Abstract
The aluminum hydroxide produced by the Bayer process contains a large amount of water which leads to the consumption of a large amount of heat for moisture removal in the calcination process, resulting in an increased energy consumption. The effects of temperature and microwave power on the dehydration ratio and the dry matter ratio of aluminum hydroxide were investigated. The characteristics of temperature variation during drying were discussed. X-ray diffraction (XRD), scanning electron microscopy (SEM), laser particle size, Fourier transform infrared (FTIR) spectroscopy, and dielectric property analyses were made to characterize the dried materials. The analysis results showed that within the range of bench-scale experimental parameters, the dehydration ratio was higher and the proportion of dry matter was lower at a higher final temperature. Within the range of pilot-scale experimental parameters, the dehydration ratio increased with the increasing microwave power from 500 W to 1500 W. XRD spectra revealed that when the final temperature exceeded 220 °C, a part of the aluminum hydroxide underwent a low-temperature phase transition to boehmite. The SEM images and a particle size analysis showed that there was no significant difference between the morphologies of the powder obtained by microwave drying and conventional drying methods. The powder obtained by both processes had an average particle size of around 80 μm. The dielectric constant and the dielectric loss of the dried material decreased greatly.
1. Introduction
Aluminum hydroxide (Aluminum hydroxide), chemical formula Al(OH)3, density 2400 kg/m3. As a process product of the aluminum production process, aluminum hydroxide occupies an important position in aluminum electrolysis production [1,2,3]. In industrial production, the main methods for the preparation of aluminum hydroxide are the Bayer method, the sintering method and the Bayer-sintering tandem method, and the water content of aluminum hydroxide attached after washing is about 6–12% [4]. Aluminum hydroxide can be transformed into γ-alumina with a stable crystalline form after high temperature calcination; however, the washed aluminum hydroxide directly into the rotary kiln calcination will be due to water removal to take away most of the heat, so that the kiln temperature drops sharply. It is reported that the drop is about 250–350 °C, resulting in about 90% of the heat loss in the calcination process, and the energy consumption increases sharply. The temperature required to remove the water from the washed aluminum hydroxide is 110–120 °C. The rapid drying or dewatering of the material prior to the calcination of aluminum hydroxide has the feasibility of reducing the energy consumption of the calcination process [4,5,6,7,8].
A microwave is an electromagnetic wave with a frequency of between 0.3–300 GHz, and there are three main bands used for heating: 2.45 GHz, 0.915 GHz, and 5.8 GHz [9]. A microwave has the characteristics of body heating, fast speed, selectivity, etc., and embodies the advantages of cleanliness, high efficiency, rapidity, energy saving, and easy control in specific applications [10]. Microwave heating is widely used in food [11], metallurgy [12,13,14], materials [15,16,17], and other industries [18,19], and its superiority and feasibility have been proved in numerous reports. The advantage of microwave heating and drying over traditional heating and drying is that microwave heating is non-contact heating with a fast heating speed, uniform heating, and selective heating characteristics, which can be used immediately, safely and efficiently. In addition, in microwave drying using the principle of dielectric loss, water molecules have a strong absorption effect on microwaves. The microwave drying process of water molecules through the dielectric loss will be microwave electromagnetic energy into thermal energy, liquid water into water vapor volatilization, and drying purposes can be achieved. The dielectric constant of aluminum hydroxide (2.2) is lower than that of water (80.4), indicating that aluminum hydroxide containing water has a better ability to absorb microwaves [20], and from the inside out of the body heating phenomenon, different from the traditional conduction, radiation, and convection, etc., will not cause excessive heating of the surface of the material, resulting in a cold center and other issues. Yan Yecheng [21] and others compared the effect of microwave and hot air drying on the quality of coal slurry and found that there is no significant change in the quality of the coal slurry under the two drying methods. Ru Saihong et al. [22] studied the effect of microwave and hot air drying on the quality of Jinxuan tea, and found that microwave drying shortened the drying time, increased the rehydration ratio, and reduced the total amount of tea polyphenols and EGCG content. Lin Guo et al. [23] used the microwave drying of wet zinc refining sludge, and found the drying and dehydration ratio could reach 99.57% under the conditions of microwave power of 600 W, a material mass of 40 g, and a microwave heating time of 3 min. Guo Lei et al. [24] used microwave drying scheelite concentrate, at a temperature of 92 °C, a microwave drying time of 174 s, a material mass of 135 g under the conditions of the relative dehydration ratio of 82.4%, and the microwave drying time and conventional electric heating drying shortened by 97.5%. Zhang Bin [25] studied the calcination process of aluminum hydroxide and alumina crystal transformation in the microwave field and found that under the premise of obtaining the same α-alumina, the calcination temperature under microwave conditions was reduced by 200 °C, and the calcination holding time was only 1/6 of the conventional method. It is known from the above literature that the use of microwave drying to deal with the removal of water from aluminum hydroxide has a theoretical and practical basis.
In this paper, the influence of the temperature of the material and microwave power on the dehydration ratio and proportion of dry matter was investigated. Moreover, the characteristics of temperature variations during the drying process were studied. XRD, SEM, FTIR spectroscopy, laser particle size, and dielectric performance tests were conducted to analyze the dried sample and to characterize aluminum hydroxide after microwave drying.
2. Materials and Experimental Methods
2.1. Experimental Materials
Water-containing aluminum hydroxide sand products were used as raw materials in the experiments, which were produced by the Aluminum Corporation of China, Zibo City, Shandong Province. Before the microwave drying experiments, three groups of parallel experiments were conducted in a drying oven to gain the free water content of the initial material according the procedure as below. The raw material of 500 g was dried at 110 °C in a drying oven for 2 h to a constant weight. (DHG-9140A, Shanghai Yiheng Technology Co., Ltd., Shanghai, China), and then to weight by analytic balance. As a result, the mass loss of the original material was 50 g. For calculation, the mass fraction of moisture in the raw materials was calculated to be 10% based on the mass loss.
