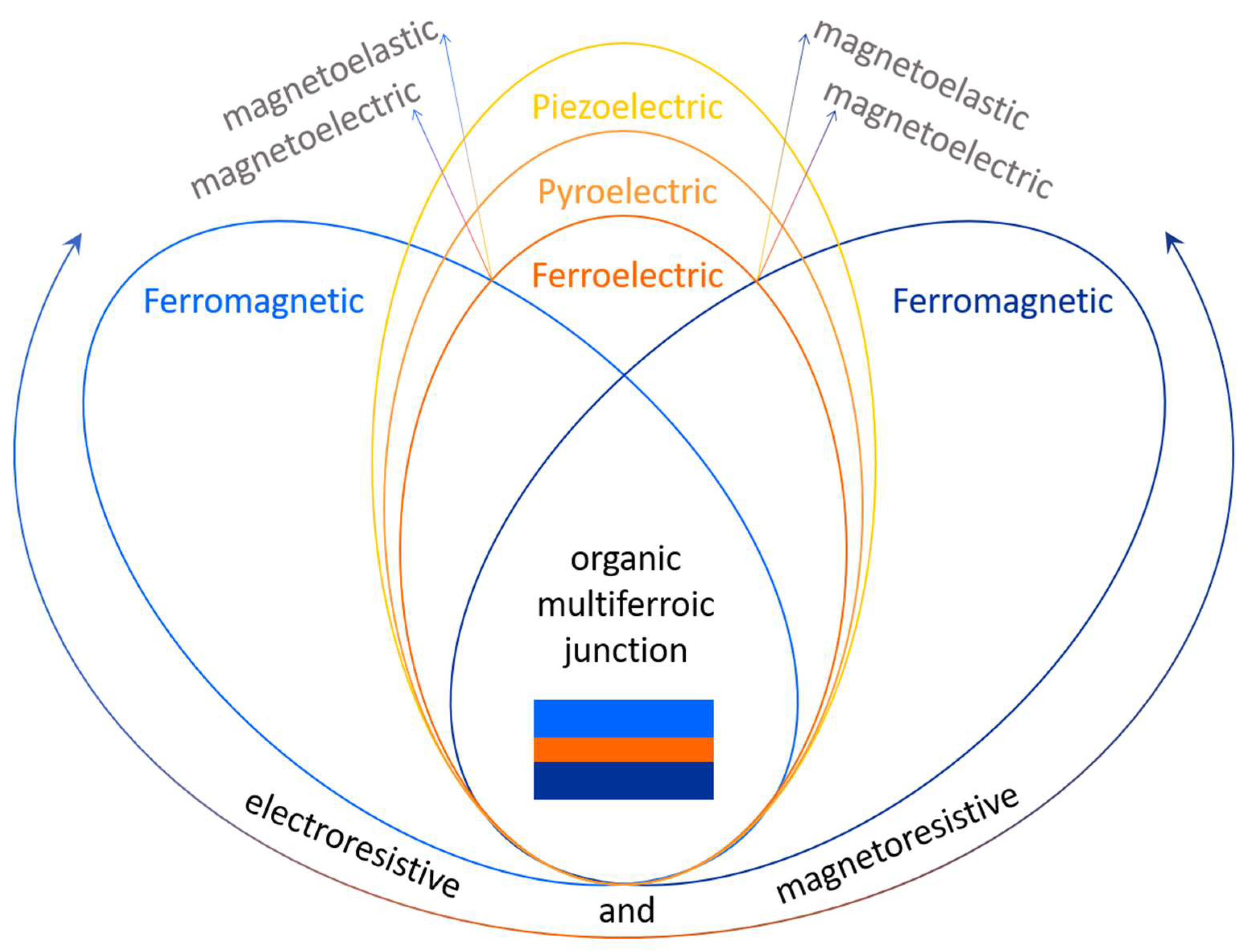
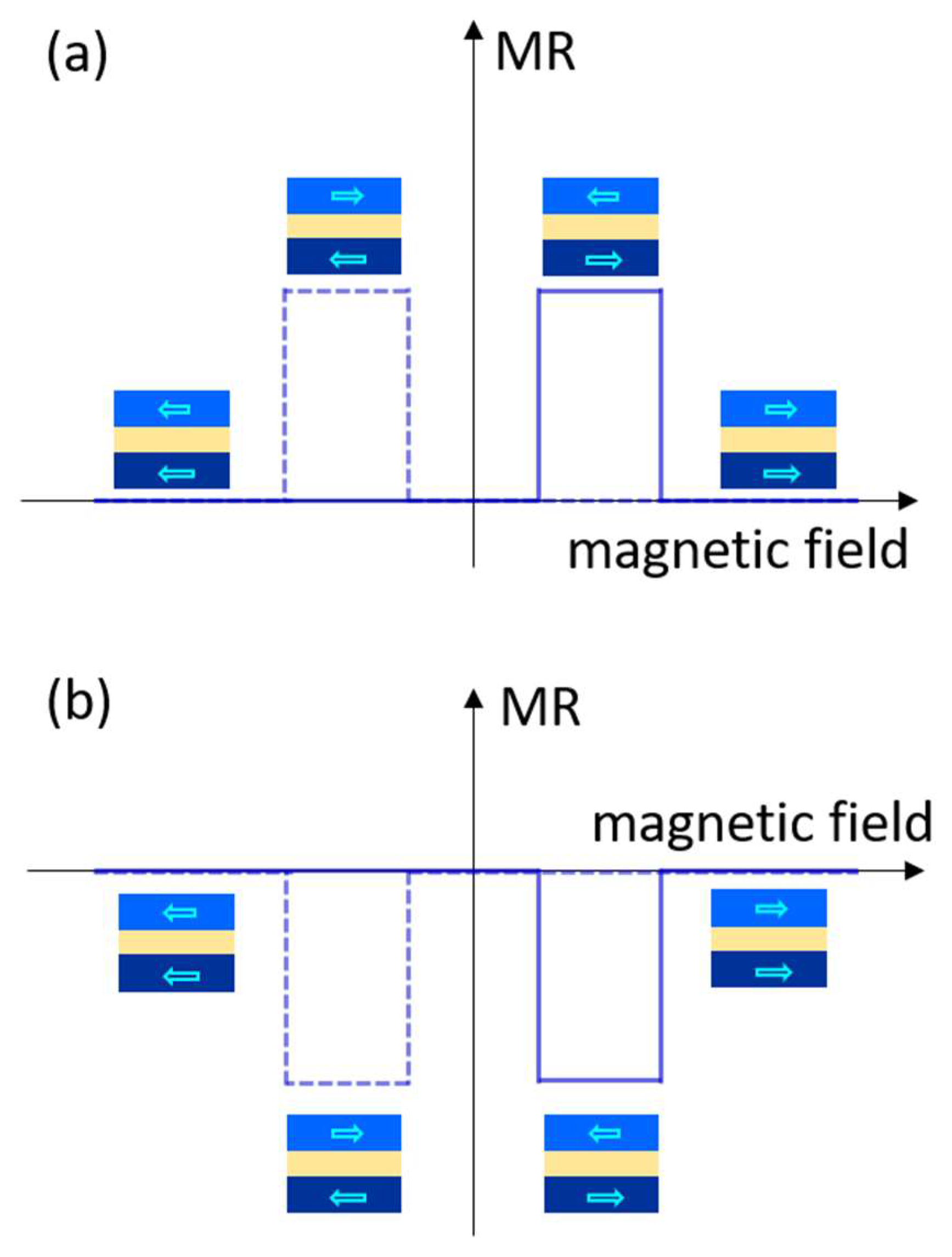
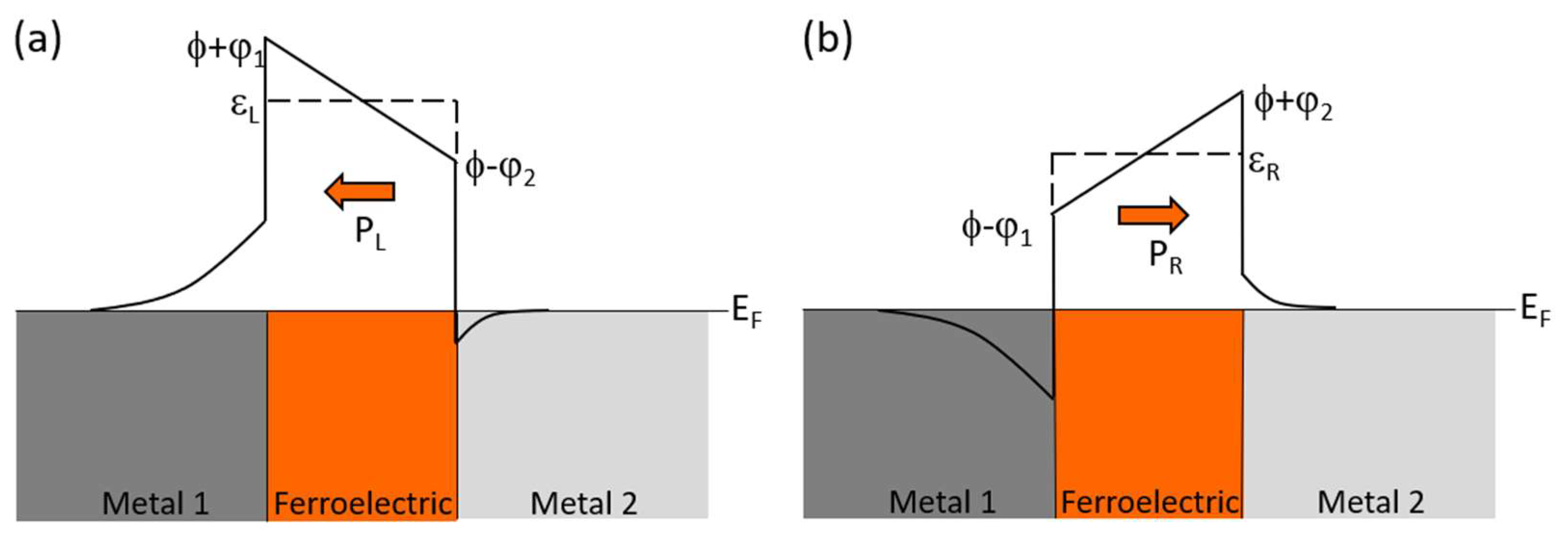
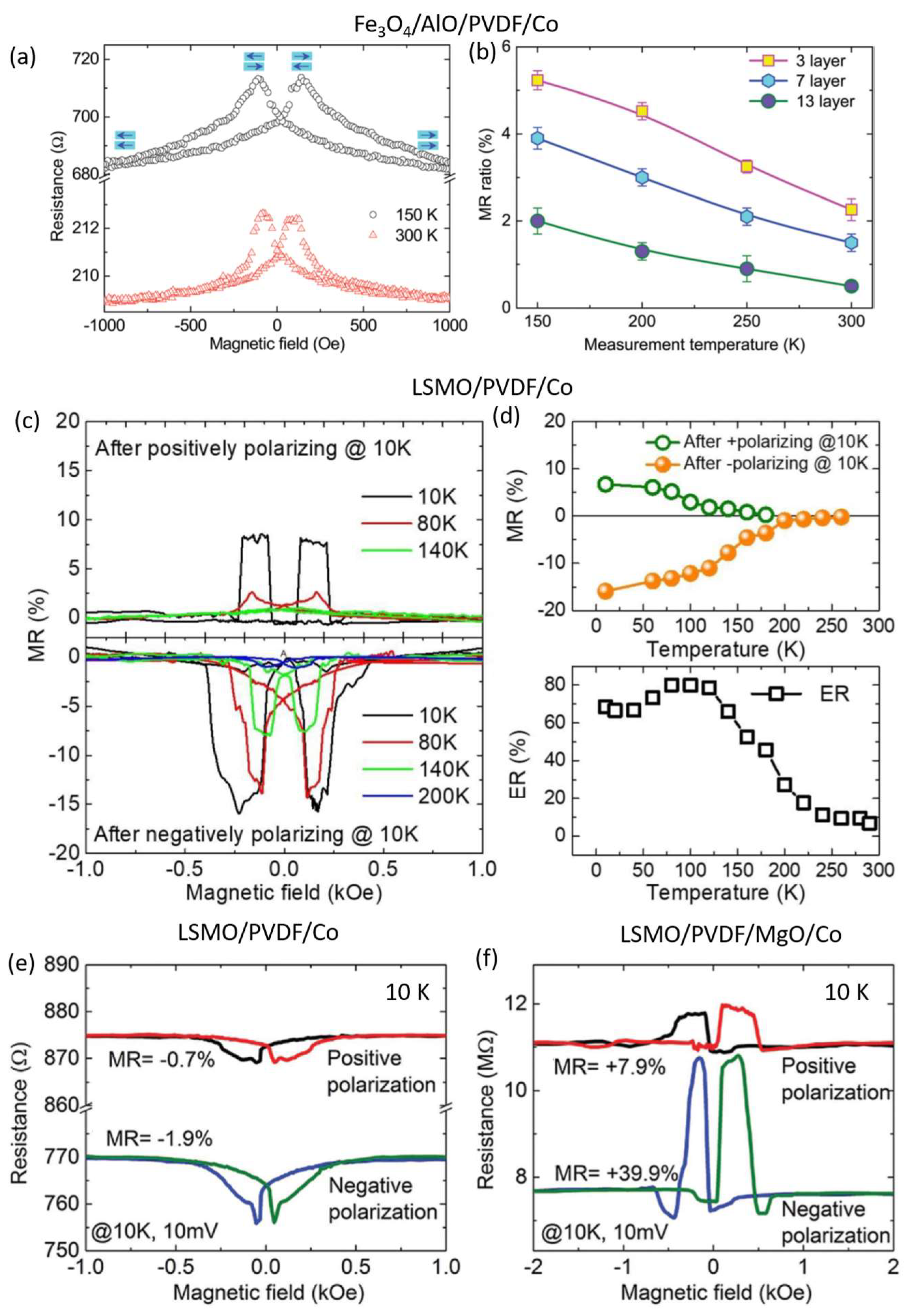
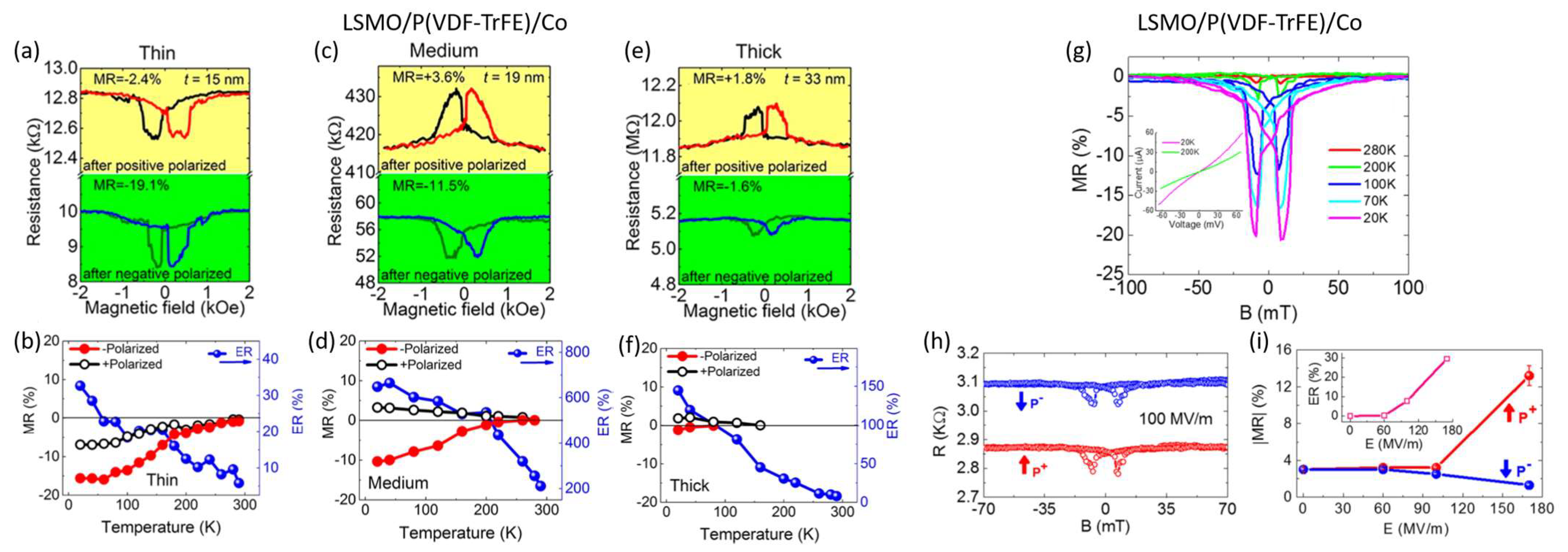
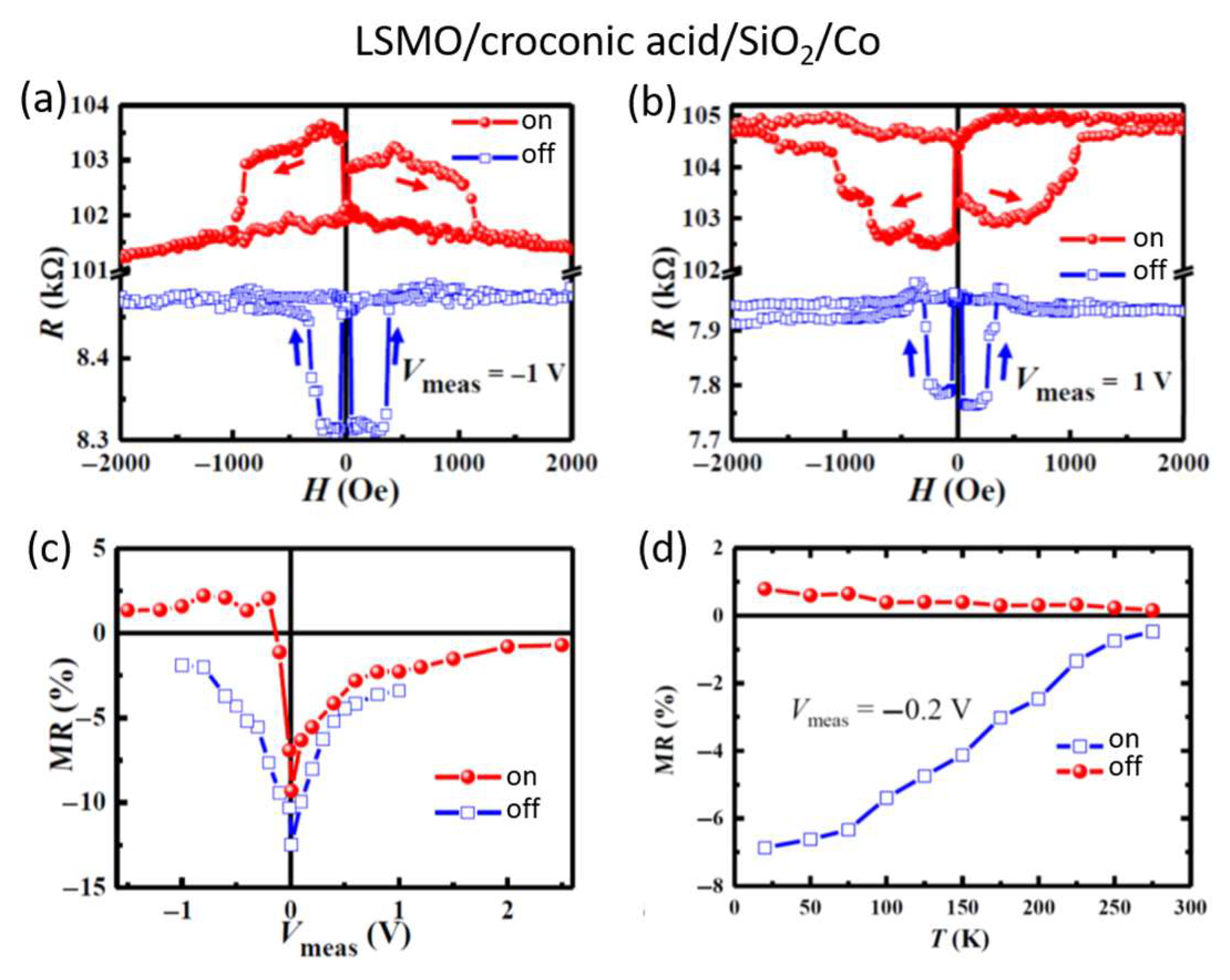
3. Organic Multiferroic Junctions
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gajek, M.; Bibes, M.; Fusil, S.; Bouzehouane, K.; Fontcuberta, J.; Barthélémy, A.; Fert, A. Tunnel junctions with multiferroic barriers. Nat. Mater. 2007, 6, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Bibes, M.; Bocher, L.; Valencia, S.; Kronast, F.; Crassous, A.; Moya, X.; Enouz-Vedrenne, S.; Gloter, A.; Imhoff, D.; et al. Ferroelectric control of spin polarization. Science 2010, 327, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Pantel, D.; Goetze, S.; Hesse, D.; Alexe, M. Reversible electrical switching of spin polarization in multiferroic tunnel junctions. Nat. Mater. 2012, 11, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Jullière, M. Tunneling between ferromagnetic films. Phys. Lett. A 1975, 54, 225–226. [Google Scholar] [CrossRef]
- Maekawa, S.; Gafvert, U. Electron tunneling between ferromagnetic films. IEEE Trans. Magn. 1982, 18, 707–708. [Google Scholar] [CrossRef]
- Zhuravlev, M.Y.; Sbirianov, R.F.; Jaswal, S.S.; Tsymbal, E.Y. Giant electroresistance in ferroelectric tunnel junctions. Phys. Rev. Lett. 2005, 94, 246802. [Google Scholar] [CrossRef]
- Kohlstedt, H.; Pertsev, N.A.; Rodriguez-Contreras, J.; Waser, R. Theoretical current-voltage characteristics of ferroelectric tunnel junctions. Phys. Rev. B 2005, 72, 125341. [Google Scholar] [CrossRef]
- Tsymbal, E.Y.; Kohlstedt, H. Tunneling across a ferroelectric. Science 2006, 313, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Dediu, V.; Murgia, M.; Matacotta, F.C.; Taliani, C.; Barbanera, S. Room temperature spin polarized injection in organic semiconductor. Solid State Commun. 2002, 122, 181–184. [Google Scholar] [CrossRef]
- Pramanik, S.; Stefanita, C.G.; Patibandla, S.; Bandyopadhyay, S.; Garre, K.; Harth, N.; Cahay, M. Observation of extremely long spin relaxation times in an organic nanowire spin valve. Nat. Nanotechnol. 2007, 2, 216–219. [Google Scholar] [CrossRef]
- Fu, K.K.; Wang, Z.; Dai, J.; Carter, M.; Hu, L. Transient electronics: Materials and devices. Chem. Mater. 2016, 28, 3527–3539. [Google Scholar] [CrossRef]
- Cosseddu, P.; Caironi, M. (Eds.) Organic Flexible Electronics: Fundamentals, Devices, and Applications; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Liang, S.; Geng, R.; Yang, B.; Zhao, W.; Subedi, R.C.; Li, X.; Han, X.; Nguyen, T.D. Curvature-enhanced spin-orbit coupling and spinterface effect in fullerene-based spin valves. Sci. Rep. 2016, 6, 19461. [Google Scholar] [CrossRef]
- Gobbi, M.; Golmar, F.; Llopis, R.; Casanova, F.; Hueso, L.E. Room-temperature spin transport in C60-based spin valves. Adv. Mater. 2011, 23, 1609–1613. [Google Scholar] [CrossRef]
- Zhang, X.; Mizukami, S.; Kubota, T.; Ma, Q.; Oogane, M.; Naganuma, H.; Ando, Y.; Miyazaki, T. Observation of a large spin-dependent transport length in organic spin valves at room temperature. Nat. Commun. 2013, 4, 1392. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Liu, W.; Guo, L.; Zhang, R.; Gu, X.; Meng, K.; Qin, Y.; Guo, A.; Yang, T.; Zhang, C.; et al. Continuous room-temperature spin-injection modulation achieved by spin-filtering competition in molecular spin valves. Adv. Mater. 2023, 35, 2300055. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Q.; Suzuk, K.; Sugihara, A.; Qin, G.; Miyazaki, T.; Mizukami, S. Magnetoresistance effect in rubrene-based spin valves at room temperature. ACS Appl. Mater. Interfaces 2015, 7, 4685–4692. [Google Scholar] [CrossRef]
- Shim, J.H.; Raman, K.V.; Park, Y.J.; Santos, T.S.; Miao, G.X.; Satpati, B.; Moodera, J.S. Large spin diffusion length in an amorphous organic semiconductor. Phys. Rev. Lett. 2008, 100, 226603. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Wang, F.; Rybicki, J.; Wohlgenannt, M.; Hutchinson, K.A. Distinguishing between tunneling and injection regimes of ferromagnet/organic semiconductor/ferromagnet junctions. Phys. Rev. B 2010, 81, 195214. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Jang, H.W.; Prigodin, V.N.; Kao, C.; Eom, C.B.; Epstein, A.J. Tunneling vs. giant magnetoresistance in organic spin valve. Synth. Met. 2010, 160, 216–222. [Google Scholar] [CrossRef]
- Li, B.; Kao, C.-Y.; Lu, Y.; Yoo, J.-W.; Prigodin, V.N.; Epstein, A.J. Room-temperature organic-based spin polarizer. Appl. Phys. Lett. 2011, 99, 153503. [Google Scholar] [CrossRef]
- Li, B.; Kao, C.-Y.; Yoo, J.-W.; Prigodin, V.N.; Epstein, A.J. Magnetoresistance in an all-organic-based spin valve. Adv. Mater. 2011, 23, 3382–3386. [Google Scholar] [CrossRef]
- Sun, X.; Gobbi, M.; Bedoya-Pinto, A.; Txoperena, O.; Federico Golmar, F.; Llopis, R.; Andrey Chuvilin, A.; Casanova, F.; Hueso, L.E. Room-temperature air-stable spin transport in bathocuproine-based spin valves. Nat. Commun. 2013, 4, 2794. [Google Scholar] [CrossRef]
- Sun, X.; Bedoya-Pinto, A.; Llopis, R.; Casanova, F.; Hueso, L.E. Flexible semi-transparent organic spin valve based on bathocuproine. Appl. Phys. Lett. 2014, 105, 083302. [Google Scholar] [CrossRef]
- Kawasugi, Y.; Ujino, T.; Tada, H. Room-temperature magnetoresistance in organic spin-valves based on a Co2MnSi Heusler alloy. Org. Electron. 2013, 14, 3186–3189. [Google Scholar] [CrossRef]
- Ikegami, T.; Kawayama, I.; Tonouchi, M.; Nakao, S.; Yamashita, Y.; Tada, H. Planar-type spin valves based on low-molecular-weight organic materials with La0.67Sr0.33MnO3 electrodes. Appl. Phys. Lett. 2008, 92, 153304. [Google Scholar] [CrossRef]
- Jiang, S.W.; Wang, P.; Jiang, S.C.; Chen, B.B.; Wang, M.; Jiang, Z.S.; Wu, D. Fabrication of lateral organic spin valves based on La0.7Sr0.3MnO3 electrodes. Spin 2014, 4, 1440008. [Google Scholar] [CrossRef]
- Mooser, S.; Cooper, J.F.K.; Banger, K.K.; Wunderlich, J.; Sirringhaus, H. Spin injection and transport in a solution-processed organic semiconductor at room temperature. Phys. Rev. B 2012, 85, 235202. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Ding, S.; Guo, S.; Cui, D.; Wang, X.; Yang, S.; Zhang, L.; Tian, X.; Wu, D.; et al. Spin injection and transport in single-crystalline organic spin valves based on TIPS-pentacene. Sci. China Mater. 2021, 64, 2795–2804. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Hukic-Markosian, G.; Wang, F.; Wojcik, L.; Li, X.-G.; Ehrenfreund, E.; Vardeny, Z.V. Isotope effect in spin response of π-conjugated polymer films and devices. Nat. Mater. 2010, 9, 345–352. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Wu, D.; Vardeny, Z.V.; Shi, J. Giant magnetoresistance in organic spin-valves. Nature 2004, 427, 821–824. [Google Scholar] [CrossRef]
- Santos, T.S.; Lee, J.S.; Migdal, P.; Lekshmi, I.C.; Satpati, B.; Moodera, J.S. Room-temperature tunnel magnetoresistance and spin-polarized tunneling through an organic semiconductor barrier. Phys. Rev. Lett. 2007, 98, 16601. [Google Scholar] [CrossRef]
- Dediu, V.; Hueso, L.E.; Bergenti, I.; Riminucci, A.; Borgatti, F.; Graziosi, P.; Newby, C.; Casoli, F.; De Jong, M.P.; Taliani, C.; et al. Room-temperature spintronic effects in Alq3-based hybrid devices. Phys. Rev. B 2008, 78, 115203. [Google Scholar] [CrossRef]
- Sun, D.; Yin, L.; Sun, C.; Guo, H.; Gai, Z.; Zhang, X.-G.; Ward, T.Z.; Cheng, Z.; Shen, J. Giant magnetoresistance in organic spin valves. Phys. Rev. Lett. 2010, 104, 236602. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, Y.J.; Lin, L.; Chen, B.B.; Yue, F.J.; Du, J.; Ding, H.F.; Zhang, F.M.; Wu, D. Room-temperature spin valve effects in La0.67Sr0.33MnO3/Alq3/Co devices. Synth. Met. 2011, 161, 1738–1741. [Google Scholar] [CrossRef]
- Yang, W.; Shi, Q.; Miao, T.; Li, Q.; Cai, P.; Liu, H.; Lin, H.; Bai, Y.; Zhu, Y.; Yu, Y.; et al. Achieving large and nonvolatile tunable magnetoresistance in organic spin valves using electronic phase separated manganites. Nat. Commun. 2019, 10, 3877. [Google Scholar] [CrossRef] [PubMed]
