Influence of Ni on the Organization and Properties of AlCoCrFeMn High-Entropy Alloys by Laser-Sintering Technique
Abstract
1. Introduction
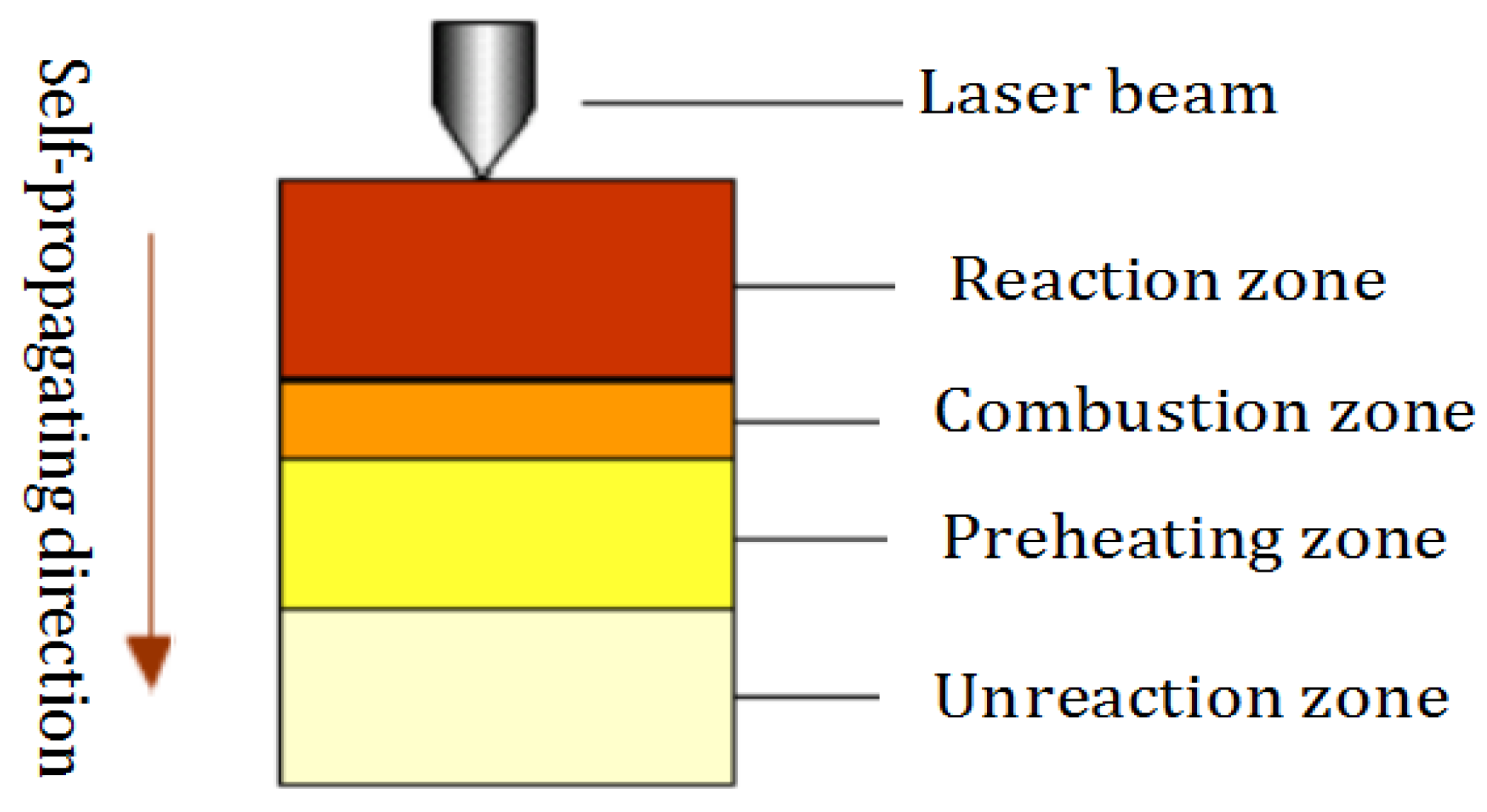
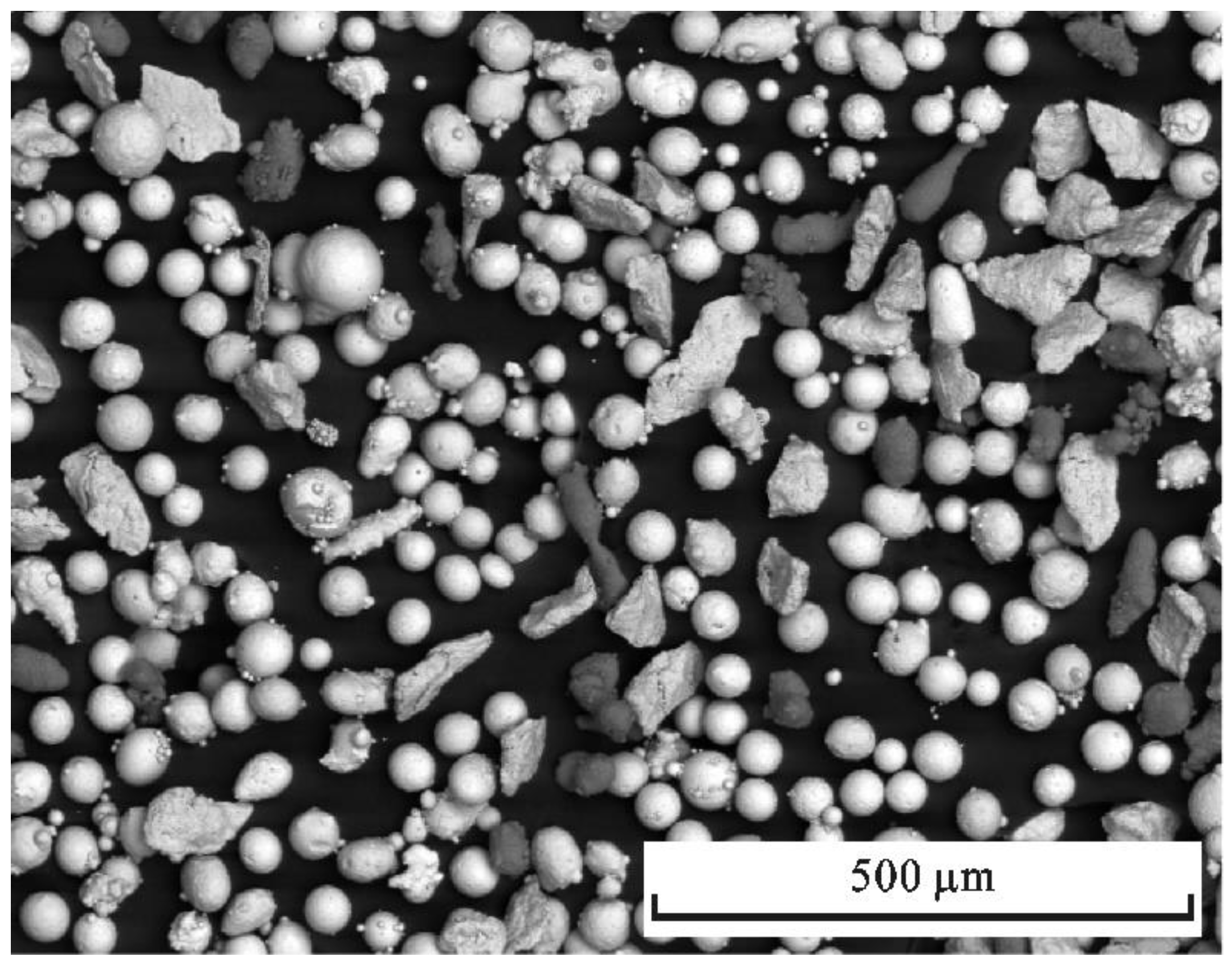
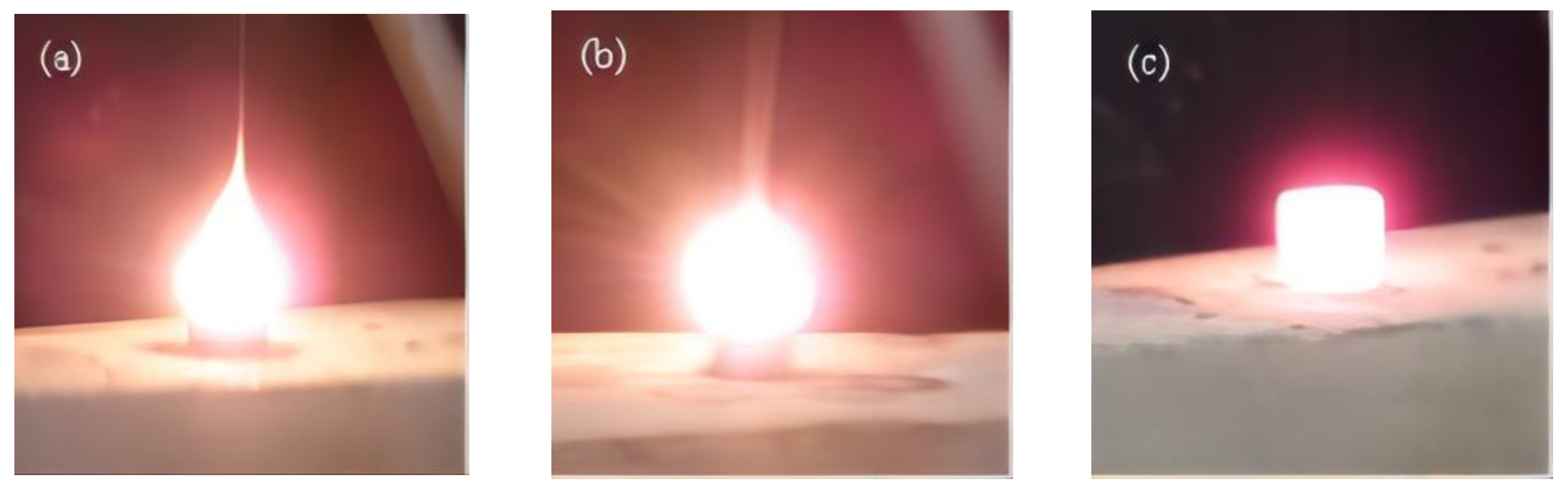
2. Experimental Materials and Method
3. Results and Analysis
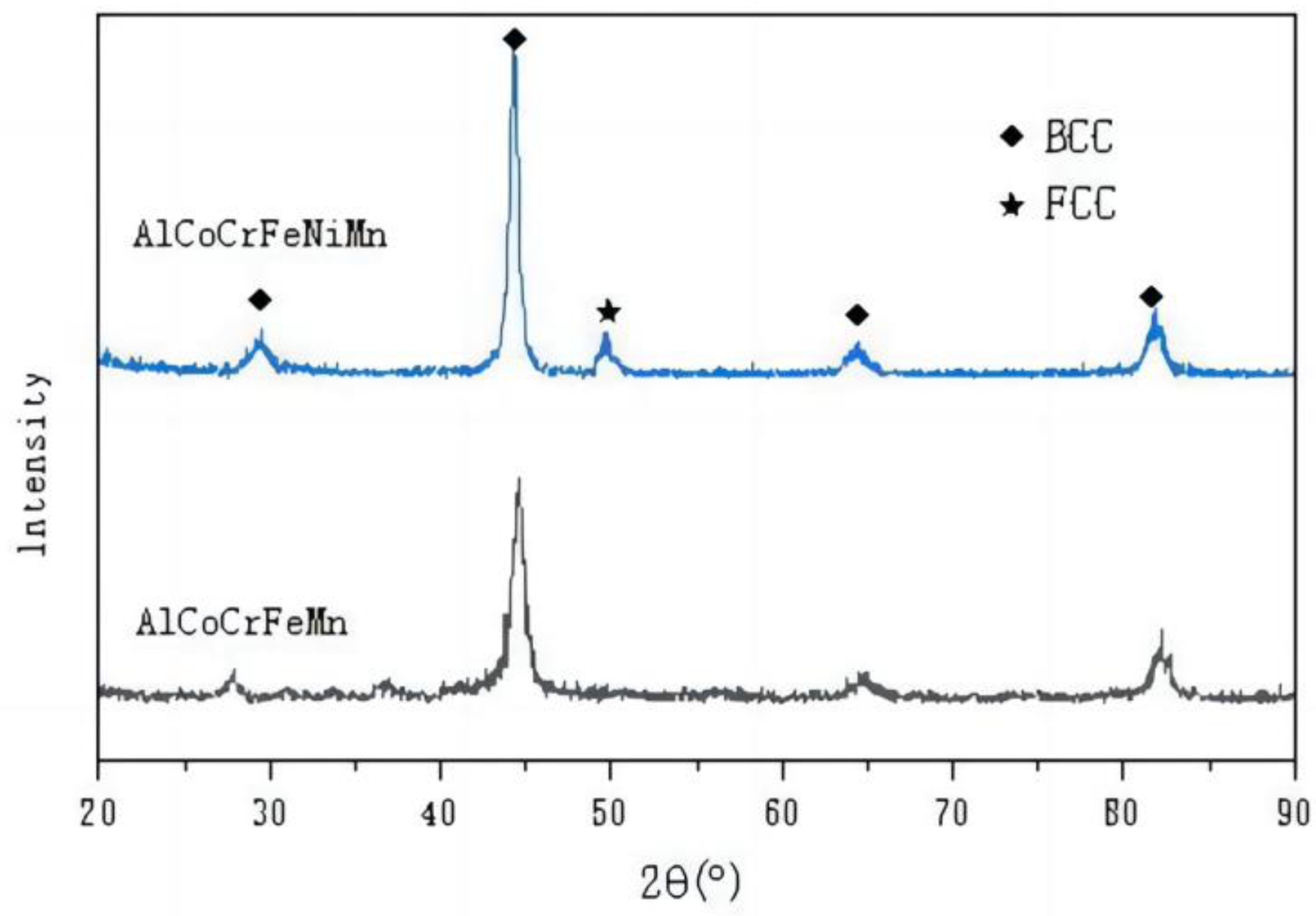
3.1. Phase Analysis of HEA
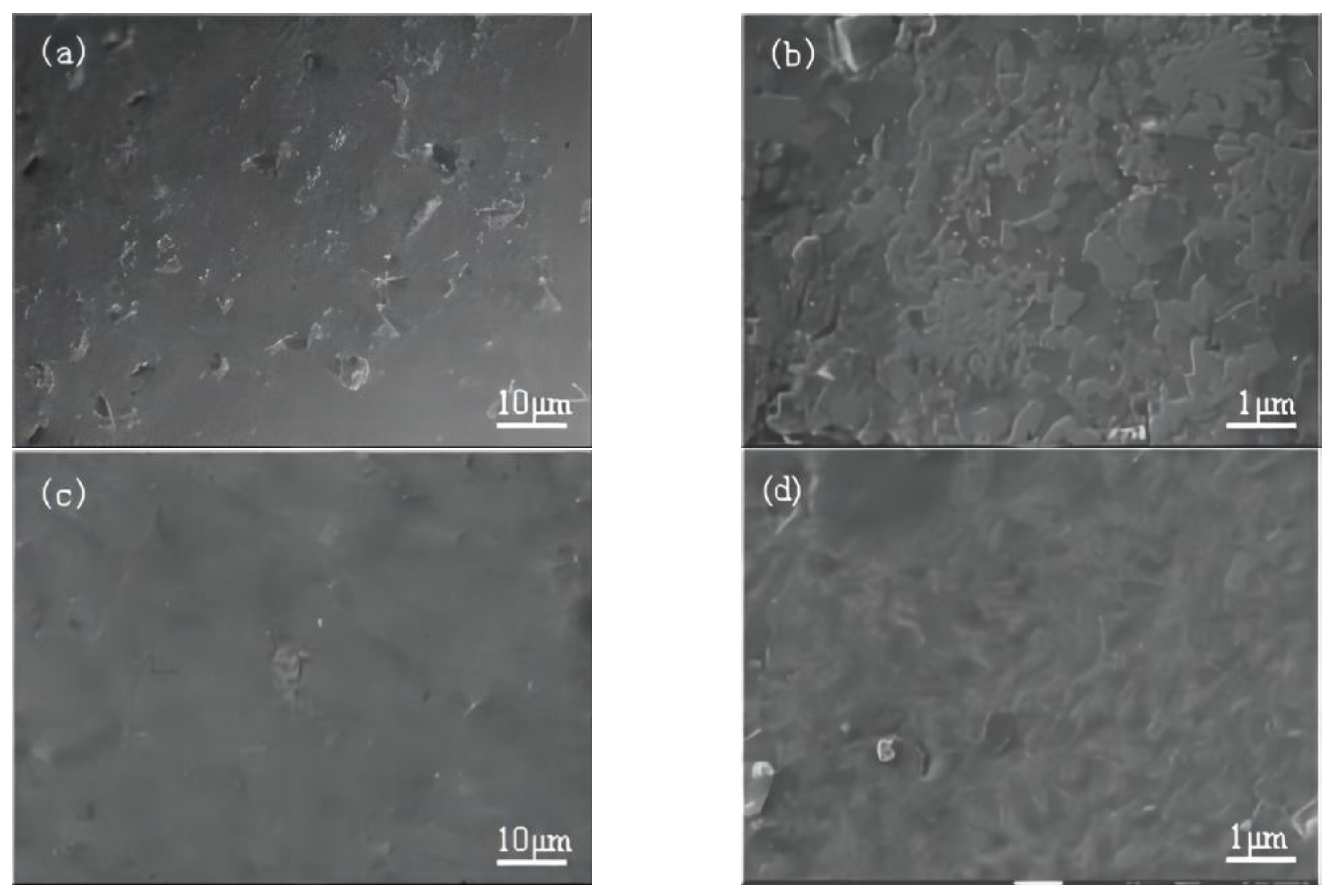
3.2. Organization Analysis of HEA
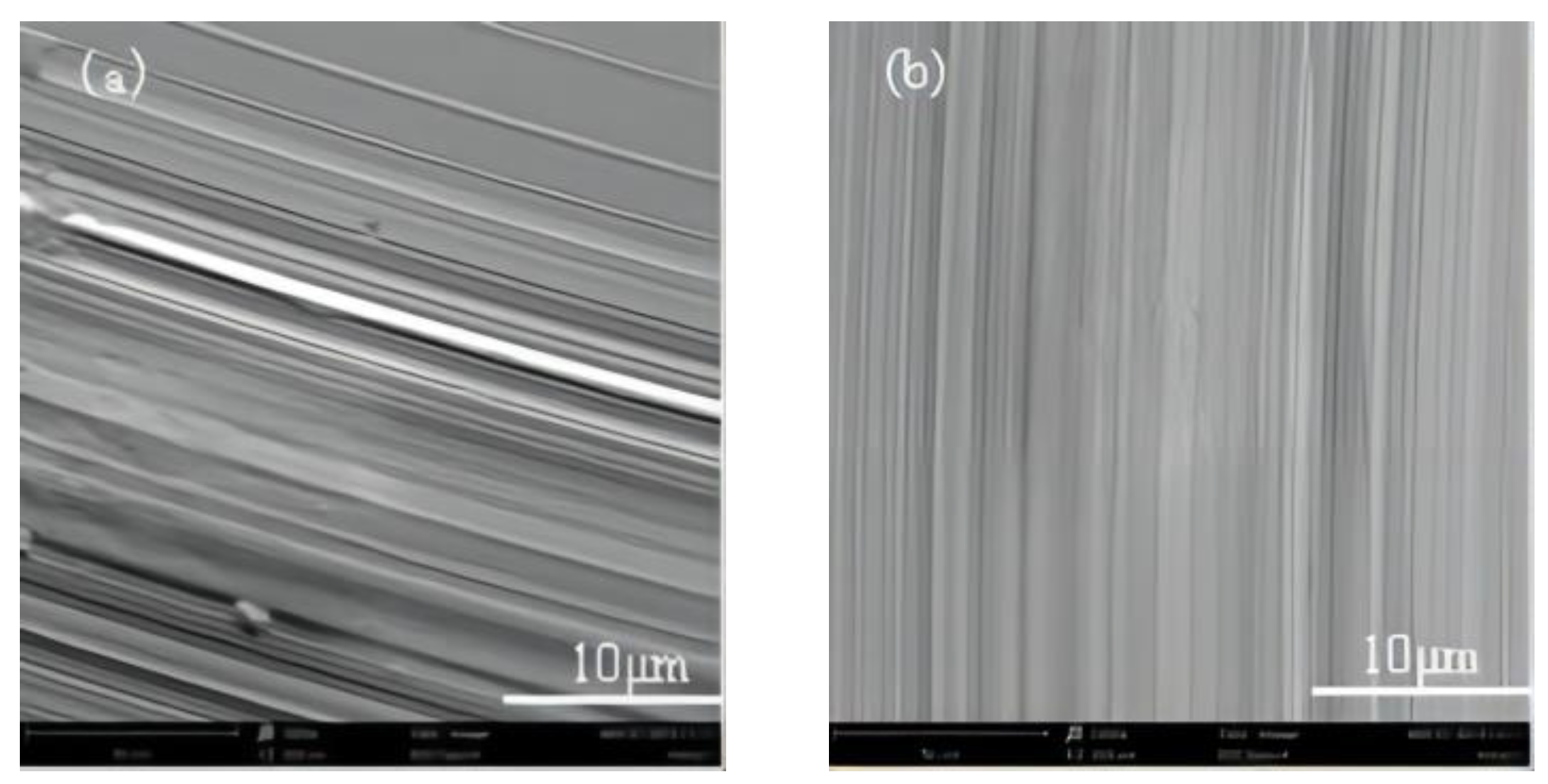
3.3. Wear Resistance Analysis of HEAs
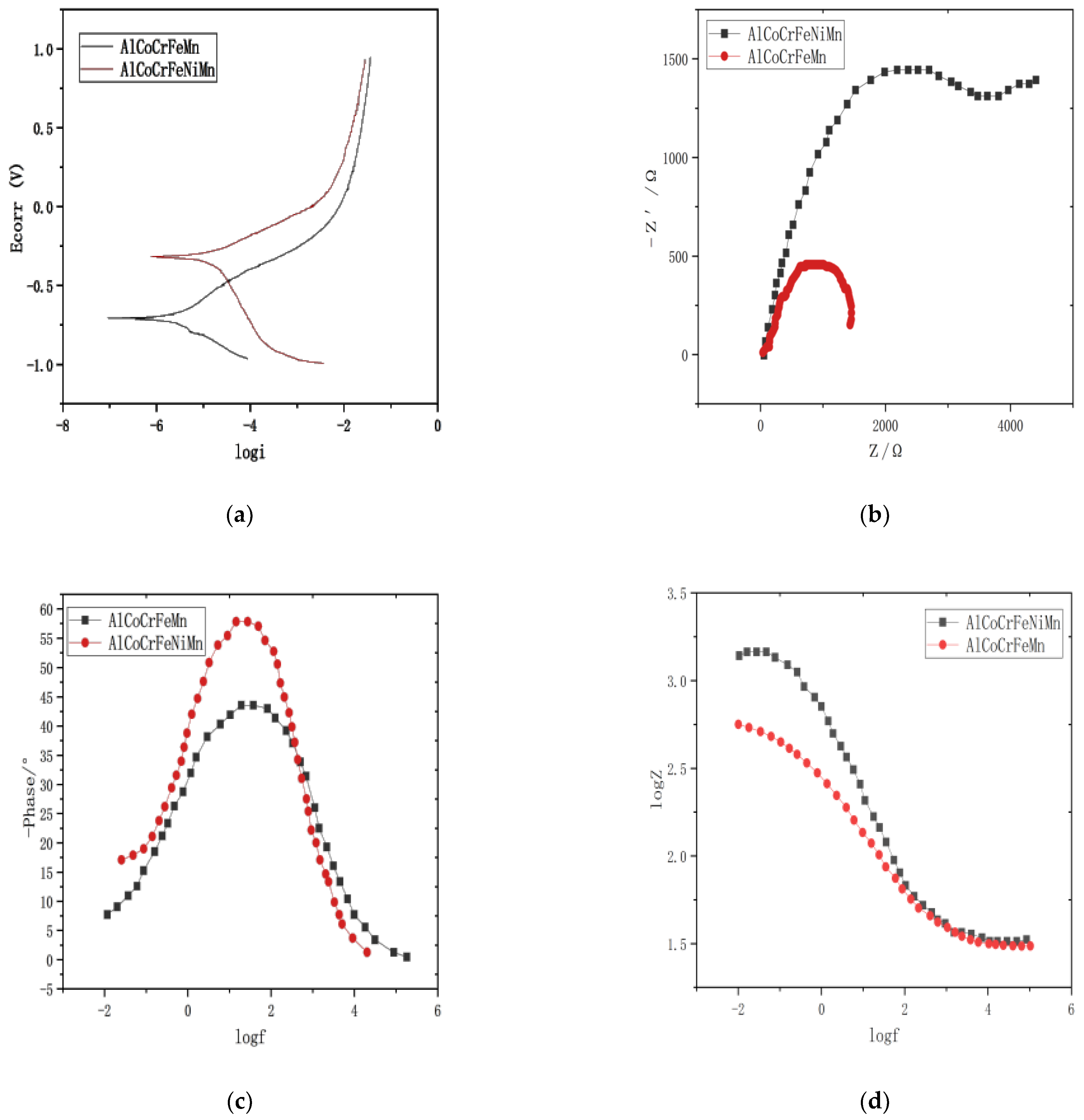
3.4. Corrosion Resistance Analysis of HEAs
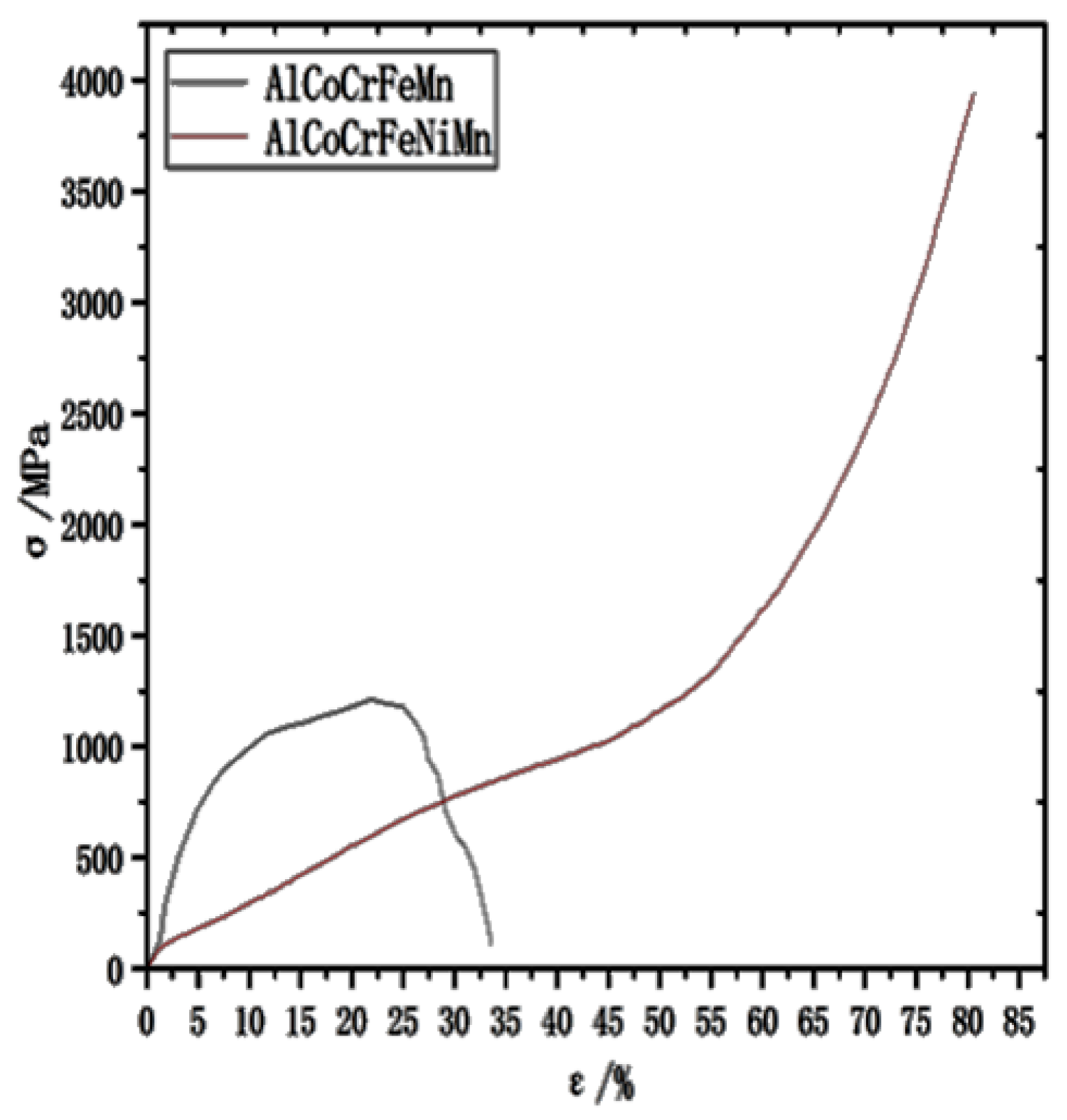
3.5. Compression Property Analysis of HEAs
4. Conclusions
- (1)