2.2. Experimental Setup and Methods
2.2.1. Experimental Setup
The drying experiment was carried out with a box type microwave high temperature reactor (Figure 1) with a power of 6 kW designed by the Kunming University of Science and Technology (Kunming, China). The power of the equipment was continuously adjustable from 0 to 6 kW, and the frequency was 2450 ± 50 MHz. During the drying process, the thermocouple was embedded in the powder sample for temperature measurement.

Figure 1.
Box type microwave high temperature reactor.
The characterization devices used in the experiment were as below, the groups on the sample surface were determined by a RAffinity-1 FTIR spectrometer (Shi-madzu Corporation, Kyoto, Japan), and the phase structure of the samples was determined by a TTR III type X-ray diffraction analyzer equipped with a rotating source in the screening angle range of 5–90° (2θ) company with a scanning speed of 2° per min. The image of the samples’ surface was obtained through a Phenom Pure high-resolution desktop scanning electron microscope equipped with a high-brightness CeB6 source, and the particle size distribution of the samples was measured by a Mastersizer 3000 intelligent laser particle size analyzer (Shanghai Spectris, Shanghai, China). In addition, the dielectric properties of the samples were measured through homemade equipment as follows.
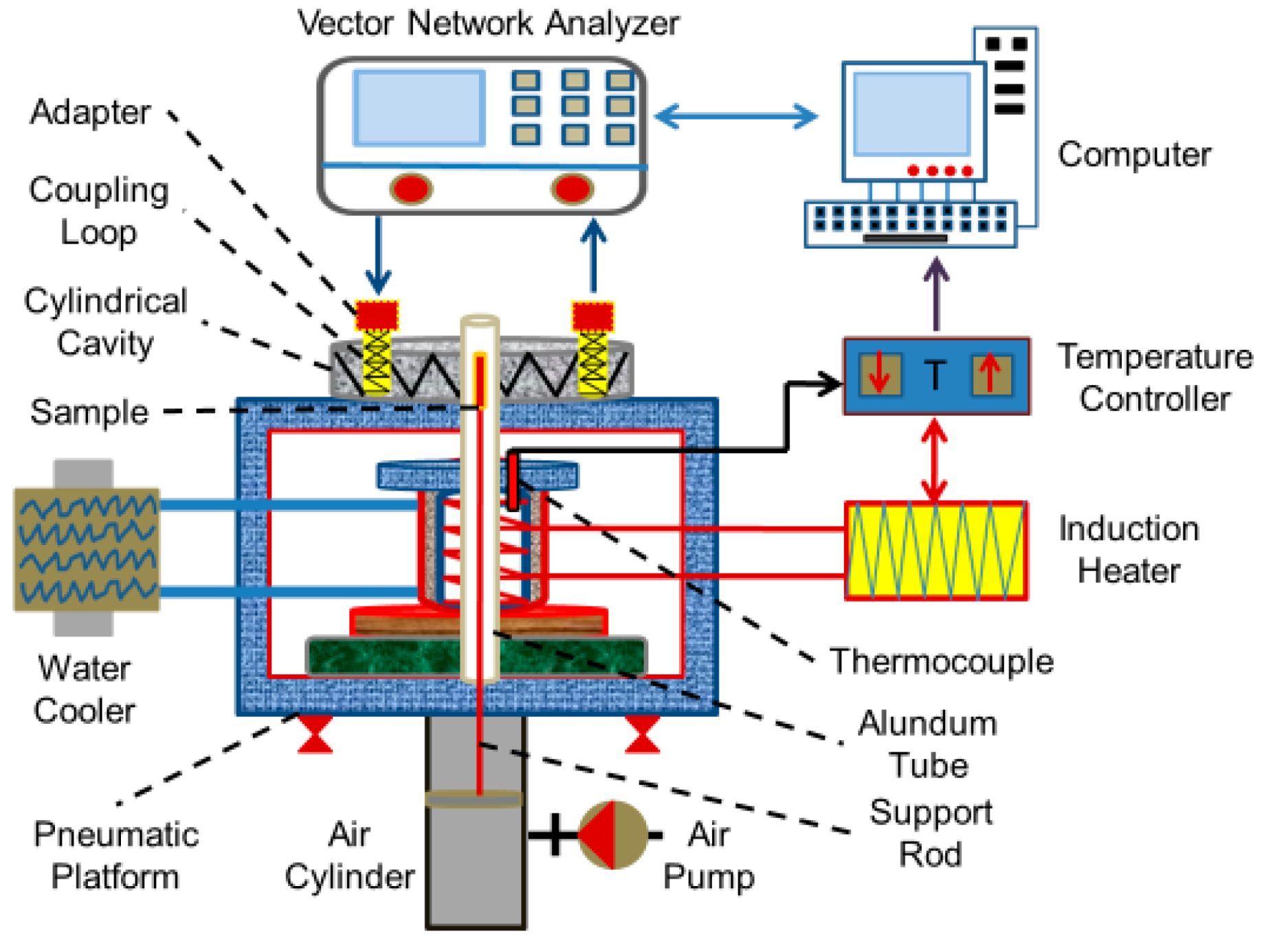
In the experiment, the resonant cavity perturbation method [14] was used to measure the dielectric constant of the sample before and after drying. The principal diagram of the measurement system is shown in Figure 2. The test system includes a vector network analyzer, a cylindrical cavity, a coupling device, a waveguide coaxial converter, an electromagnetic induction heater, a quartz sleeve, a sample lifting platform, a circulating cooling system, and a computer software system. The temperature range of the measuring system is 25~1000 °C and the microwave frequency is 2450 (±50) MHz. The dielectric constant (ε) consists of the real (ε′ dielectric constant) and imaginary (ε″ dielectric loss factor) parts of the complex dielectric constant and is defined by the follow equation:
ε = ε′ − jε″
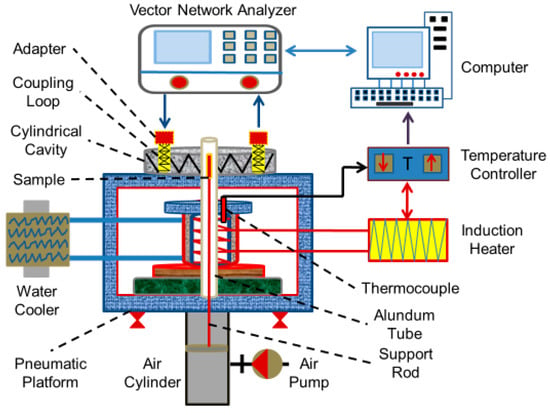
Figure 2.