- Angervo, I.; Saloaro, M.; Palonen, H.; Huhtinen, H.; Paturi, P.; Mäkelä, T.; Majumdar, S. Giant magnetoresistance response in Sr2FeMoO6 based organic spin valves. Appl. Surf. Sci. 2022, 589, 152854. [Google Scholar] [CrossRef]
- Zhang, X.; Mizukami, S.; Ma, Q.; Kubota, T.; Oogane, M.; Naganuma, H.; Ando, Y.; Miyazaki, T. Spin-dependent transport behavior in C60 and Alq3 based spin valves with a magnetite electrode (invited). J. Appl. Phys. 2014, 115, 172608. [Google Scholar] [CrossRef]
- Drew, A.J.; Hoppler, J.; Schulz, L.; Pratt, F.L.; Desai, P.; Shakya, P.; Kreouzis, T.; Gillin, W.P.; Suter, A.; Morley, N.A.; et al. Direct measurement of the electronic spin diffusion length in a fully functional organic spin valve by low-energy muon spin rotation. Nat. Mater. 2009, 8, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bergenti, I.; Borgatti, F.; Calbucci, M.; Riminucci, A.; Cecchini, R.; Graziosi, P.; MacLaren, D.A.; Giglia, A.; Rueff, J.P.; Céolin, D.; et al. Oxygen impurities link bistability and magnetoresistance in organic spin valves. ACS Appl. Mater. Interfaces 2018, 10, 8132–8140. [Google Scholar] [CrossRef]
- Cucinotta, G.; Poggini, L.; Pedrini, A.; Bertani, F.; Cristiani, N.; Torelli, M.; Graziosi, P.; Cimatti, I.; Cortigiani, B.; Otero, E.; et al. Tuning of a vertical spin valve with a monolayer of single molecule magnets. Adv. Funct. Mater. 2017, 27, 1703600. [Google Scholar] [CrossRef]
- Poggini, L.; Cucinotta, G.; Pradipto, A.-M.; Scarrozza, M.; Barone, P.; Caneschi, A.; Graziosi, P.; Calbucci, M.; Cecchini, R.; Dediu, A.A.; et al. An Organic Spin Valve Embedding a Self-Assembled Monolayer of Organic Radicals. Adv. Mater. Interfaces 2016, 3, 1500855. [Google Scholar] [CrossRef]
- Morley, N.A.; Rao, A.; Dhandapani, D.; Gibbs, M.R.J.; Grell, M.; Richardson, T. Room temperature organic spintronics. J. Appl. Phys. 2008, 103, 07F306. [Google Scholar] [CrossRef]
- Ding, S.; Tian, Y.; Dong, H.; Zhu, D.; Hu, W. Anisotropic magnetoresistance in NiFe-based polymer spin valves. ACS Appl. Mater. Interfaces 2019, 11, 11654–11659. [Google Scholar] [CrossRef]
- Ding, S.; Tian, Y.; Liu, X.; Zou, Y.; Dong, H.; Mi, W.; Hu, W. Unveiling the role of Fe3O4 in polymer spin valve near Verwey transition. Nano Res. 2021, 14, 304–310. [Google Scholar] [CrossRef]
- Majumdar, S.; Laiho, R.; Laukkanen, P.; Väyrynen, I.J.; Majumdar, H.S.; Österbacka, R. Application of regioregular polythiophene in spintronic devices: Effect of interface. Appl. Phys. Lett. 2006, 89, 122114. [Google Scholar] [CrossRef]
- Ding, S.; Tian, Y.; Li, Y.; Mi, W.; Dong, H.; Zhang, X.; Hu, W.; Zhu, D. Inverse magnetoresistance in polymer spin valves. ACS Appl. Mater. Interfaces 2017, 9, 15644–15651. [Google Scholar] [CrossRef]
- Geng, R.; Subedi, R.C.; Luong, H.M.; Pham, M.T.; Huang, W.; Li, X.; Hong, K.; Shao, M.; Xiao, K.; Hornak, L.A.; et al. Effect of charge localization on the effective hyperfine interaction in organic semiconducting polymers. Phys. Rev. Lett. 2018, 120, 086602. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, S.; Tian, Y.; Wang, J.; Chen, Y.; Zhao, T.; Hu, F.; Hu, W.; Shen, B. The in situ optimization of spinterface in polymer spin valve by electronic phase separated oxides. Small 2023, 19, 2303375. [Google Scholar] [CrossRef]
- Ding, S.; Tian, Y.; Wang, H.; Zhou, Z.; Mi, W.; Ni, Z.; Zou, Y.; Dong, H.; Gao, H.; Zhu, D.; et al. Reliable spin valves of conjugated polymer based on mechanically transferrable top electrodes. ACS Nano 2018, 12, 12657–12664. [Google Scholar] [CrossRef]
- Yu, D.; Ding, S.; Li, J.; Mi, W.; Tian, Y.; Hu, W. Molecular spinterface in F4TCNQ-doped polymer spin valves. J. Mater. Chem. C 2022, 10, 2608–2615. [Google Scholar] [CrossRef]
- Geng, R.; Roy, A.; Zhao, W.; Subedi, R.C.; Li, X.; Locklin, J.; Nguyen, T.D. Engineering of spin injection and spin transport in organic spin valves using π-conjugated polymer brushes. Adv. Funct. Mater. 2016, 26, 3999–4006. [Google Scholar] [CrossRef]
- Wang, F.J.; Yang, C.G.; Valy Vardeny, Z.; Li, X.G. Spin response in organic spin valves based on La2/3Sr1/3MnO3 electrodes. Phys. Rev. B 2007, 75, 245324. [Google Scholar] [CrossRef]
- Matsuzaka, M.; Sasaki, Y.; Hayashi, K.; Misawa, T.; Komine, T.; Akutagawa, T.; Fujioka, M.; Nishii, J.; Kaiju, H. Room-temperature magnetoresistance in Ni78Fe22/C8-BTBT/Ni78Fe22 nanojunctions fabricated from magnetic thin-film edges using a novel technique. Nanoscale Adv. 2022, 4, 4739. [Google Scholar] [CrossRef]
- Luo, Z.; Song, X.; Liu, X.; Lu, X.; Yao, Y.; Zeng, J.; Li, Y.; He, D.; Zhao, H.; Gao, L.; et al. Revealing the key role of molecular packing on interface spin polarization at two-dimensional limit in spintronic devices. Sci. Adv. 2023, 9, eade9126. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Chen, F.; Zhang, F. Excellent spin transport in spin valves based on the conjugated polymer with high carrier mobility. Sci. Rep. 2015, 5, 9355. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Lin, Z.; Zheng, Y.; Jiang, Q.; Zheng, N.; Zhang, W.; Kui-juan Jin, K.-j.; Yu, G. Tuning charge carrier and spin transport properties via structural modification of polymer semiconductors. ACS Appl. Mater. Interfaces 2019, 11, 30089–30097. [Google Scholar] [CrossRef]
- Li, D.; Zheng, Y.; Yang, M.; Wei, C.; Liu, X.; Zheng, N.; Zhang, W.; Kuijuan Jin, K.; Yu, G. Molecular and interfacial adjustment of magnetoresistance in organic spin valves using isoindigo-based polymers. ACS Mater. Lett. 2022, 4, 1065–1073. [Google Scholar] [CrossRef]
- Zheng, N.; Lin, Z.; Zheng, Y.; Li, D.; Yang, J.; Zhang, W.; Wang, L.; Yu, G. Room-temperature stable organic spin valves using solution-processed ambipolar naphthalenediimide-based conjugated polymers. Org. Electron. 2020, 81, 105684. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, X.; Zheng, Y.; Li, D.; Lin, Z.; Zhang, W.; Jin, K.-j.; Yu, G. Negative Magnetoresistance Behavior in Polymer Spin Valves Based on Donor-Acceptor Conjugated Molecules. Adv. Mater. Interfaces 2020, 7, 2000868. [Google Scholar] [CrossRef]
- Prieto-Ruiz, J.P.; Gómez Miralles, S.; Prima-García, H.; López-Muñoz, A.; Riminucci, A.; Graziosi, P.; Aeschlimann, M.; Cinchetti, M.; Dediu, V.A.; Coronado, E. Enhancing light emission in interface engineered spin-OLEDs through spin-polarized injection at high voltages. Adv. Mater. 2019, 31, 1806817. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Zhang, W.; Yang, Z.; Li, D.; Liu, M.; Jin, K.; Wang, L.; Yu, G. Magnetoresistance in organic spin valves based on acid-exfoliated 2D covalent organic frameworks thin films. Angew. Chem. Int. Ed. 2023, 62, e202308921. [Google Scholar] [CrossRef]
- Xu, W.; Szulczewski, G.J.; LeClair, P.; Navarrete, I.; Schad, R.; Miao, G.; Guo, H.; Gupta, A. Tunneling magnetoresistance observed in LSMO/organic molecule/Co junctions. Appl. Phys. Lett. 2007, 90, 072506. [Google Scholar] [CrossRef]
- Bairagi, K.; Garcia Romero, D.; Calavalle, F.; Catalano, S.; Zuccatti, E.; Llopis, R.; Casanova, F.; Hueso, L.E. Room-temperature operation of a p-type molecular spin photovoltaic device on a transparent substrate. Adv. Mater. 2020, 32, 1906908. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, T.; Katz, H.E.; Reich, D.H. Effects of carrier mobility and morphology in organic semiconductor spin valves. J. Appl. Phys. 2009, 105, 07C708. [Google Scholar] [CrossRef]
- Jiang, S.W.; Wang, P.; Chen, B.B.; Zhou, Y.; Ding, H.F.; Wu, D. Tuning carrier mobility without spin transport degrading in copper-phthalocyanine. Appl. Phys. Lett. 2015, 107, 042407. [Google Scholar] [CrossRef]
- Tong, J.; Ruan, L.; Yao, X.; Qin, G.; Zhang, X. Defect states dependence of spin transport in iron phthalocyanine spin valves. Phys. Rev. B 2019, 99, 054406. [Google Scholar] [CrossRef]