- The organizational structure of AlCoCrFeNiMn HEA materials is the equiaxial crystal structure; compared with AlCoCrFeMn, the surface is more dense, the metal composition of the segregation phenomenon has been suppressed, and a small amount of the FCC phase appears in the composition of the alloy.
- (2)
- Compared with AlCoCrFeMn HEAs, the self-corrosion potential of AlCoCrFeNiMn is more positive, the pitting potential is higher, and the corrosion current density is smaller, thus expanding the passivation zone, and showing better corrosion resistance in 3.5% NaCl solution.
- (3)
- The addition of Ni promotes the formation of the FCC phase in the alloy, which greatly improves the wear resistance and yield strength of the HEA, making its comprehensive mechanical properties better.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J. Nanostructured High Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, K.P.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2. [Google Scholar] [CrossRef]
- Liang, X.B.; Guo, W.; Chen, Y.X. Microstructure and mechanical properties of FeCrNiCoCu(B) high-entropy alloy coatings. Mater. Sci. Forum. 2011, 24, 70–73. [Google Scholar] [CrossRef]
- Sui, Y.; Feng, K.; Cheng, C.; Qi, J.; He, Y.; Wei, F.; Meng, Q.; Sun, Z.; Shen, C. Interaction between Ti-48Al Alloy and Coating Materials during the Solidification Process. Rare Metal Mater. Eng. 2016, 45, 3062–3067. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy Design Strategies and Future Trend in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.; Ma, S. Guidelines in predicting phase formation of high-entropy alloys. Mrs Commun. 2014, 4, 57–62. [Google Scholar] [CrossRef]