Schematic diagram of dielectric constant measurement system.
The loss tangent (tanδ) is a parameter that describes how well the material absorbs microwave energy and is the ratio of the dielectric loss factor to the dielectric constant [18,19].
2.2.2. Experimental Methods
The numbers of the experiments on the microwave drying of aluminum hydroxide and related parameters are shown in Table 1. The detail of the experiments is described as below:

Table 1.
Experimental parameters.
- (a)
- The continuous cyclic experiments were carried out with a sample mass of 50 g with different microwave powers and processing times of 20, 30, and 40 min, with the aim of investigating the effect of drying time on the drying effect, as well as the effect of microwave power and initial material mass on the properties of the output of the dried material. The continuous cycle experiments described refer to experiments without time intervals, as shown in Table 1. L1-2 was started immediately after the end of L1-1, followed by L1-3, and so on, until all nine case experiments were completed.
- (b)
- Under the conditions of a sample mass of 250 g, a processing time of 30 min, and a material target temperature of 150 °C, the effects of microwave power changes on the material heating rate, drying effect, and the corresponding changes in material properties were investigated. Among them, the design of the microwave heating and drying of the maximum temperature of 150 °C, with a drying time of 30 min, taking into account the reasons for energy consumption, that is, when the processing time has been full of 30 min and the temperature is lower than 150 °C, to stop the microwave and take out the material weighed and analyzed. When the temperature rises to 150 °C but the time is less than 30 min, the use of heat preservation to extend the processing time to 30 min.
2.2.3. Calculation Methods
During the experiment, a 50 mL curved alumina ceramic crucible was used to fill 50 g of material in the bench-scale experiment, and a 300 mL curved alumina ceramic crucible was used to fill 250 g of material in the pilot-scale experiment. A certain mass of material was first weighed and placed in a crucible and then dried in a microwave cavity. After the experiments, the samples were taken out from microwave cavity and weighed by analytic balance, and then stored in a drying oven for subsequent analysis.
In this study, the mass loss ratio and the proportion of dry matter were used to indicate the drying effect.
The mass loss ratio () is the ratio of mass loss of material to initial mass before drying, and it is calculated by:
The proportion of dry matter (δ) is the percentage of material weights before and after drying, and its expression is:
The dehydration ratio () is the ratio of mass loss after drying to moisture content before drying, and it is calculated by:
wherein, (g) is the material mass before drying and (g) is the material mass after drying.
3. Results and Analysis
3.1. Microwave Drying Process and Results
3.1.1. Analysis of Microwave Heating Characteristics
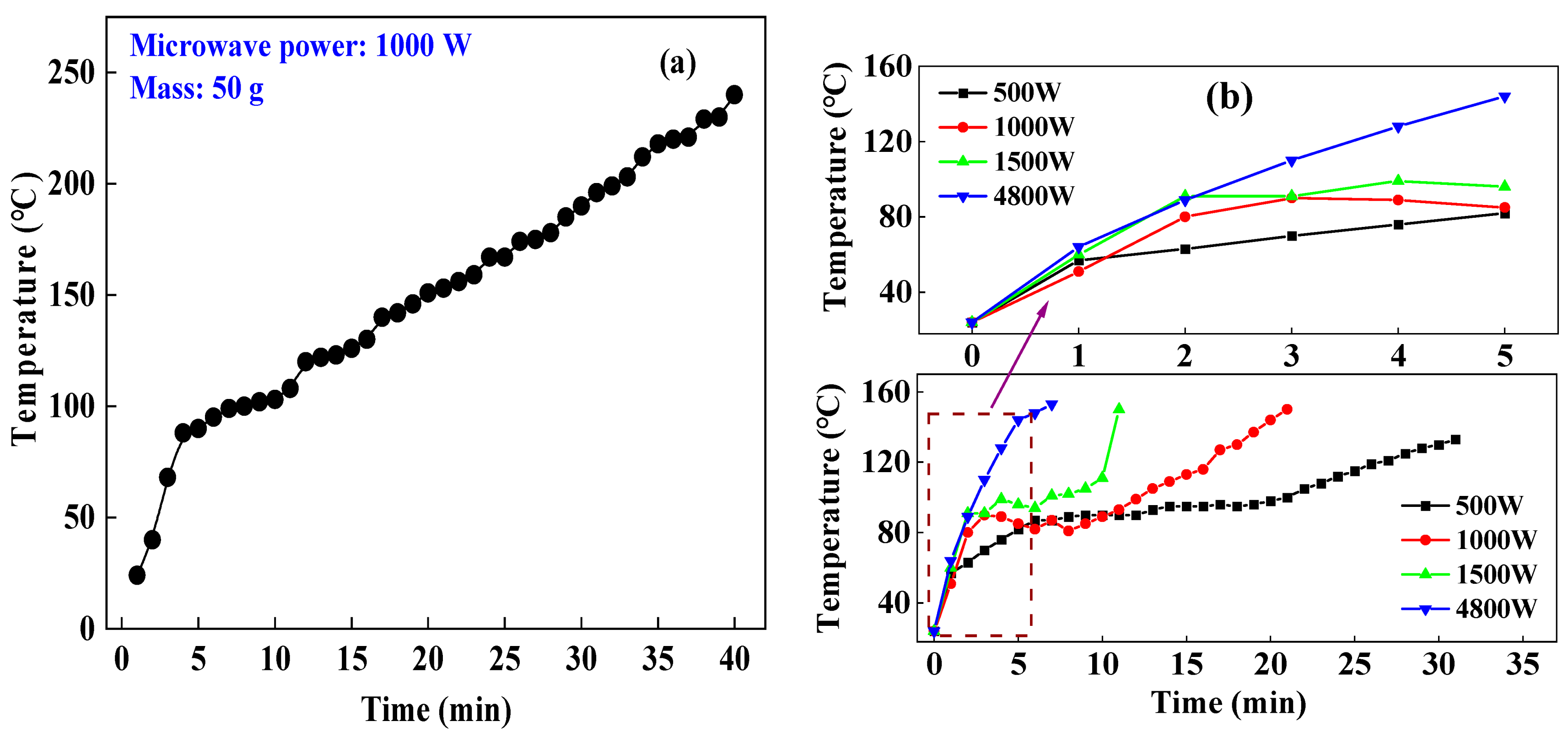
The corundum crucible with aluminum hydroxide samples that were weighed with a balance was put in the microwave oven for drying experiments under the given parameters provided in Table 1. The temperature changes of the samples during experiments are illustrated in Figure 3a,b. We take the temperature change in experiment L3-3 (Figure 3a) as an example. The temperature of the materials (50 g) rapidly increased to 97 °C in the first 5 min of the drying process under the conditions of a microwave power of 1000 W with an experimental time of 40 min, showing an average heating rate of 16.8 °C/min. In the following process, the average heating rate was 4.0 °C/min. At 2450 MHz and room temperature, the dielectric constant, tangent loss, and dielectric loss of water are 80.4, 9.889, and 0.123, respectively. The results revealed that in the initial stage of the experiment, the aluminum hydroxide contained moisture, and the water molecules were polar molecules with good microwave absorbing capacity [18,19,20]. The materials absorbed a large number of microwaves and were heated to 110 °C rapidly. After the free water in the materials was evaporated at its boiling point, the moisture content was reduced, and the materials were heated at a slower rate in the microwave field.
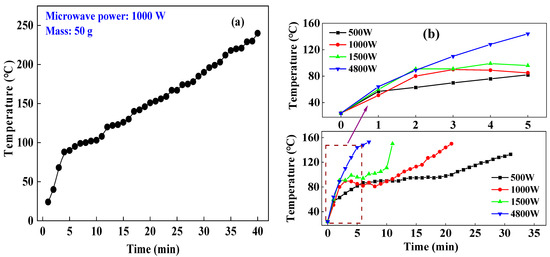
Figure 3.
Heating characteristics of aluminum hydroxide samples, (a) 50 g; (b) 250 g.
Figure 3b shows the experimental results under the parameters as follows: a microwave powers of 500 W, 1000 W, 1500 W, and 4800 W, a material mass of 250 g, and a target temperature of 150 °C. At microwave powers of 500 W, 1000 W, 1500 W, and 4800 W, it took 21 min, 12 min, 7 min, and 3 min for the materials to be heated to 110 °C from 24 °C, respectively. The average heating rates were 3.6 °C/min, 5.6 °C/min, 11.0 °C/min, and 28.7 °C/min through calculation based on experimental data, respectively. These data indicate that when the material masses are the same, a higher microwave power can increase the heating rate of materials and shorten the drying time. However, in the following stage of the experimental process, the average heating rates were 3.3 °C/min, 5.6 °C/min, 12.3 °C/min, and 10.8 °C/min, respectively. Those results indicate that the physical and chemical properties of the material have changed, which presents different microwave absorption capabilities during the heating process and lead to a different heating rate corresponding to different microwave power.