- Sun, X.; Bedoya-Pinto, A.; Mao, Z.; Gobbi, M.; Yan, W.; Guo, Y.; Atxabal, A.; Llopis, R.; Yu, G.; Liu, Y.; et al. Active morphology control for concomitant long distance spin transport and photoresponse in a single organic device. Adv. Mater. 2016, 28, 2609–2615. [Google Scholar] [CrossRef]
- Hong, J.Y.; Chang, S.-H.; Ou Yang, K.H.; Yeh, P.C.; Shiu, H.W.; Chen, C.-H.; Chiang, W.C.; Lin, M.T. A multifunctional molecular spintronic platform with magnetoresistive and memristive responses via a self-assembled monolayer. J. Appl. Phys. 2019, 125, 142905. [Google Scholar] [CrossRef]
- Bowen, M.; Bibes, M.; Barthélémy, A.; Contour, J.-P.; Anane, A.; Lemaı̂tre, Y.; Fert, A. Nearly total spin polarization in La2/3Sr1/3MnO3 from tunneling experiments. Appl. Phys. Lett. 2003, 82, 233–235. [Google Scholar] [CrossRef]
- Uehara, M.; Mori, S.; Chen, C.; Cheong, S.W. Percolative phase separation underlies colossal magnetoresistance in mixed-valent manganites. Nature 1999, 399, 560–563. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, J.; Xu, Y.; Wang, S.; Yu, J.; Cao, S.; Jing, C.; Shen, X. Action of strong coupling on steplike magnetization and transport properties in phase-separated manganite. Appl. Phys. Lett. 2005, 87, 232501. [Google Scholar] [CrossRef]
- Stanciu, A.E.; Schinteie, G.; Kuncser, A.C.; Locovei, C.; Trupina, L.; Iacob, N.; Leca, A.; Borca, B.; Kuncser, V. Magnetic properties of nanosized Fe and FeCo systems on trenched Mo templates. Coatings 2022, 12, 1366. [Google Scholar] [CrossRef]
- Borca, B.; Fruchart, O.; David, P.; Rousseau, A.; Meyer, C. Kinetic self-organization of trenched templates for the fabrication of versatile ferromagnetic nanowires. Appl. Phys. Lett. 2007, 90, 142507. [Google Scholar] [CrossRef]
- Borca, B.; Fruchart, O.; Cheynis, F.; Hasegawa, M.; Meyer, C. Growth and magnetism of self-organized arrays of Fe(110) wires formed by deposition on kinetically grooved W(110). Surf. Sci. 2007, 601, 4358–4361. [Google Scholar] [CrossRef]
- Borca, B.; Fruchart, O.; Kritsikis, E.; Cheynis, F.; Rousseau, A.; David, P.; Meyer, C.; Toussaint, J.-C. Tunable magnetic properties of arrays of Fe(110) nanowires grown on kinetically grooved W(110) self-organized templates. J. Magn. Magn. Mater. 2010, 322, 257–264. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kimura, T.; Sawada, H.; Terakura, K.; Takura, Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Ray, S.; Kumar, A.; Sarma, D.D.; Cimino, R.; Turchini, S.; Zennaro, S.; Zema, N. Electronic and magnetic structures of Sr2FeMoO6. Phys. Rev. Lett. 2001, 87, 097204. [Google Scholar] [CrossRef]
- Dedkov, Y.S.; Rüdiger, U.; Güntherodt, G. Evidence for the half-metallic ferromagnetic state of Fe3O4 by spin-resolved photoelectron spectroscopy. Phys. Rev. B 2002, 65, 064417. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Tolea, F.; Tolea, M.; Sofronie, M.; Valeanu, M. Distribution of plates׳ sizes tell the thermal history in a simulated martensitic-like phase transition. Solid State Commun. 2015, 213–214, 37–41. [Google Scholar] [CrossRef]
- Sofronie, M.; Tolea, F.; Tolea, M.; Popescu, B.; Valeanu, M. Magnetic and magnetostrictive properties of the ternary Fe67.5Pd30.5Ga2 ferromagnetic shape memory ribbons. J. Phys. Chem. Solids 2020, 142, 109446. [Google Scholar] [CrossRef]
- Jourdan, M.; Minár, J.; Braun, J.; Kronenberg, A.; Chadov, S.; Balke, B.; Gloskovskii, A.; Kolbe, M.; Elmers, H.J.; Schönhense, G.; et al. Direct observation of half-metallicity in the Heusler compound Co2MnSi. Nat. Commun. 2014, 5, 3974. [Google Scholar] [CrossRef]
- Tedrow, P.M.; Meservey, R. Spin polarization of electrons tunneling from films of Fe, Co, Ni, and Gd. Phys. Rev. B 1973, 7, 318. [Google Scholar] [CrossRef]
- Monsma, D.J.; Parkin, S.S.P. Spin polarization of tunneling current from ferromagnet/Al2O3 interfaces using copper-doped aluminum superconducting films. Appl. Phys. Lett. 2000, 77, 720–722. [Google Scholar] [CrossRef]
- Paraskevopoulos, D.; Meservey, R.; Tedrow, P.M. Spin polarization of electrons tunneling from 3D ferromagnetic metals and alloys. Phys. Rev. B 1977, 16, 4907. [Google Scholar] [CrossRef]
- Soulen, R.J., Jr.; Byers, J.M.; Osofsky, M.S.; Nadgorny, B.; Ambrose, T.; Cheng, S.F.; Broussard, P.R.; Tanaka, C.T.; Nowak, J.; Moodera, J.S.; et al. Measuring the spin polarization of a metal with a superconducting point contact. Science 1998, 282, 85–88. [Google Scholar] [CrossRef]
- Barraud, C.; Seneor, P.; Mattana, R.; Fusil, S.; Bouzehouane, K.; Deranlot, C.; Graziosi, P.; Hueso, L.; Bergenti, I.; Dediu, V.; et al. Unravelling the role of the interface for spin injection into organic semiconductors. Nat. Phys 2010, 6, 615–620. [Google Scholar] [CrossRef]
- Jiang, J.S.; Pearson, J.E.; Bader, S.D. Absence of spin transport in the organic semiconductor Alq3. Phys. Rev. B 2008, 77, 035303. [Google Scholar] [CrossRef]
- Metzelaars, M.; Schleicher, S.; Hattori, T.; Borca, B.; Matthes, F.; Sanz, S.; Bürgler, D.E.; Rawson, J.; Schneider, C.M.; Kögerler, P. Cyclophane with eclipsed pyrene units enables construction of spin interfaces with chemical accuracy. Chem. Sci. 2021, 12, 8430–8437. [Google Scholar] [CrossRef]
- Schleicher, S.; Borca, B.; Rawson, J.; Matthes, F.; Bürgler, D.E.; Kögerler, P.; Schneider, C.M. Ultra-high vacuum deposition of pyrene molecules on metal surfaces. Phys. Status Solidi B 2018, 255, 1800235. [Google Scholar] [CrossRef]
- Atodiresei, N.; Brede, J.; Lazić, P.; Caciuc, V.; Hoffmann, G.; Wiesendanger, R.; Blügel, S. Design of the local spin polarization at the organic-ferromagnetic interface. Phys. Rev. Lett. 2010, 105, 066601. [Google Scholar] [CrossRef] [PubMed]
- Raman, K.V.; Kamerbeek, A.M.; Mukherjee, A.; Atodiresei, N.; Sen, T.K.; Lazić, P.; Caciuc, V.; Michel, R.; Stalke, D.; Mandal, S.K.; et al. Interface-engineered templates for molecular spin memory devices. Nature 2013, 493, 509–513. [Google Scholar] [CrossRef]
- Lennartz, M.C.; Caciuc, V.; Atodiresei, N.; Karthäuser, S.; Blügel, S. Electronic mapping of molecular orbitals at the molecule-metal interface. Phys. Rev. Lett. 2010, 105, 066801. [Google Scholar] [CrossRef] [PubMed]
- Michnowicz, T.; Borca, B.; Pétuya, R.; Schendel, V.; Pristl, M.; Pentegov, I.; Kraft, U.; Klauk, H.; Wahl, P.; Mutombo, P.; et al. Controlling single molecule conductance by a locally induced chemical reaction on individual thiophene units. Angew. Chem. Int. Ed. 2020, 59, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Borca, B.; Michnowicz, T.; Aguilar-Galindo, F.; Pétuya, R.; Pristl, M.; Schendel, V.; Pentegov, I.; Kraft, U.; Klauk, H.; Wahl, P.; et al. Chiral and catalytic effects of site-specific molecular adsorption. J. Phys. Chem. Lett. 2023, 14, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Su, T.A.; Neupane, M.; Steigerwald, M.L.; Venkataraman, L.; Nuckolls, C. Chemical principles of single-molecule electronics. Nat. Rev. Mater. 2016, 1, 16002. [Google Scholar] [CrossRef]
- Ando, K.; Watanabe, S.; Mooser, S.; Saitoh, E.; Sirringhaus, H. Solution-processed organic spin–charge converter. Nat. Mater. 2013, 12, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.W.; Liu, S.; Wang, P.; Luan, Z.Z.; Tao, X.D.; Ding, H.F.; Wu, D. Exchange-dominated pure spin current transport in Alq3 molecules. Phys. Rev. Lett. 2015, 115, 086601. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, T.; Qi, D.-C.; Tong, W.; Xu, L.; Zhu, J.; Zhang, Z.; Xu, H.; Zhang, W.; Guo, Y.; et al. Quantitative study of spin relaxation in rubrene thin films by inverse spin Hall effect. Appl. Phys. Lett. 2019, 115, 053301. [Google Scholar] [CrossRef]
- Jiang, J.S.; Pearson, J.E.; Bader, S.D. Direct determination of energy level alignment and charge transport at metal−Alq3 interfaces via ballistic-electron-emission spectroscopy. Phys. Rev. Lett. 2011, 106, 156807. [Google Scholar] [CrossRef]
- Gobbi, M.; Bedoya-Pinto, A.; Golmar, F.; Llopis, R.; Casanova, F.; Hueso, L.E. C60-based hot-electron magnetic tunnel transistor. Appl. Phys. Lett. 2012, 101, 102404. [Google Scholar] [CrossRef]