- Roy, U.; Roy, H.; Daoud, H. Fracture toughness and fracture micromechanismin a cast AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2014, 132, 186–189. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Wei Li Ping Liu Peter, K. Microstructures and properties of high-entropy alloy films and coatings. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar]
- George, E.P.; Raabe, D.; Ritchie, R.O. High entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhao, J.H.; Jin, R.H.; Jix, L.; Duan, T.Z.; Zhuang, S.D.; Zhao, Z.X. Effect of Al content on the erosion and corrosion resistance of CoCrFeNiTi_(0.5) high entropy alloy coatings. Mater. Rev. 2023, 37, 202–207. [Google Scholar]
- Sriharitha, R.; Murty, B.S.; Kottada, R.S. Phase formation in mechanically alloyed AlxCoCrCuFeNi (x = 0.45, 1, 2.5, 5 mol) high entropy alloys. Intermetallics 2013, 32, 119–126. [Google Scholar] [CrossRef]
- Nong, Z.; Li, H.; Wang, J. Study on thermal stability of AlCrFeNiTi high-entropy alloy. Rare Metal Mater. Eng. 2018, 47, 191–196. [Google Scholar]
- Wang, R.; Zhang, K.; Davies, C. Evolution of microstructure, mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy prepared by direct laser fabrication. J. Alloys Compd. 2017, 694, 971–981. [Google Scholar] [CrossRef]
- Sharma, A.; Deshmukh, S.A.; Liaw, P.K.; Balasubramanian, G. Crystallization kinetics in AlxCrCoFeNi (0 ≤ x ≤ 40) high-entropy alloys. Scripta Mater. 2017, 141, 54–57. [Google Scholar] [CrossRef]
- Shi, X. Effect of elemental Cu on the organizational properties of CuxAlFeNiCrTi (x = 0, 0.5, 1.0) high entropy alloys. Mater. Prot. 2018, 51, 58–61. [Google Scholar]