3.1.2. Effect of Microwave Power on Dehydration Ratio
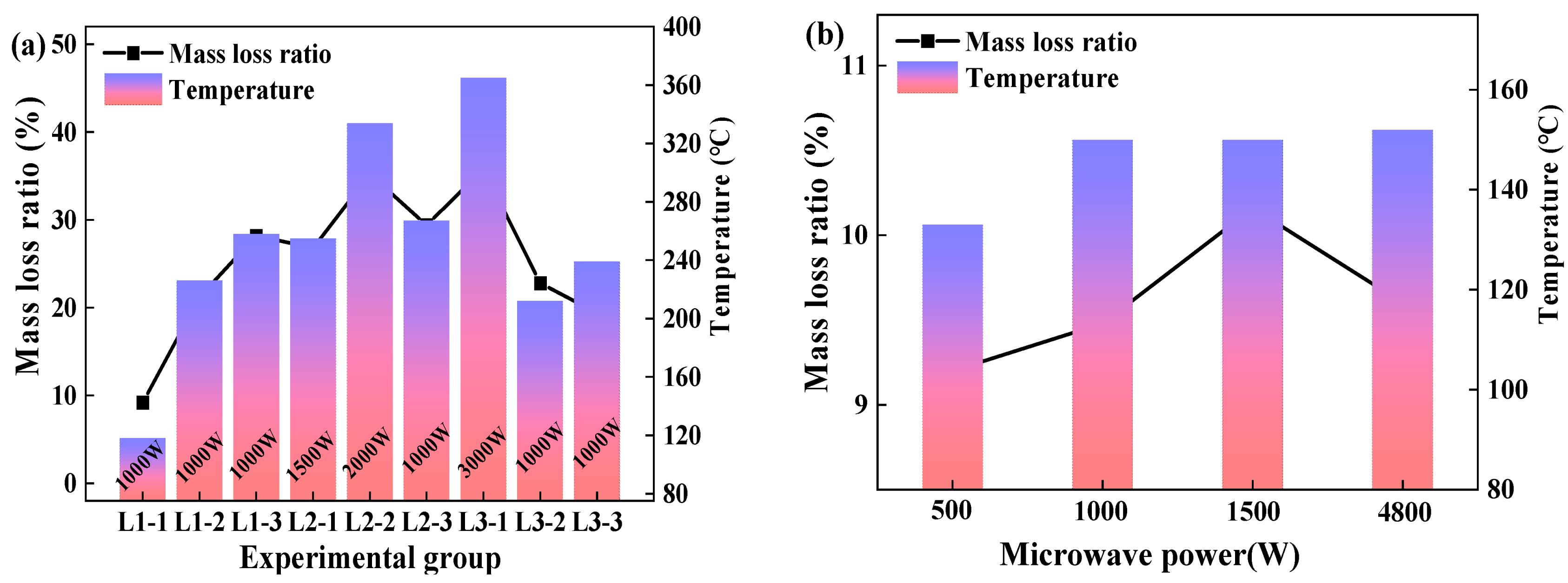
The experimental results of the continuous cyclic experiments with a material mass of 50 g were shown in Figure 4a. In view of the L1 group experiments, the mass loss ratio calculated according to Equation (1) was positively correlated with the drying time. In experiment L1-1, where the drying time was set as 20 min, the material temperature reached 118 °C and the dehydration ratio was 90.8% via calculating the experiment data with Equation (3). It can also be seen from Figure 4a that the material temperature changed differently among the different groups of continuous cyclic experiments because all the experiments were conducted in the same microwave cavity without cooling. Nevertheless, as shown in Figure 4a, it also could be seen that the temperature of the material was increased with microwave power and heating time; thus, the mass loss ratio showed the same change trend as the temperature. When the material temperature was above 160 °C, the mass loss ratio far exceeded 10% that was the moisture content of the material. This is possibly attributed to the removal of crystalline water from the material, or the thermal decomposition or other reactions of aluminum hydroxide. This point would be examined through the phase composition analysis via XRD.
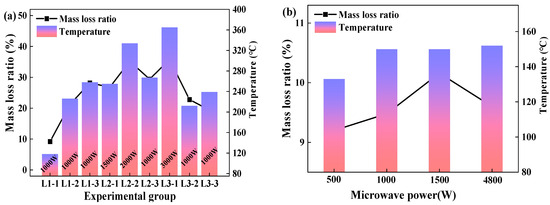
Figure 4.
Characteristics of dehydration ratio (a) 50 g; (b) 250 g.
Figure 4b illustrates the impact of microwave power on the dehydration ratio and material temperature. 250 g of the samples were heated for 30 min and the target temperature was set as 150 °C. Based on the experimental results, the influence of microwave power on the dehydration ratio was investigated. In Figure 4b, the mass loss ratio increased with the increase of the microwave power from 500 to 1500 W, and it peaked at 10.16%. In the experiments where the microwave power was set as 4800 W, the materials reached the target temperature of 150 °C after 7 min of microwave drying, showing a dehydration ratio of 9.60%. Thus, we could infer that the higher microwave power used during the experiment could quickly heat the material to the target temperature, but the dehydration effect was influenced by time and microwave power. If enough energy was supplied to the cavity, the moisture could be totally separated from the material. As a result, even though a microwave power of 4800 W could heat the material to the target temperature of 150 °C in 7 min, it could not separate all the free water from the material. Hence, considering the economy of energy consumption, the matching relationship between the material quality, microwave power, and processing time must be considered.