- Gobbi, M.; Pietrobon, L.; Atxabal, A.; Bedoya-Pinto, A.; Sun, X.; Golmar, F.; Llopis, R.F.; Casanova, F.; Hueso, L.E. Determination of energy level alignment at metal/molecule interfaces by in-device electrical spectroscopy. Nat. Commun. 2014, 5, 4161. [Google Scholar] [CrossRef] [PubMed]
- Cinchetti, M.; Heimer, K.; Wüstenberg, J.P.; Andreyev, O.; Bauer, M.; Lach, S.; Ziegler, C.; Gao, Y.; Aeschlimann, M. Determination of spin injection and transport in a ferromagnet/organic semiconductor heterojunction by two-photon photoemission. Nat. Mater. 2009, 8, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Ananthavel, S.P.; Waldeck, D.H.; Naaman, R. Asymmetric scattering of polarized electrons by organized organic films of chiral molecules. Science 1999, 283, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.R.; Matthes, F.; Schneider, C.M.; Ernst, K.-H.; Bürgler, D.E. Spin-selective electron transport through single chiral molecules. Small 2023, 2308233. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.R.; Matthes, F.; Caciuc, V.; Atodiresei, N.; Schneider, C.M.; Ernst, K.-H.; Bürgler, D.E. Enantioselective adsorption on magnetic surfaces. Adv. Mater. 2024, 36, 2308666. [Google Scholar] [CrossRef]
- Safari, M.R.; Matthes, F.; Ernst, K.-H.; Bürgler, D.E.; Schneider, C.M. Deposition of chiral heptahelicene molecules on ferromagnetic Co and Fe thin-film substrates. Nanomaterials 2022, 12, 3281. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, C.; Zhang, Y.; Yang, L.; Yan, Y. Efficient spin selectivity in self-sssembled superhelical conducting polymer microfibers. ACS Nano 2020, 14, 6607–6615. [Google Scholar] [CrossRef] [PubMed]
- Garnica, M.; Stradi, D.; Barja, S.; Calleja, F.; Díaz, C.; Alcamí, M.; Martín, N.; Vázquez de Parga, A.L.; Martín, F.; Miranda, R. Long-range magnetic order in a purely organic 2D layer adsorbed on epitaxial graphene. Nat. Phys. 2013, 9, 368–374. [Google Scholar] [CrossRef]
- Garnica, M.; Calleja, F.; Vázquez de Parga, A.L.; Miranda, R. Mapping spin distributions in electron acceptor molecules adsorbed on nanostructured graphene by the Kondo effect. Surf. Sci. 2014, 630, 356–360. [Google Scholar] [CrossRef]
- Mugarza, A.; Krull, C.; Robles, R.; Stepanow, S.; Ceballos, G.; Gambardella, P. Spin coupling and relaxation inside molecule–metal contacts. Nat. Commun. 2011, 2, 490. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Richter, C.A. Organic spin-valves and beyond: Spin injection and transport in organic semiconductors and the effect of interfacial engineering. Adv. Mater. 2017, 29, 1602739. [Google Scholar] [CrossRef]
- Jiang, A.Q.; Wang, C.; Jin, K.J.; Liu, X.B.; Scott, J.F.; Hwang, C.S.; Tang, T.A.; Lu, H.B.; Yang, G.Z. A resistive memory in semiconducting BiFeO3 thin-film capacitors. Adv. Mater. 2011, 23, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Blom, P.W.M.; Wolf, R.M.; Cillessen, J.F.M.; Krijn, M.P.C.M. Ferroelectric Schottky diode. Phys. Rev. Lett. 1994, 73, 2107. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Bibes, M. Ferroelectric tunnel junctions for information storage and processing. Nat. Commun. 2014, 5, 4289. [Google Scholar] [CrossRef]
- Yamada, H.; Garcia, V.; Fusil, S.; Boyn, S.; Marinova, M.; Gloter, A.; Xavier, S.; Grollier, J.; Jacquet, E.; Carrétéro, C.; et al. Giant electroresistance of super-tetragonal BiFeO3-based ferroelectric tunnel junctions. ACS Nano 2013, 7, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, J.; Nie, F.; Zhang, N.; Yu, T.; Zhao, L.; Shi, C.; Zhang, P.; He, B.; Lü, W.; et al. Giant electroresistance in ferroelectric tunnel junctions via high-throughput designs: Toward high-performance neuromorphic computing. ACS Appl. Mater. Interfaces 2024, 16, 1015–1024. [Google Scholar] [CrossRef]
- Tian, B.B.; Wang, J.L.; Fusil, S.; Liu, Y.; Zhao, X.L.; Sun, S.; Shen, H.; Lin, T.; Sun, J.L.; Duan, C.G.; et al. Tunnel electroresistance through organic ferroelectrics. Nat. Commun. 2016, 7, 11502. [Google Scholar] [CrossRef]
- Majumdar, S.; Chen, B.; Qin, Q.H.; Majumdar, H.S.; van Dijken, S. Electrode dependence of tunneling electroresistance and switching stability in organic ferroelectric P(VDF-TrFE)-based tunnel junctions. Adv. Funct. Mater. 2018, 28, 1703273. [Google Scholar] [CrossRef]
- Majumdar, S.; Tan, H.; Qin, Q.H.; van Dijken, S. Energy-efficient organic ferroelectric tunnel junction memristors for neuromorphic computing. Adv. Electron. Mater. 2019, 5, 1800795. [Google Scholar] [CrossRef]
- He, S.; Guo, M.; Dan, Z.; Lan, S.; Ren, W.; Zhou, L.; Wang, Y.; Liang, Y.; Zheng, Y.; Pan, J.; et al. Large-area atomic-smooth polyvinylidene fluoride Langmuir-Blodgett film exhibiting significantly improved ferroelectric and piezoelectric responses. Sci. Bull. 2021, 66, 1080–1090. [Google Scholar] [CrossRef]
- Tu, L.; Yuan, S.; Xu, J.; Yang, K.; Wang, P.; Cui, X.; Zhang, X.; Wang, J.; Zhan, Y.-Q.; Zheng, L.-R. A wide-range operating synaptic device based on organic ferroelectricity with low energy consumption. RSC Adv. 2018, 8, 26549. [Google Scholar] [CrossRef]
- Kim, K.L.; Lee, W.; Hwang, S.K.; Joo, S.H.; Cho, S.M.; Song, G.; Cho, S.H.; Jeong, B.; Hwang, I.; Ahn, J.-H.; et al. Epitaxial growth of thin ferroelectric polymer films on graphene layer for fully transparent and flexible nonvolatile memory. Nano Lett. 2016, 16, 334–340. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, H.; Xu, J.; Yu, C.; Xiao, H.; Wang, R.; Xu, L.; Zeng, Z.; Liang, S. Emulation of synaptic behavior by organic ferroelectric tunnel junctions. Phys. Lett. A 2021, 392, 127138. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tokura, Y. Organic ferroelectrics. Nat. Mater 2008, 7, 357–366. [Google Scholar] [CrossRef]
- Tayi, A.S.; Kaeser, A.; Matsumoto, M.; Aida, T.; Stupp, S.I. Supramolecular ferroelectrics. Nat. Chem. 2015, 7, 281–294. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, R.-G. Ferroelectric metal–organic frameworks. Chem. Rev. 2012, 112, 1163–1195. [Google Scholar] [CrossRef]
- Goldsmith, G.J.; White, J.G. Ferroelectric behavior of thiourea. J. Chem. Phys. 1959, 31, 1175–1187. [Google Scholar] [CrossRef]
- Kamishima, Y.; Akishige, Y.; Hashimoto, M. Ferroelectricity activity on organic crystal trichloroacetamide. J. Phys. Soc. Jpn. 1991, 60, 2147–2150. [Google Scholar] [CrossRef]
- Bordeaux, D.; Bornarel, J.; Capiomont, A.; Lajzerowicz-Bonneteau, J. New ferroelastic-ferroelectric compound: Tanane. Phys. Rev. Lett. 1973, 31, 314–317. [Google Scholar] [CrossRef]
- Kroupa, J.; Vanẽk, P.; Krupkova, R.; Zikmund, Z. Dielectric and optical properties of weak ferroelectric cyclohexan-1,1′-diacetic acid. Ferroelectrics 1997, 202, 229–234. [Google Scholar] [CrossRef]
- Szklarz, P.; Bator, G. Pyroelectric properties of tricyclohexylmethanol (TCHM) single crystal. J. Phys. Chem. Solids 2005, 66, 121–125. [Google Scholar] [CrossRef]
- Terauchi, H.; Kojima, T.; Sakaue, K.; Tajiri, F.; Maeda, H. Structural phase transition of benzil. J. Chem. Phys. 1982, 76, 612–615. [Google Scholar] [CrossRef]
- Gruner-Bauer, P.; Dormann, E. The ferroelectric low-temperature phase of single crystals of the substituted diacetylene 1,6-bis(2,4-dinitrophenoxy)-2,4-hexadiyne (DNP). J. Phys. Condens. Matter. 1992, 4, 5599–5609. [Google Scholar] [CrossRef]
- Feder, J. Two-dimensional ferroelectricity. Ferroelectrics 1976, 12, 71–84. [Google Scholar] [CrossRef]
- Horiuchi, S.; Kumai, R.; Tokura, Y. Hydrogen-bonding molecular chains for high-temperature ferroelectricity. Adv. Mater. 2011, 23, 2098–2103. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tokunaga, Y.; Giovannetti, G.; Picozzi, S.; Itoh, H.; Shimano, R.; Kumai, R.; Tokura, Y. Above-room-temperature ferroelectricity in a single-component molecular crystal. Nature 2010, 463, 789–792. [Google Scholar] [CrossRef]
- Horiuchi, S.; Kagawa, F.; Hatahara, K.; Kobayashi, K.; Kumai, R.; Murakami, Y.; Tokura, Y. Above-room-temperature ferroelectricity and antiferroelectricity in benzimidazoles. Nat. Commun. 2012, 3, 1308. [Google Scholar] [CrossRef]