- An, Y.-J.; Zhu, L.; Jin, S.-H.; Lu, J.-J.; Liu, X.-Y. Laser-Ignited Self-Propagating Sintering of AlCrFeNiSi High-Entropy Alloys: An Improved Technique for Preparing High-Entropy Alloys. Metals 2019, 9, 438. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.P. Progress of Porous Silicon Nitride Ceramics Prepared via Self-propagating High Temperature Synthesis. J. Inorg. Mater. 2022, 37, 853–864. [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2010, 10, 534–538. [Google Scholar] [CrossRef]


| Element | Al | Co | Cr | Fe | Mn |
|---|---|---|---|---|---|
| Nominal | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| A | 8.94 | 12.68 | 27.51 | 24.46 | 26.41 |
| B | 2.68 | 3.46 | 60.45 | 13.66 | 19.75 |
| C | 5.08 | 7.56 | 44.78 | 19.76 | 22.82 |
| D | 6.98 | 9.15 | 39.58 | 20.35 | 23.94 |
| Element | Al | Co | Cr | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| Nominal | 16.66 | 16.66 | 16.66 | 16.66 | 16.66 | 16.66 |
| A | 18.04 | 16.14 | 16.45 | 13.96 | 19.56 | 15.85 |
| B | 17.94 | 15.88 | 15.77 | 14.78 | 18.69 | 16.94 |
| C | 17.02 | 15.65 | 16.06 | 14.97 | 18.82 | 17.48 |
| Alloy Name | Ecorr/V | Icorr/(A·cm−2) | Corrosion Rate/(mm·a−1) |
|---|---|---|---|
| AlCoCrFeMn | −0.718 | 3.413 × 10−5 | 9.68 × 10−3 |
| AlCoCrFeNiMn | −0.325 | 1.643 × 10−5 | 2.08 × 10−3 |
| Alloy Name | Compression Rate/% | Yielding Strength/MPa | Compressive Strength/MPa |
|---|---|---|---|
| AlCoCrFeMn | 34 | 150 | 1280 |
| AlCoCrFeNiMn | 83 | 780 | 3920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Jiang, B.; Jiang, C.; Liu, H.; Li, Y. Influence of Ni on the Organization and Properties of AlCoCrFeMn High-Entropy Alloys by Laser-Sintering Technique. Coatings 2024, 14, 684. https://doi.org/10.3390/coatings14060684
An Y, Jiang B, Jiang C, Liu H, Li Y. Influence of Ni on the Organization and Properties of AlCoCrFeMn High-Entropy Alloys by Laser-Sintering Technique. Coatings. 2024; 14(6):684. https://doi.org/10.3390/coatings14060684
Chicago/Turabian StyleAn, Yajun, Bojin Jiang, Chuanjiu Jiang, Haocheng Liu, and Yiming Li. 2024. "Influence of Ni on the Organization and Properties of AlCoCrFeMn High-Entropy Alloys by Laser-Sintering Technique" Coatings 14, no. 6: 684. https://doi.org/10.3390/coatings14060684
APA StyleAn, Y., Jiang, B., Jiang, C., Liu, H., & Li, Y. (2024). Influence of Ni on the Organization and Properties of AlCoCrFeMn High-Entropy Alloys by Laser-Sintering Technique. Coatings, 14(6), 684. https://doi.org/10.3390/coatings14060684