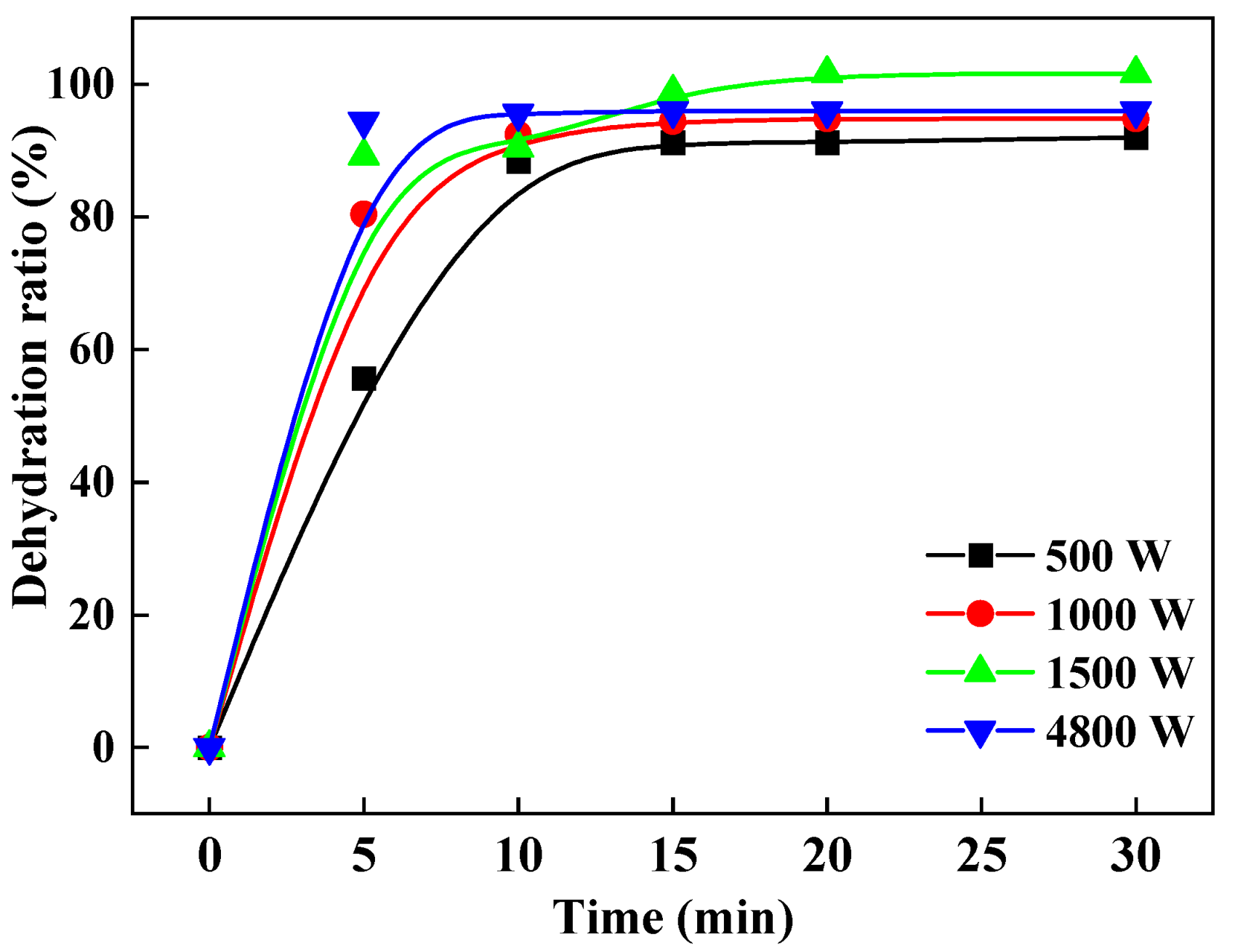
In the experiments where 250 g of aluminum hydroxide materials were tested, the microwave was stopped every 5 min so that the samples could be taken out and weighed. The dehydration ratios are calculated according to Equation (3), and the results that the change of the dehydration ratio with time are shown in Figure 5. It is evident that after 5 min of drying, at the beginning of the 5 min, the dehydration ratio at a microwave power of 4500 W was the highest (94.4%), compared with that at other microwave powers. The dehydration ratios at all studied microwave powers reached above 90% after 15 min. At microwave powers of 500 W, 1000 W, and 1500 W, the dehydration ratios were 92.00%, 94.80%, and 101.60% after 30 min of drying, respectively. As a result, we could infer that the higher microwave power could quickly heat the material to the removal temperature of free water, and the nature of drying is that the water absorbs energy to reach the boiling point and thus evaporates and separates from the dry materials. The increased microwave power strengthened the internal electric field of the material, accelerated its heating, and increased its temperature, thereby promoting the faster removal of more moisture from the materials.
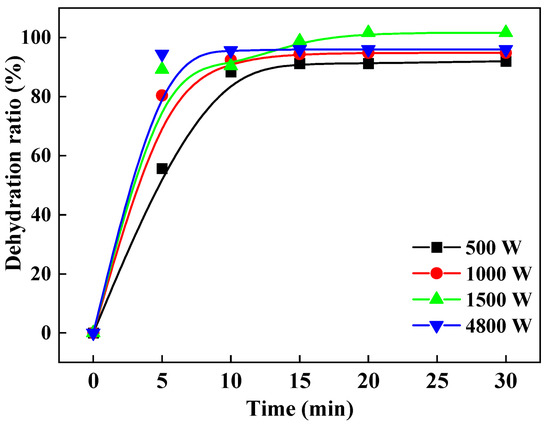
Figure 5.
Variation of dehydration ratio with drying time under different microwave powers.
It has been reported that Fick’s second diffusion law is often used to describe the drying process involved in various materials [26]. The diffusion of water in aluminum hydroxide during drying can be described by Fick’s second diffusion law. Correspondingly, with the increase of microwave heating power, the diffusion coefficient of aluminum hydroxide also increases. This is because the faster the heating rate, the more significant the diffusion coefficient of aluminum hydroxide. The diffusion coefficient increases linearly in the microwave power range of 500~4800 W. The more significant the effective diffusion coefficient is, the faster the reaction rate is, which further indicates that microwave heating has a strong selectivity in accelerating the reaction rate. [26,27,28]
By comparing the results between experiments that tested 50 g and 250 g of aluminum hydroxide samples, it was found that microwave power and drying time should be increased properly while more materials were dried in order to achieve the complete removal of moisture from the materials. Meanwhile, the temperature should be adjusted properly so that the material properties do not change in the drying process.