- Dutta, S.; Vikas; Yadav, A.; Boomishankar, R.; Bala, A.; Kumar, V.; Chakraborty, T.; Elizabeth, S.; Munshi, P. Record-high thermal stability achieved in a novel single-component all-organic ferroelectric crystal exhibiting polymorphism. Chem. Commun. 2019, 55, 9610–9613. [Google Scholar] [CrossRef]
- Liao, W.-Q.; Deng, B.-B.; Wang, Z.-X.; Cheng, T.-T.; Hu, Y.-T.; Cheng, S.-P.; Xiong, R.-G. Optically induced ferroelectric polarization switching in a molecular ferroelectric with reversible photoisomerization. Adv. Sci. 2021, 8, 2102614. [Google Scholar] [CrossRef]
- Socol, M.; Trupina, L.; Galca, A.-C.; Chirila, C.; Stan, G.E.; Vlaicu, A.-M.; Stanciu, A.E.; Boni, A.G.; Botea, M.; Stanculescu, A.; et al. Electro-active properties of nanostructured films of cytosine and guanine nucleobases. Nanotechnology 2021, 32, 415702. [Google Scholar] [CrossRef]
- Zelenovskii, P.S.; Vasileva, D.S.; Vasilev, S.G.; Kopyl, S.; Kholkin, A. Ferroelectricity in glycine: A mini-review. Front. Mater. 2022, 9, 918890. [Google Scholar] [CrossRef]
- Bdikin, I.; Heredia, A.; Neumayer, S.M.; Bystrov, V.S.; Gracio, J.; Rodriguez, B.J.; Kholkin, A.L. Local piezoresponse and polarization switching in nucleobase thymine microcrystals. J. Appl. Phys. 2015, 118, 072007. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Zhu, X.; Shen, J. Light-induced ferroelectricity in bioinspired self-assembled diphenylalanine nanotubes/microtubes. Angew. Chem. Int. Ed. 2013, 52, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, A.J. Ferroelectric polymers. Science 1983, 220, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Ishida, K.; Kubono, A.; Horiuchi, T.; Yamada, H.; Matsushige, K. Remanent polarization of evaporated films of vinylidene fluoride oligomers. J. Appl. Phys. 2003, 93, 2866–2870. [Google Scholar] [CrossRef]
- Furukawa, T. Ferroelectric properties of vinylidene fluoride copolymers. Phase Transit. 1989, 18, 143–211. [Google Scholar] [CrossRef]
- He, X.; Yao, K.; Gan, B.K. Phase transition and properties of a ferroelectric poly(vinylidene fluoride-hexafluoropropylene) copolymer. J. Appl. Phys. 2005, 97, 084101. [Google Scholar] [CrossRef]
- Katz, D.; Gelfandbein, V. Ferroelectric behaviour of α-nylon 11. J. Phys. D Appl. Phys. 1982, 15, L115. [Google Scholar] [CrossRef]
- Yanakaa, A.; Sakai, W.; Kinashi, K.; Tsutsum, N. Ferroelectric performance of nylons 6–12, 10–12, 11–12, and 12–12. RSC Adv. 2020, 10, 15740–15750. [Google Scholar] [CrossRef]
- Bahgat, A.A.; Sayyah, S.M.; Abd-Elsalam, H.M. Study of ferroelectricity in polyaniline. Int. J. Polym. Mater. 2003, 52, 499–515. [Google Scholar] [CrossRef]
- Okamoto, H.; Mitani, T.; Tokura, Y.; Koshihara, S.; Komatsu, T.; Iwasa, Y.; Koda, T.; Saito, G. Anomalous dielectric response in tetrathiafulvalene-p-chloranil as observed in temperature- and pressure-induced neutral-to-ionic phase transition. Phys. Rev. B 1991, 43, 8224–8232. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Okimoto, Y.; Kumai, R.; Tokura, Y. Anomalous valence fluctuation near a ferroelectric transition in an organic charge-transfer complex. J. Phys. Soc. Jpn 2000, 69, 1302–1305. [Google Scholar] [CrossRef]
- Horiuchi, S.; Ishii, F.; Kumai, R.; Okimoto, Y.; Tachibana, H.; Nagaosa, N.; Tokura, Y. Ferroelectricity near room temperature in co-crystals of nonpolar organic molecules. Nat. Mater. 2005, 4, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Kumai, R.; Tokura, Y. A supramolecular ferroelectric realized by collective proton transfer. Angew. Chem. Int. Ed. 2007, 46, 3497–3501. [Google Scholar] [CrossRef]
- Szafrański, M.; Katrusiak, A.; McIntyre, G.J. Ferroelectric order of parallel bistable hydrogen bonds. Phys. Rev. Lett. 2002, 89, 215507. [Google Scholar] [CrossRef]
- Hoshino, S.; Mitsui, T.; Jona, F.; Pepinsky, R. Dielectric and thermal study of tri-glycine sulfate and tri-glycine fluoberyllate. Phys. Rev. 1957, 107, 1255–1258. [Google Scholar] [CrossRef]
- Khanduri, P.C.; Upadhyay, T.C. Study of ferroelectric phase transition in triglycine fluoberyllate crystal. Ferroelectrics 2024, 618, 375–384. [Google Scholar] [CrossRef]
- Levstik, A.; Filipič, C.; Blinc, R. Critical exponents of ferroelectric TSCC determined from dielectric measurements. Solid State Commun. 1976, 18, 1231–1234. [Google Scholar] [CrossRef]
- Valasek, J. Piezo-electric and allied phenomena in Rochelle salt. Phys. Rev. 1921, 17, 475–481. [Google Scholar] [CrossRef]
- Yang, Y.; Xi, Z.; Dong, Y.; Zheng, C.; Hu, H.; Li, X.; Jiang, Z.; Lu, W.-C.; Wu, D.; Wen, Z. Spin-filtering ferroelectric tunnel junctions as multiferroic synapses for neuromorphic computing. ACS Appl. Mater. Interfaces 2020, 12, 56300–56309. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yang, H.; Yang, H.; Tao, B.; Djeffal, A.; Chshiev, M.; Huang, W.; Li, X.; Ferri, A.; Desfeux, R.; et al. Ferroelectric control of organic/ferromagnetic spinterface. Adv. Mater. 2016, 28, 10204–10210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, J.; Zhu, H.; Wang, Z.; Zhou, L.; Wang, S.; Miyashita, T.; Mitsuishi, M.; Qin, G. Room temperature magnetoresistance effects in ferroelectric poly(vinylidene fluoride) spin valves. J. Mater. Chem. C 2017, 5, 5055. [Google Scholar] [CrossRef]
- Xiao, C.; Sun, H.; Cheng, L.; Devaux, X.; Ferri, A.; Huang, W.; Desfeux, R.; Li, X.-G.; Migot, S.; Chshiev, M.; et al. Temperature dependence of transport mechanisms in organic multiferroic tunnel junctions. J. Phys. D Appl. Phys. 2020, 53, 325301. [Google Scholar] [CrossRef]
- Subedi, R.C.; Geng, R.; Luong, H.M.; Huang, W.; Li, X.; Hornak, L.A.; Nguyen, T.D. Large magnetoelectric effect in organic ferroelectric copolymer-based multiferroic tunnel junctions. Appl. Phys. Lett. 2017, 110, 053302. [Google Scholar] [CrossRef]
- Liang, S.; Yu, Z.; Devaux, X.; Ferri, A.; Huang, W.; Yang, H.; Desfeux, R.; Li, X.; Migot, S.; Chaudhuri, D.; et al. Quenching of spin polarization switching in organic multiferroic tunnel junctions by ferroelectric “ailing-channel” in organic barrier. ACS Appl. Mater. Interfaces 2018, 10, 30614–30622. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, X.; Koten, M.A.; Shield, J.E.; Chen, X.; Yun, Y.; N’Diaye, A.T.; Hong, X.; Xu, X. Spin rectification and electrically controlled spin transport in molecular-ferroelectrics-based spin valves. Phys. Rev. Appl. 2020, 13, 064011. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Chakraborty, A.; D’Olimpio, G.; Fujii, J.; Ge, A.; Zhou, Y.; Liu, C.; Agarwal, A.; Vobornik, I.; et al. Terahertz nonlinear Hall rectifiers based on spin-polarized topological electronic states in 1T-CoTe2. Adv. Mater. 2023, 35, 2209557. [Google Scholar] [CrossRef]
- Jugovac, M.; Cojocariu, I.; Sánchez-Barriga, J.; Gargiani, P.; Valvidares, M.; Feyer, V.; Blügel, S.; Bihlmayer, G.; Perna, P. Inducing single spin-polarized flat bands in monolayer graphene. Adv. Mater. 2023, 35, 2301441. [Google Scholar] [CrossRef]
- Chakraborty, A.; Fujii, J.; Kuo, C.-N.; Lue, C.S.; Politano, A.; Vobornik, I.; Agarwal, A. Observation of highly anisotropic bulk dispersion and spin-polarized topological surface states in CoTe2. Phys. Rev. B 2023, 107, 085406. [Google Scholar] [CrossRef]
- Muñiz Cano, B.; Ferreiros, Y.; Pantaleón, P.A.; Dai, J.; Tallarida, M.; Figueroa, A.I.; Marinova, V.; García-Díez, K.; Mugarza, A.; Valenzuela, S.O.; et al. Experimental demonstration of a magnetically induced warping transition in a topological insulator mediated by rare-earth surface dopants. Nano Lett. 2023, 23, 6249–6258. [Google Scholar] [CrossRef]
- Vobornik, I.; Sarkar, A.B.; Zhang, L.; Boukhvalov, D.W.; Ghosh, B.; Piliai, L.; Kuo, C.-N.; Mondal, D.; Fujii, J.; Lue, C.S.; et al. Kitkaite NiTeSe, an ambient-stable layered Dirac semimetal with low-energy type-II Fermions with application capabilities in spintronics and optoelectronics. Adv. Funct. Mater. 2021, 31, 2106101. [Google Scholar] [CrossRef]
- Jugovac, M.; Cojocariu, I.; Brondin, C.A.; Crotti, A.; Petrović, M.; Bonetti, S.; Locatelli, A.; Menteş, T.O. Coupling borophene to graphene in air-stable heterostructures. Adv. Electron. Mater. 2023, 9, 2300136. [Google Scholar] [CrossRef]
- Fujii, J.; Ghosh, B.; Vobornik, I.; Sarkar, A.B.; Mondal, D.; Kuo, C.-N.; Bocquet, F.C.; Zhang, L.; Boukhvalov, D.W.; Lue, C.S.; et al. Mitrofanovite Pt3Te4: A topological metal with termination-dependent surface band structure and strong spin polarization. ACS Nano 2021, 15, 14786–14793. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.; Schlickum, U.; Klappenberger, F.; Zoppellaro, G.; Klyatskaya, S.; Ruben, M.; Barth, J.V.; Brune, H. Using metal-organic templates to steer the growth of Fe and Co nanoclusters. Appl. Phys. Lett. 2008, 93, 243102. [Google Scholar] [CrossRef]
- Barja, S.; Stradi, D.; Borca, B.; Garnica, M.; Díaz, C.; Rodriguez-García, J.M.; Alcamí, M.; Vázquez de Parga, A.L.; Martín, F.; Miranda, R. Ordered arrays of metal–organic magnets at surfaces. J. Phys. Condens. Matter. 2013, 25, 484007. [Google Scholar] [CrossRef]
- Martín-Fuentes, C.; Parreiras, S.O.; Urgel, J.I.; Rubio-Giménez, V.; Muñiz Cano, B.; Moreno, D.; Lauwaet, K.; Valvidares, M.; Valbuena, M.A.; Gargiani, P.; et al. On-surface design of a 2D cobalt-organic network preserving large orbital magnetic moment. J. Am. Chem. Soc. 2022, 144, 16034–16041. [Google Scholar] [CrossRef] [PubMed]
- Schlickum, U.; Klappenberger, F.; Decker, R.; Zoppellaro, G.; Klyatskaya, S.; Ruben, M.; Kern, K.; Brune, H.; Barth, J.V. Surface-confined metal−organic nanostructures from Co-directed assembly of linear terphenyl-dicarbonitrile linkers on Ag(111). J. Phys. Chem. C 2010, 114, 15602–15606. [Google Scholar] [CrossRef]
- Cojocariu, I.; Carlotto, S.; Jugovac, M.; Floreano, L.; Casarin, M.; Feyer, V.; Schneider, C.M. Distortion-driven spin switching in electron-doped metal porphyrins. J. Mater. Chem. C 2022, 10, 9748–9757. [Google Scholar] [CrossRef]
- Stradi, D.; Borca, B.; Barja, S.; Garnica, M.; Díaz, C.; Rodríguez-García, J.M.; Alcamí, M.; Vázquez de Parga, A.L.; Miranda, R.; Martín, F. Understanding the self-assembly of TCNQ on Cu(111): A combined study based on scanning tunnelling microscopy experiments and density functional theory simulations. RSC Adv. 2016, 6, 15071–15079. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Auwärter, W.; Ecija, D.; Seufert, K.; Rusponi, S.; Houwaart, T.; Sautet, P.; Bocquet, M.-L.; Thakur, P.; Stepanow, S.; et al. Restoring the Co magnetic moments at interfacial Co-porphyrin arrays by site-selective uptake of iron. ACS Nano 2015, 9, 3605–3616. [Google Scholar] [CrossRef]
- Ballav, N.; Wäckerlin, C.; Siewert, D.; Oppeneer, P.M.; Jung, T.A. Emergence of on-surface magnetochemistry. J. Phys. Chem. Lett. 2013, 4, 2303–2311. [Google Scholar] [CrossRef]
- Cuxart, M.G.; Valbuena, M.A.; Robles, R.; Moreno, C.; Bonell, F.; Sauthier, G.; Imaz, I.; Xu, H.; Nistor, C.; Barla, A.; et al. Molecular approach for engineering interfacial interactions in magnetic/topological insulator heterostructures. ACS Nano 2020, 14, 6285–6294. [Google Scholar] [CrossRef]
- Bystrov, V.; Paramonova, E.; Meng, X.; Shen, H.; Wang, J.; Lin, T.; Fridkin, V. Ferroelectric thin films and composites based on polyvinylidene fluoride and graphene layers: Molecular dynamics study. Coatings 2024, 14, 356. [Google Scholar] [CrossRef]
- Bychkov, I.; Belim, S.; Maltsev, I.; Shavrov, V. Phase transition and magnetoelectric effect in 2D ferromagnetic films on a ferroelectric substrate. Coatings 2021, 11, 1325. [Google Scholar] [CrossRef]
- Chylarecka, D.; Kim, T.K.; Tarafder, K.; Müller, K.; Gödel, K.; Czekaj, I.; Wäckerlin, C.; Cinchetti, M.; Ali, M.E.; Piamonteze, C.; et al. Indirect magnetic coupling of manganese porphyrin to a ferromagnetic cobalt substrate. J. Phys. Chem. C 2011, 115, 1295–1301. [Google Scholar] [CrossRef]
- Aryal, U.K.; Ahmadpour, M.; Turkovic, V.; Rubahn, H.-G.; Di Carlo, A.; Madsen, M. 2D materials for organic and perovskite photovoltaics. Nano Energy 2022, 94, 106833. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Pillai, S.C. Two-dimensional (2D) electrode materials for supercapacitors. Mater. Today Proc. 2021, 41, 498–505. [Google Scholar] [CrossRef]
- Hao, G.; Mosey, A.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, T.; Zhang, X.; Zhang, J.; N’Diaye, A.T.; Cheng, R.; et al. Nonvolatile voltage controlled molecular spin state switching. Appl. Phys. Lett. 2019, 114, 032901. [Google Scholar] [CrossRef]
- Borca, B.; Bartha, C. Advances of nanoparticles and thin films. Coatings 2022, 12, 1138. [Google Scholar] [CrossRef]
| Organic Material | System (Thickness) | MR/Temp. (K) | Ref. |
|---|---|---|---|
 Fullerene | LSMO(50 nm)/C60(120 nm)/Co(15 nm) | −13.3%/20 K | [13] |
| LSMO(50 nm)/C60(180 nm)/Co(15 nm) | ~−7%/20 K | [13] | |
| LSMO(50 nm)/C60(180 nm)/Co(15 nm) | ~−4.5%/120 K | [13] | |
| LSMO(50 nm)/C60(180 nm)/Co(15 nm) | ~−1%/290 K | [13] | |
| Co(15 nm)/AlOx(1 nm)/C60(5 nm)/Py(20 nm) | 9%/300 K | [14] | |
| Co(15 nm)/AlOx(1 nm)/C60(28 nm)/Py(20 nm) | 5.5%/300 K | [14] | |
| Co(15 nm)/AlOx(1 nm)/C60(18 nm)/Py(20 nm) | ~14%/80 K | [14] | |
| Fe3O4/AlOx/C60(80 nm)/Co(10 nm) | 6.9%/150 K | [15] | |
| Fe3O4/AlOx/C60(80 nm)/Co(10 nm) | 5.3%/300 K | [15] | |
| Fe3O4/AlOx/C60(110 nm)/Co(10 nm) | ~2.2%/150 K | [15] | |
| Fe3O4/AlOx/C60(110 nm)/Co(10 nm) | ~0.5%/300 K | [15] | |
| LSMO(50 nm)/C70(120 nm)/Co(15 nm) | −8.9%/20 K | [13] | |
| LSMO(50 nm)/C70(180 nm)/Co(15 nm) | ~−6%/20 K | [13] | |
| LSMO(50 nm)/C70(180 nm)/Co(15 nm) | ~−4%/120 K | [13] | |
| LSMO(50 nm)/C70(180 nm)/Co(15 nm) | ~−0.8%/290 K | [13] | |
 PC71BM | Co(20 nm)/LiF(1.4 nm)/PC71BM(45 nm)/Py(12 nm) | ~6.2%/300 K | [16] |
| Co(20 nm)/LiF(1.5 nm)/PC71BM(45 nm)/Py(12 nm) | ~1.3%/300 K | [16] | |
| Co(20 nm)/LiF(1.6 nm)/PC71BM(45 nm)/Py(12 nm) | ~−0.6%/300 K | [16] | |
| Co(20 nm)/LiF(1.8 nm)/PC71BM(45 nm)/Py(12 nm) | ~−2.4%/300 K | [16] | |
| Co(20 nm)/LiF(2 nm)/PC71BM(45 nm)/Py(12 nm) | ~−1.2%/300 K | [16] | |
 Rubrene | Fe3O4(100 nm)/AlOx(2 nm)/rubrene(2 nm)/Co(10 nm) | 6%/300 K | [17] |
| Fe3O4(100 nm)/AlOx(2 nm)/rubrene(2 nm)/Co(10 nm) | 11%/150 K | [17] | |
| Fe3O4(100 nm)/AlOx(2 nm)/rubrene(6 nm)/Co(10 nm) | 3.3%/300 K | [17] | |
| Fe3O4(100 nm)/AlOx(2 nm)/rubrene(6 nm)/Co(10 nm) | 9%/150 K | [17] | |
| Co(8 nm)/Al2O3(0.5 nm)/rubrene(4.6 nm)/Fe(10 nm)/CoO(1.5 nm) | 6%/295 K | [18] | |
| Co(8 nm)/Al2O3(0.5 nm)/rubrene(4.6 nm)/Fe(10 nm)/CoO(1.5 nm) | 13%/80 K | [18] | |
| Co(8 nm)/Al2O3(0.5 nm)/rubrene(4.6 nm)/Fe(10 nm)/CoO(1.5 nm) | 16%/4.2 K | [18] | |
| Co(15 nm)/AlOx(2.5 nm)/rubrene(5 nm)/Fe(15 nm) | ~−0.058%/100 K | [19] | |
| Co(15 nm)/AlOx(2.5 nm)/rubrene(10 nm)/Fe(15 nm) | ~−0.058%/100 K | [19] | |
| Co(15 nm)/AlOx(2.5 nm)/rubrene(5 nm)/Fe(15 nm) | ~−0.025%/300 K | [19] | |
| Co(15 nm)/AlOx(2.5 nm)/rubrene(10 nm)/Fe(15 nm) | ~−0.035%/300 K | [19] | |
| LSMO(50 nm)/LAO(1.2 nm)/rubrene(5 nm)/Fe(30 nm) | ~12.5%/10 K | [20] | |
| LSMO(50 nm)/LAO(1.2 nm)/rubrene(5 nm)/Fe(30 nm) | ~3.5%/150 K | [20] | |
| LSMO(50 nm)/LAO(1.2 nm)/rubrene(5 nm)/Fe(30 nm) | ~0.1%/250 K | [20] | |
| LSMO(50 nm)/LAO(1.2 nm)/rubrene(20 nm)/Fe(30 nm) | ~0.2%/10 K | [20] | |
 Rubrene/TCNE | Fe(50 nm)/rubrene(10 nm)/V[TCNE]x(300 nm) | −0.06%/100 K | [21] |
| Fe(50 nm)/rubrene(10 nm)/V[TCNE]x(300 nm) | −0.02%/200 K | [21] | |
| Fe(50 nm)/rubrene(10 nm)/V[TCNE]x(300 nm) | 0.01%/300 K | [21] | |
| Al(50 nm)/V[TCNE]x(50 nm)/rubrene(10 nm)/V[TCNE]x(300 nm)/Al(30 nm) | −0.035%/100 K | [22] | |
| Al(50 nm)/V[TCNE]x(50 nm)/rubrene(10 nm)/V[TCNE]x(300 nm)/Al(30 nm) | −0.01%/200 K | [22] | |
 BCP | Co(11 nm)/AlOx(1.5 nm)/BCP(5 nm)/Py(11 nm) | ~4.5%/300 K | [23] |
| Co(11 nm)/AlOx(1.5 nm)/BCP(10 nm)/Py(11 nm) | ~4%/300 K | [23] | |
| Co(11 nm)/AlOx(1.5 nm)/BCP(30 nm)/Py(11 nm) | ~4%/300 K | [23] | |
| Co(11 nm)/AlOx(1.5 nm)/BCP(10 nm)/Py(11 nm) bending/in air | ~3%/300 K | [24] | |