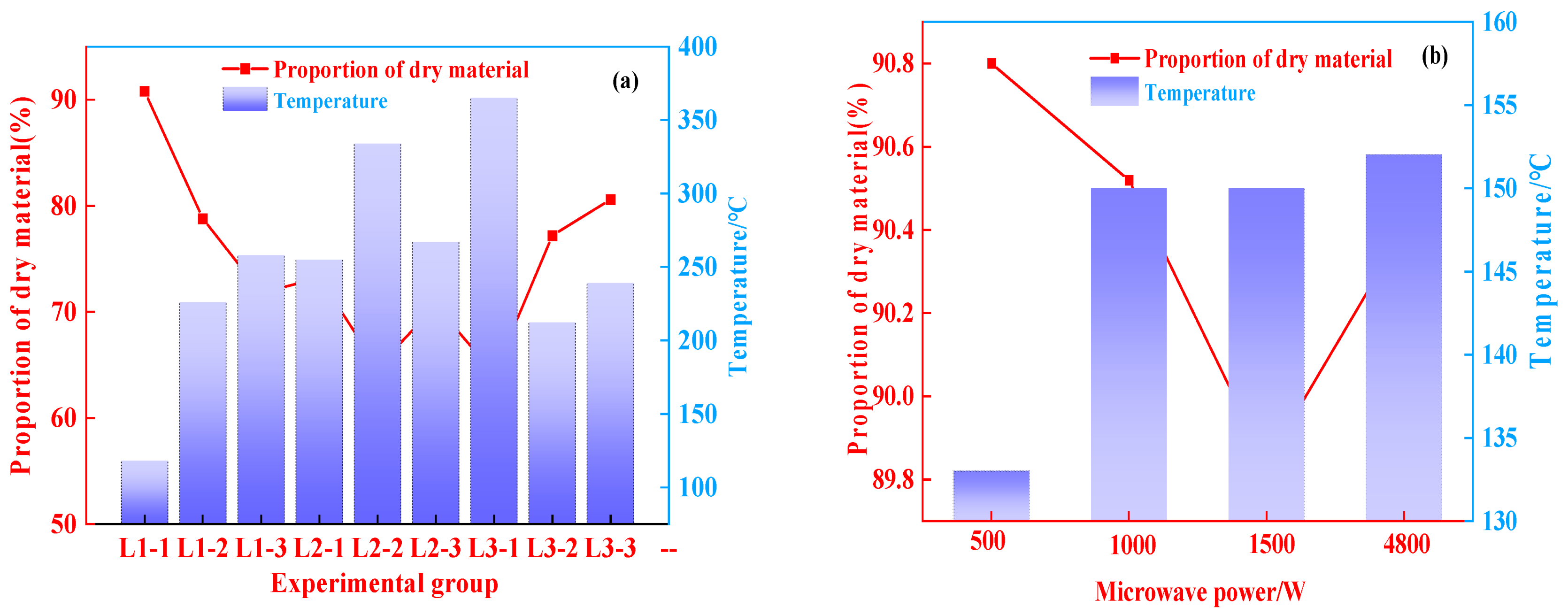
3.1.3. Effect of Microwave Power on Proportion of Dry Matter
Figure 6 shows how drying time and microwave power affect the proportion of dry matter. According to Figure 6a, the proportion of dry matter was negatively correlated with the final temperature. The proportion of dry matter was low at a high processing temperature. It can be seen from Figure 6b that the proportion of dry matter showed a descending trend with the increase of the microwave power from 500 to 1500 W. For 250 g of aluminum hydroxide materials, the proportion of dry matter was 90.4% after 7 min of microwave drying at power of 4800 W. The results indicate that the temperature and microwave power should be controlled during the drying process in order to prevent the excessive mass loss of the materials caused by overheating in the microwave field. Such mass loss is mainly derived from the removal of crystallization water from aluminum hydroxide, low-temperature thermal decomposition, and low-temperature phase transition, and it causes changes in the material properties [29].
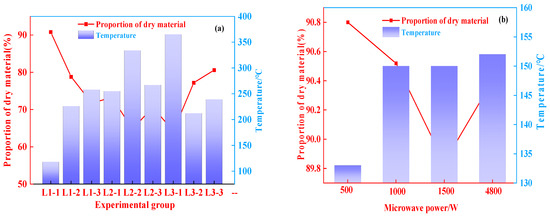
Figure 6.
Variation characteristics of dry material proportion, (a) 50 g; (b) 250 g.
3.2. Characterization Analysis
3.2.1. Phase Analysis
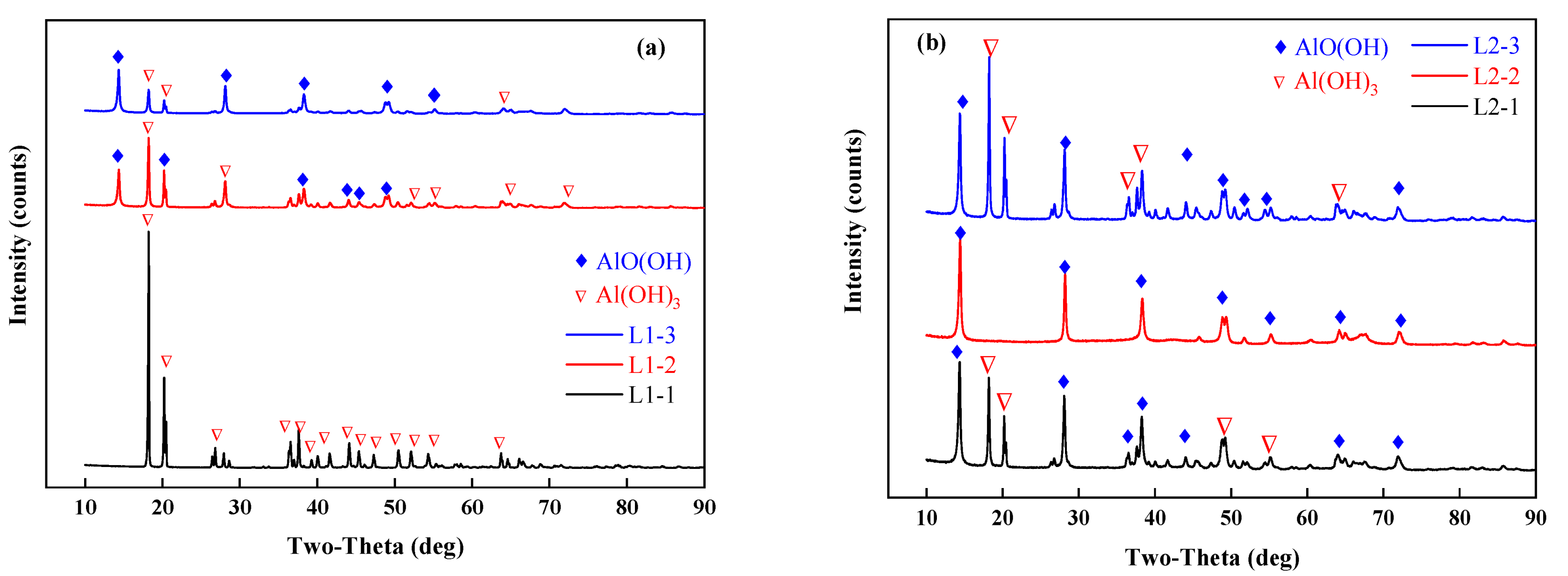