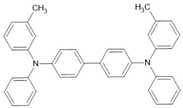 TPD | Co2MnSi(20 nm)/TPD(200 nm)/Co(7 nm) | 7.8%/300 K | [25] |
| Co2MnSi(20 nm)/TPD(200 nm)/Co(7 nm) | ~11%/150 K | [25] | |
| Co2MnSi(20 nm)/TPD(200 nm)/Co(7 nm) | 10.7%/5 K | [25] | |
| LSMO/TPD(200 nm)/Co(7 nm) | ~1.5%/250 K | [25] | |
| LSMO/TPD(200 nm)/Co(7 nm) | ~5%/150 K | [25] | |
| LSMO/TPD(200 nm)/Co(7 nm) | 19%/5 K | [25] | |
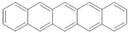 pentacene | LSMO/pentacene(200 nm)/LSMO | ~5.5%/5.3 K | [26] |
| LSMO/pentacene(30 nm)/LSMO | ~2%/9 K | [27] | |
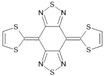 BTQBT | LSMO/BTQBT(200 nm)/LSMO | ~8%/10 K | [26] |
| LSMO/BTQBT(50 nm)/LSMO | ~28%/9.1 K | [26] | |
 TIPS-Pentacene | CoPt(10 nm)/TIPS-pentacene(75 nm)/AlOx(2 nm)/Co(10 nm) | −0.08%/300 K | [28] |
| CoPt(10 nm)/TIPS-pentacene(40 nm)/AlOx(2 nm)/Co(10 nm) | ~−0.45%/300 K | [28] | |
| CoPt(10 nm)/TIPS-pentacene(40 nm)/AlOx(2 nm)/Co(10 nm) | ~−0.05%/175 K | [28] | |
| CoPt(10 nm)/TIPS-pentacene(100 nm)/AlOx(2 nm)/Co(10 nm) | ~−0.05%/300 K | [28] | |
| LSMO/TIPS-pentacene single crystal(269 nm)/Co(10 nm) | ~−17%/30 K | [28] | |
| LSMO/TIPS-pentacene single crystal(457 nm)/Co(10 nm) | ~−1.5%/30 K | [29] | |
| LSMO/TIPS-pentacene single crystal(269 nm)/Co(10 nm) | ~−2.8%/100 K | [29] | |
| LSMO/TIPS-pentacene polycryst. film(97 nm)/Co(10 nm) | ~−6%/30 K | [29] | |
| LSMO/TIPS-pentacene polycryst. film(97 nm)/Co(10 nm) | ~−3.5%/100 K | [29] | |
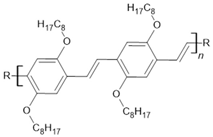 DOO-PPV | LSMO(200 nm)/Hydrogen-DOO-PPV(25 nm)/Co(15 nm) | 2%/10 K | [30] |
| LSMO(200 nm)/Deuterium-DOO-PPV(25 nm)/Co(15 nm) | ~40%/10 K | [30] | |
| LSMO(200 nm)/H/D-DOO-PPV(25 nm)/Co(15 nm) | ~5%/10 K | [30] | |
| LSMO(200 nm)/H/D-DOO-PPV(25 nm)/Co(15 nm) | ~2.5%/150 K | [30] | |
| LSMO(200 nm)/H/D-DOO-PPV(25 nm)/Co(15 nm) | ~0.2%/300 K | [30] | |
| LSMO(200 nm)/H-DOO-PPV(55 nm)/Co(15 nm) | ~0.2%/10 K | [30] | |
| LSMO(200 nm)/D-DOO-PPV(55 nm)/Co(15 nm) | ~2.5%/10 K | [30] | |
 Alq3 | LSMO(100 nm)/Alq3(130 nm)/Co(3.5 nm) | −40%/11 K | [31] |
| LSMO(100 nm)/Alq3(160 nm)/Co(3.5 nm) | ~−14%/11 K | [31] | |
| LSMO(100 nm)/Alq3(200 nm)/Co(3.5 nm) | ~−7%/11 K | [31] | |
| LSMO(100 nm)/Alq3(250 nm)/Co(3.5 nm) | ~−2%/11 K | [31] | |
| LSMO(100 nm)/Alq3(160 nm)/Co(3.5 nm) | ~−2%/130 K | [31] | |
| LSMO(100 nm)/Alq3(160 nm)/Co(3.5 nm) | ~−0.3%/230 K | [31] | |
| Co(8 nm)/Al2O3(0.6 nm)/Alq3(1.6 nm)/Py(10 nm) | 4.6%/300 K | [32] | |
| Co(8 nm)/Al2O3(0.6 nm)/Alq3(1.6 nm)/Py(10 nm) | 6.8%/77 K | [32] | |
| Co(8 nm)/Al2O3(0.6 nm)/Alq3(1.6 nm)/Py(10 nm) | 7.5%/4.2 K | [32] | |
| LSMO(15–20 nm)/Alq3 (300 nm)/Al2O3 (2 nm)/Co(35 nm) | ~−0.1%/20 K | [33] | |
| LSMO(15–20 nm)/Alq3 (200 nm)/Al2O3 (2 nm)/Co(35 nm) | ~−5%/20 K | [33] | |
| LSMO(15–20 nm)/Alq3 (100 nm)/Al2O3 (2 nm)/Co(35 nm) | −11%/20 K | [33] | |
| LSMO(15–20 nm)/Alq3 (100 nm)/Al2O3 (2 nm)/Co(35 nm) | −6%/100 K | [33] | |
| LSMO(15–20 nm)/Alq3 (100 nm)/Al2O3 (2 nm)/Co(35 nm) | −0.15%/300 K | [33] | |
| LSMO/Alq3 (23 nm)/Co-nanodots/Co(7 nm) | −1%/10 K | [34] | |
| LSMO/Alq3 (67 nm)/Co-nanodots/Co(7 nm) | −7%/10 K | [34] | |
| LSMO/Alq3 (93 nm)/Co-nanodots/Co(7 nm) | −300%/10 K | [34] | |
| LSMO/Alq3 (135 nm)/Co-nanodots/Co(7 nm) | −13%/10 K | [34] | |
| LSMO/Alq3 (93 nm)/Co(7 nm) | −35%/10 K | [34] | |
| LSMO/Alq3 (135 nm)/Co(7 nm) | −4%10 K | [34] | |
| LSMO/Alq3 (40 nm)/Co(20 nm) | ~−18%/100 K | [35] | |
| LSMO/Alq3 (40 nm)/Co(20 nm) | ~−10%/150 K | [35] | |
| LSMO/Alq3 (40 nm)/Co(20 nm) | −0.07%/300 K | [35] | |
| LPCMO(60 nm)/Alq3(60 nm)/Co(10 nm) | 100–440%(1−7T)/10 K | [36] | |
| LPCMO(60 nm)/Alq3(60 nm)/Co(10 nm) | ~200%(7T)/25 K | [36] | |
| LPCMO(60 nm)/Alq3(60 nm)/Co(10 nm) | ~100%(7T)/75 K | [36] | |
| LPCMO(60 nm)/Alq3(60 nm)/Co(10 nm) | ~1%(7T)/150 K | [36] | |
| LSMO/Alq3(60 nm)/Co(10 nm) | −37%/10 K | [36] | |
| SFMO(150 nm)/Alq3(45 nm)/Co(16 nm) | 30%/10 K | [37] | |
| SFMO(150 nm)/Alq3(45 nm)/Co(16 nm) | 25%/100 K | [37] | |
| SFMO(150 nm)/Alq3(45 nm)/Co(16 nm) | 10%/200 K | [37] | |
| SFMO(150 nm)/Alq3(45 nm)/Co(16 nm) | 2%/300 K | [37] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(2 nm)/Co(10 nm) | ~12.5%/150 K | [38] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(2 nm)/Co(10 nm) | ~6%/300 K | [38] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(10 nm)/Co(10 nm) | ~4%/150 K | [38] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(10 nm)/Co(10 nm) | ~2%/300 K | [38] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(20 nm)/Co(10 nm) | ~1%/150 K | [38] | |
| Fe3O4(110 nm)/AlOx(2 nm)/Alq3(20 nm)/Co(10 nm) | ~0.5%/300 K | [38] | |
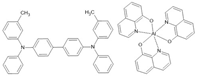 TPD/Alq3 | FeCo(17 nm)/TPD(50 nm)/Alq3(200 nm)/LiF(1.9 nm)/NiFe(17 nm) | ~0.3%/40 K | [39] |
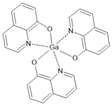 Gaq3 | LSMO(20 nm)/Gaq3 (10–15 nm)/AlOx(2 nm)/Co(7 nm) electrically controlled-high-resistive state (oxygen migration) | −2%/100 K | [40] |
| LSMO(20 nm)/Gaq3 (10–15 nm)/AlOx(2 nm)/Co(7 nm) electrically controlled-high-resistive state (oxygen migration) | −8%/100 K | [40] | |
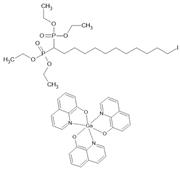 IC12H2(PO3Et2)2/ Gaq3 | LSMO/IC12H2(PO3Et2)2(ML)/Gaq3(40 nm)/AlOx(2 nm)/Co | ~−1.9%/200 K | [41] |
| LSMO/IC12H2(PO3Et2)2(ML)/Gaq3(40 nm)/ AlOx(2 nm)/Co | ~−9.8%/100 K | [41] | |
| LSMO/IC12H2(PO3Et2)2(ML)/Gaq3(40 nm)/ AlOx(2 nm)/Co | ~−18%/10 K | [41] | |
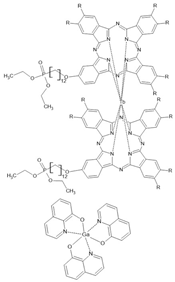 Tb[Pc(C12OPO3Et2)]2 /Gaq3 | LSMO/Tb[Pc(C12OPO3Et2)]2(ML)/Gaq3(40 nm)/AlOx(2 nm)/Co | ~−2%/200 K | [41] |
| LSMO/Tb[Pc(C12OPO3Et2)]2(ML)/Gaq3(40 nm)/AlOx(2 nm)/Co | ~−9.3%/100 K | [41] | |
| LSMO/Tb[Pc(C12OPO3Et2)]2(ML)/Gaq3(40 nm)/AlOx(2 nm)/Co | ~−13%/100 K | [41] | |
 NitPO/Gaq3 | LSMO(15 nm)/NitPO(ML)/Gaq3(150 nm)/AlOx(2 nm)/Co | −4.34%/100 K | [42] |
| LSMO(15 nm)/NitPO(ML)/Gaq3(150 nm)/AlOx(2 nm)/Co | −7.1%/3 K | [42] | |
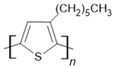 P3HT | Fe50Co50(20 nm)/P3HT(75 nm)/Ni81Fe19(20 nm) | 0.1%/300 K | [43] |
| Fe50Co50(40 nm)/P3HT(150 nm)/Ni81Fe19(20 nm) | −0.04%/300 K | [43] | |
| Ni81Fe19(12 nm)/P3HT(30 nm)/AlOx(1 nm)/Co(10 nm) | ~1%/10 K | [44] | |
| Fe3O4(80 nm)/P3HT(12 nm)/AlOx(1 nm)/Co(12 nm) | ~2.3%/100 K | [45] | |
| Fe3O4(80 nm)/P3HT(12 nm)/AlOx(1 nm)/Co(12 nm) | ~0.75%/200 K | [45] | |
| Fe3O4(80 nm)/P3HT(25 nm)/AlOx(1 nm)/Co(12 nm) | ~1.75%/100 K | [45] | |
| LSMO/P3HT(100 nm)/Co(10 nm) | 80%/5 K | [46] | |
| LSMO/P3HT(100 nm)/Co(10 nm) | 1.5%/300 K | [46] | |
| LSMO(100 nm)/P3HT(80 nm)/AlOx(1 nm)/Co(10 nm) | −0.2%/2 K 15.6%/2 K | [47] | |
| LSMO/P3HTannealed(45 nm)/Co | ~15%/20 K | [48] | |
| LSMO/P3HTannealed(72 nm)/Co | ~11%/20 K | [48] | |
| LSMO/P3HTannealed(103 nm)/Co | ~8%/20 K | [48] | |
| LSMO/P3HTannealed(175 nm)/Co | ~4%/20 K | [48] | |
| LSMO/P3HT(45 nm)/Co | ~12%/20 K | [48] | |
| LSMO/P3HT(72 nm)/Co | ~6%/20 K | [48] | |
| LSMO/P3HT(103 nm)/Co | ~3%/20 K | [48] | |
| LSMO/P3HT(175 nm)/Co | ~2%/20 K | [48] | |
| LPCMO(60 nm)/P3HT(30 nm)/AlOx/Co(10 nm) | 93%/30 K | [49] | |
| LPCMO(60 nm)/P3HT(30 nm)/AlOx/Co(10 nm) | ~53%/50 K | [49] | |
| LPCMO(60 nm)/P3HT(30 nm)/AlOx/Co(10 nm) | ~30%/75 K | [49] | |
| LSMO(100 nm)/P3HT(20 nm)/AlOx(1 nm)/Co(10 nm) | ~7.5%/2 K | [50] | |
| LSMO(100 nm)/P3HT(20 nm)/AlOx(1 nm)/Co(10 nm) | ~4%/5 K | [50] | |
| LSMO(100 nm)/P3HT(20 nm)/AlOx(1 nm)/Co(10 nm) | ~3%/10 K | [50] | |
| LSMO(100 nm)/P3HT(20 nm)/AlOx(1 nm)/Co(10 nm) | ~0.1%/30 K | [50] | |
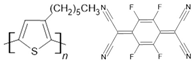 P3HT/F4-TCNQ | LSMO(100 nm)/P3HT(40 nm)/F4TCNQ dopant/Co(15 nm) | ~19%/2 K | [51] |
| LSMO(100 nm)/P3HT(40 nm)/F4TCNQ dopant/Co(15 nm) | ~16%/5 K | [51] | |
| LSMO(100 nm)/P3HT(40 nm)/F4TCNQ dopant/Co(15 nm) | ~14%/10 K | [51] | |
| LSMO(100 nm)/P3HT(40 nm)/F4TCNQ dopant/Co(15 nm) | ~13%/15 K | [51] | |
| LSMO(100 nm)/P3HT(40 nm)/F4TCNQ dopant/Co(15 nm) | ~11%/20 K | [51] | |
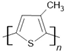 P3MT | LSMO/P3MT(15 nm)/Co(15 nm) | ~−43%/20 K | [52] |
| LSMO/P3MT(15 nm)/Co(15 nm) | ~−0.3%/280 K | [52] | |
 T6 | LSMO/T6(70–140 nm)/LSMO | 15–30%/300 K | [9] |
| LSMO/T6(200 nm)/LSMO | 7–10%/300 K | [9] | |
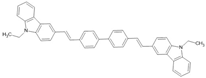 CVB | LSMO/CVB(100 nm)/Co | ~−15%/14 K | [53] |
| LSMO/CVB(100 nm)/Co | ~−3.5%/140 K | [53] | |
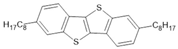 C8-BTBT | Ni78Fe22/C8-BTBT(2 nm)/Ni78Fe22 | ~−0.3%/300 K | [54] |
| Ni78Fe22/C8-BTBT(4 nm)/Ni78Fe22 | ~0.5%/300 K | [54] | |
| Py/C8-BTBT(1ML)/Co | ~−1%/10 K | [55] | |
| Py/C8-BTBT(2ML)/Co | ~−12%/10 K | [55] | |
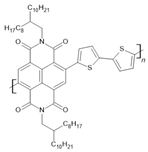 P(NDI2OD-T2) | LSMO(100 nm)/P(NDI2OD-T2)(35 nm)/AlOx(1.5 nm)/Co(10 nm) | ~30%/4.2 K | [56] |
| LSMO(100 nm)/P(NDI2OD-T2)(35 nm)/AlOx(1.5 nm)/Co(10 nm) | ~18%/50 K | [56] | |
| LSMO(100 nm)/P(NDI2OD-T2)(35 nm)/AlOx(1.5 nm)/Co(10 nm) | ~5%/150 K | [56] | |
| LSMO(100 nm)/P(NDI2OD-T2)(35 nm)/AlOx(1.5 nm)/Co(10 nm) | ~1%/250 K | [56] | |
| LSMO(100 nm)/P(NDI2OD-T2)(100 nm)/AlOx(1.5 nm)/Co(10 nm) | ~10%/4.2 K | [56] | |
| LSMO(100 nm)/P(NDI2OD-T2)(100 nm)/AlOx(1.5 nm)/Co(10 nm) | ~5%/50 K | [56] | |
| LSMO(100 nm)/P(NDI2OD-T2)(100 nm)/AlOx(1.5 nm)/Co(10 nm) | ~2%/150 K | [56] | |
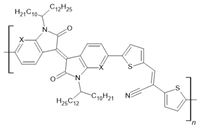 PIID-CNTVT-C1: X=C PAIID-CNTVT-C1: X=N | LSMO(50 nm)/PIID-CNTVT-C1(40 nm)/Py(10 nm) | ~19%/50 K | [57] |
| LSMO(50 nm)/PIID-CNTVT-C1(40 nm)/Py(10 nm) | ~14%/100 K | [57] | |
| LSMO(50 nm)/PIID-CNTVT-C1(40 nm)/Py(10 nm) | ~8%/150 K | [57] | |
| LSMO(50 nm)/PIID-CNTVT-C1(40 nm)/Py(10 nm) | ~3%/200 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C1(40 nm)/Py(10 nm) | ~25%/50 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C1(40 nm)/Py(10 nm) | ~17%/100 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C1(40 nm)/Py(10 nm) | ~10%/150 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C1(40 nm)/Py(10 nm) | ~5%/200 K | [57] | |
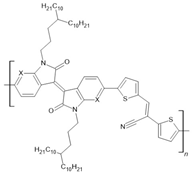 PIID-CNTVT-C3: X=C-H PAIID-CNTVT-C3: X=N | LSMO(50 nm)/PIID-CNTVT-C3(40 nm)/Py(10 nm) | ~16%/50 K | [57] |
| LSMO(50 nm)/PIID-CNTVT-C3(40 nm)/Py(10 nm) | ~12%/100 K | [57] | |
| LSMO(50 nm)/PIID-CNTVT-C3(40 nm)/Py(10 nm) | ~6%/150 K | [57] | |
| LSMO(50 nm)/PIID-CNTVT-C3(40 nm)/Py(10 nm) | ~2%/200 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C3(40 nm)/Py(10 nm) | ~23%/50 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C3(40 nm)/Py(10 nm) | ~15%/100 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C3(40 nm)/Py(10 nm) | ~10%/150 K | [57] | |
| LSMO(50 nm)/PAIID-CNTVT-C3(40 nm)/Py(10 nm) | ~5%/200 K | [57] | |
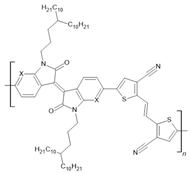 PIID-TVTCN: X=C-H PAIID-TVTCN: X=N PFIID-TVTCN: X=C-F | LSMO(30 nm)/PIID-TVTCN(25 nm)/Co(10 nm) | ~−23%/50 K | [58] |
| LSMO(30 nm)/PIID-TVTCN(25 nm)/Co(10 nm) | ~−8%/200 K | [58] | |
| LSMO(30 nm)/PAIID-TVTCN(25 nm)/Co(10 nm) | ~−10%/50 K | [58] | |
| LSMO(30 nm)/PAIID-TVTCN(25 nm)/Co(10 nm) | ~−3%/200 K | [58] | |
| LSMO(30 nm)/PFIID-TVTCN(25 nm)/Co(10 nm) | ~−16%/50 K | [58] | |
| LSMO(30 nm)/PFIID-TVTCN(25 nm)/Co(10 nm) | ~−5%/200 K | [58] | |
| LSMO(30 nm)/PIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~10%/50 K | [58] | |
| LSMO(30 nm)/PIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~4%/200 K | [58] | |
| LSMO(30 nm)/PAIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~17%/50 K | [58] | |
| LSMO(30 nm)/PAIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~5%/200 K | [58] | |
| LSMO(30 nm)/PFIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~10%/50 K | [58] | |
| LSMO(30 nm)/PFIID-TVTCN(25 nm)/AlOx(2 nm)/Co(10 nm) | ~3%/200 K | [58] | |
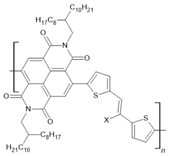 PNVT-8: X=H PNVT-CN-8:X=CN | NiFe(12 nm)/PNVT-8(15 nm)/Co(12 nm) | ~0.35%/10 K | [59] |
| NiFe(12 nm)/PNVT-8(15 nm)/Co(12 nm) | ~0.18/150 K | [59] | |
| NiFe(12 nm)/PNVT-8(15 nm)/Co(12 nm) | ~0.12/300 K | [59] | |
| NiFe(12 nm)/PNVT-CN-8(15 nm)/Co(12 nm) | ~0.22/10 K | [59] | |
| NiFe(12 nm)/PNVT-CN-8(15 nm)/Co(12 nm) | ~0.1%/150 K | [59] | |
| NiFe(12 nm)/PNVT-CN-8(15 nm)/Co(12 nm) | ~0.08%/300 K | [59] | |
| NiFe(12 nm)/Au(3 nm)/PNVT-CN-8(25 nm)/Co(12 nm) | ~−0.1%/100 K | [60] | |
| NiFe(12 nm)/Au(3 nm)/PNVT-CN-8(25 nm)/Co(12 nm) | ~−0.08%/300 K | [60] | |
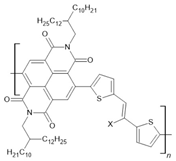 PNVT-10: X=H PNVT-CN-10:X=CN | NiFe(12 nm)/PNVT-10(15 nm)/Co(12 nm) | ~0.19%/10 K | [59] |
| NiFe(12 nm)/PNVT-10(15 nm)/Co(12 nm) | ~0.07/150 K | [59] | |
| NiFe(12 nm)/PNVT-10(15 nm)/Co(12 nm) | ~0.02/300 K | [59] | |
| NiFe(12 nm)/PNVT-CN-10(15 nm)/Co(12 nm) | ~0.13%/10 K | [59] | |
| NiFe(12 nm)/PNVT-CN-10(15 nm)/Co(12 nm) | ~0.05/150 K | [59] | |
| NiFe(12 nm)/PNVT-CN-10(15 nm)/Co(12 nm) | ~0.02/200 K | [59] | |
| NiFe(12 nm)/Au(3 nm)/PNVT-CN-10(25 nm)/Co(12 nm) | ~0.05%/10 K | [60] | |
| NiFe(12 nm)/Au(3 nm)/PNVT-CN-10(25 nm)/Co(12 nm) | ~−0.03%/100 K | [60] | |
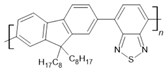 F8BT | LSMO(20 nm)/F8BT(45 nm)/MoOx(3 nm)/Co(20 nm) magneto-electroluminescence | ~9%/20 K ~2.4%/20 K | [61] |
| LSMO(20 nm)/F8BT(45 nm)/MoOx(3 nm)/Co(20 nm) | ~7%/30 K | [61] | |
| LSMO(20 nm)/F8BT(45 nm)/MoOx(3 nm)/Co(20 nm) magneto-electroluminescence | ~3%/100 K ~0.4%/100 K | [61] | |
| LSMO(20 nm)/F8BT(45 nm)/MoOx(3 nm)/Co(20 nm) | ~1%/150 K | [61] | |
 Py-Np | LSMO/Py-Np(90 nm)/Co(20 nm) | ~−27%/30 K | [62] |
| LSMO/Py-Np(90 nm)/Co(20 nm) | ~−19%/100 K | [62] | |
| LSMO/Py-Np(90 nm)/Co(20 nm) | ~−9%/150 K | [62] | |
| LSMO/Py-Np(90 nm)/Co(20 nm) | ~−2%/200 K | [62] | |
 TPP | LSMO(50 nm)/TPP(20 nm)/Co(5 nm) | ~−15%/80 K | [63] |
| LSMO(50 nm)/TPP(20 nm)/Co(5 nm) | ~−6%/80 K | [63] | |
| LSMO(50 nm)/TPP(20 nm)/Co(5 nm) | ~−3%/80 K | [63] | |
 H2Pc | Co(12 nm)/AlOx(1.5 nm)/H2Pc(90 nm)/Py spin-photovoltaic-resistive levels control with light intensity | ~7%/300 K | [64] |
 CuPc | Fe(25 nm)/CuPc(100 nm)/Co(5 nm) | 6.4%/40 K | [65] |
| Fe(25 nm)/CuPc(100 nm)/Co(5 nm) | 3.2%/80 K | [65] | |
| Fe(25 nm)/CuPc(100 nm)/Co(5 nm) | 1.8%/120 K | [65] | |
| LSMO/CuPc(110 nm)/Co | ~−6%/10 K | [66] | |
| LSMO/CuPc(110 nm)/Co | ~−4.5%/110 K | [66] | |
| LSMO/CuPc(110 nm)/Co | ~−1%/300 K | [66] | |
| LSMO/CuPc(50 nm)/Co | ~−20%/10 K | [66] | |
| LSMO/CuPc(160 nm)/Co | ~−2.5%/10 K | [66] | |
| LSMO/CuPc(200 nm)/Co | ~−0.5%/10 K | [66] | |
 FePc | Co/FePc(50 nm)/Co | 4%/10 K | [67] |
| Co/FePc(50 nm)/Co | 1.2%/50 K | [67] | |
| Co/FePc(50 nm)/Co | 0.5%/100 K | [67] | |
 F16CuPc | Co/AlOx/F16CuPc(15 nm)/Py | ~4%/300 K | [68] |
| Co/AlOx/F16CuPc(90 nm)/Py Spin-photovoltaic (vacuum or air) | ~4%/295 K −30/−25%/295 K | [68] | |
 BDMT | NiFe(15 nm)/FeCo(10 nm)/BDMT(1 nm)/AlOx(1 nm)FeCo(35 nm) | 2.4%/20 K | [69] |
| NiFe(15 nm)/FeCo(10 nm)/BDMT(1 nm)/AlOx(1 nm)/FeCo(35 nm) | 1.9%/100 K | [69] | |
| NiFe(15 nm)/FeCo(10 nm)/BDMT(1 nm)/AlOx(1 nm)/FeCo(35 nm) | 0.25%/300 K | [69] |
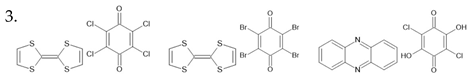
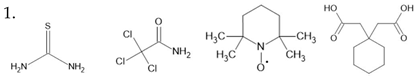 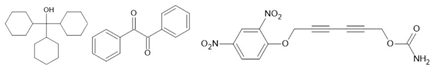 thiourea [129] TCAA [130] TEMPO [131] CDA [132] TCHM [133] Benzil [134] DNP [135] | ||
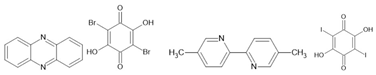
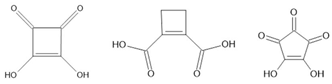  squaric acid [136] CBDC [137] croconic acid [138] PhMDA [137] 3-HPLN [137] MBI [139] DC-MBI [139] | ||
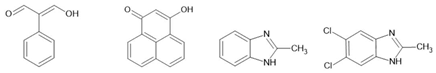
 1P [140] SF-PFA [141] |  |  guanine [142] glycine [143] thymine [144] FF [145] |
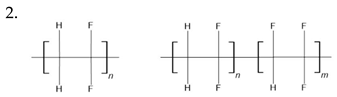 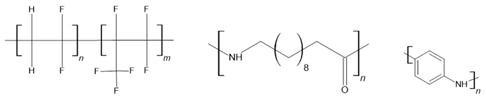 PVDF [146,147] P(VDF-TrFE) [148] P(VDF-HFP) [149] nylon 11 [150] 6,10,11,12-12 [151] PANI [152] | ||
  TTF/CA [153,154] TTF/BA [154] Phz/H2ca [155] Phz/H2ba [155] DMBP/H2ia [156] | ||
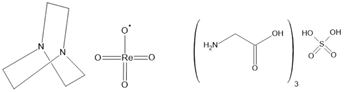 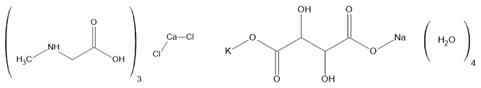 Hdabco/ReO4 [157] TGS [158,159] TSCC [160] Rochelle salt [161] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borca, B. Advances in Organic Multiferroic Junctions. Coatings 2024, 14, 682. https://doi.org/10.3390/coatings14060682
Borca B. Advances in Organic Multiferroic Junctions. Coatings. 2024; 14(6):682. https://doi.org/10.3390/coatings14060682
Chicago/Turabian StyleBorca, Bogdana. 2024. "Advances in Organic Multiferroic Junctions" Coatings 14, no. 6: 682. https://doi.org/10.3390/coatings14060682