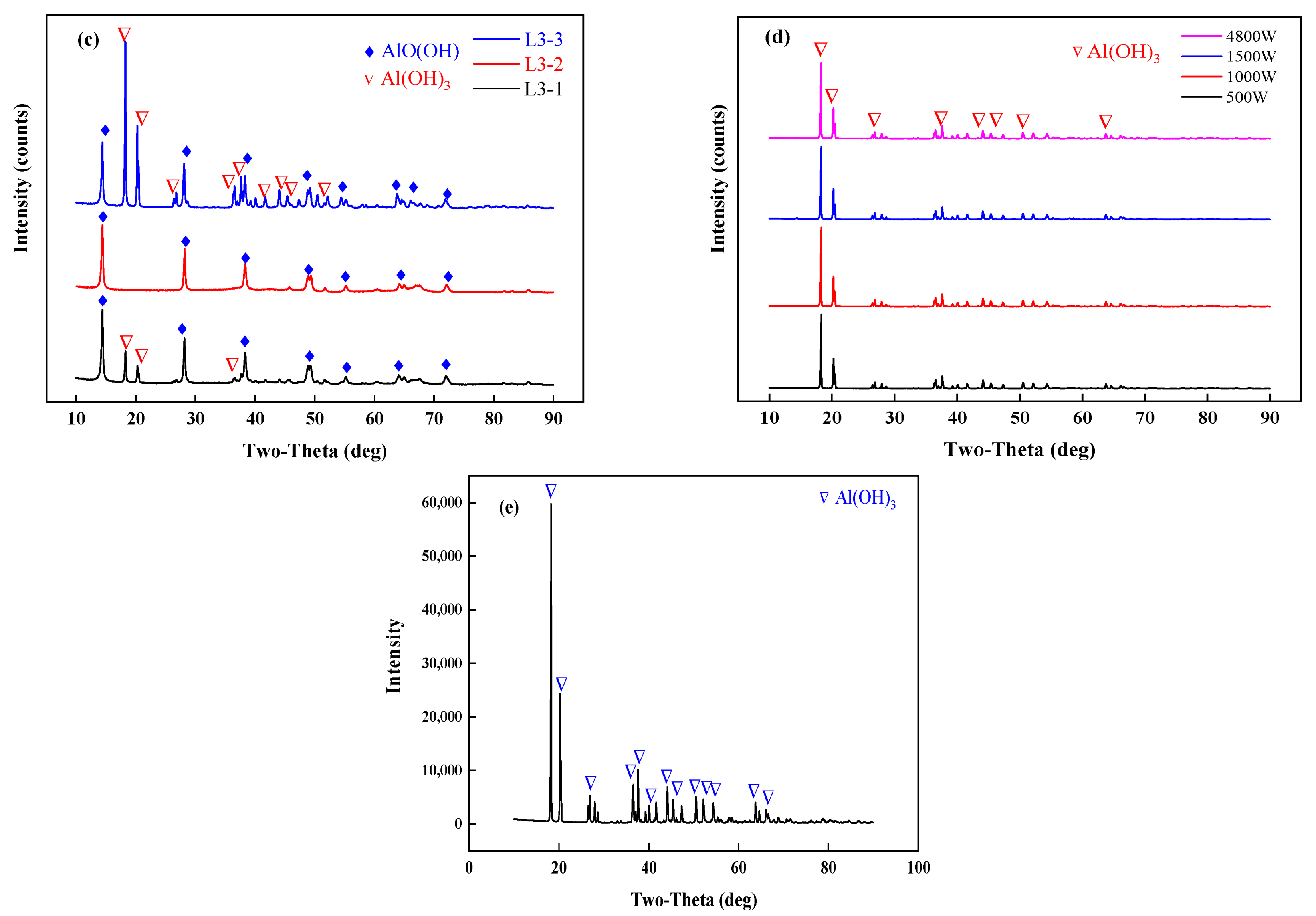
An X-ray diffractometer (TTRA III, Rigaku, Tokyo, Japan) was used to characterize the samples before and after drying. The X-ray generator operates at 18 kW and is irradiated by CuKα (λ = 1.54056 A) at a pressure of 40 kV and a current of 200 mA. The sample is scanned at a scanning rate of 4°/min in the range of 10–90°. The results of the XRD analysis are shown in Figure 7. In experiments L1, 2, and 3, where 50 g of the samples were tested, the morphologies of the phases in the dried samples differed due to different final temperatures. The dried samples consisted of aluminum hydroxide and boehmite (AlO(OH)). As shown in Figure 6 and Figure 7, when the final temperature exceeded 220 °C, the dehydrated aluminum hydroxide in the dried samples underwent a low-temperature phase transition to boehmite. The XRD analysis of the pilot-scale experiments with the temperature controlled at 150 °C showed that a low-temperature phase transition did not occur, and in the main phase, the aluminum hydroxide in the dried samples did not transform into another substance. The blast drying oven was used for comparison with microwave drying. It was found that the materials dried for 2 h at 110 °C in the blast drying oven did not undergo a phase transition.
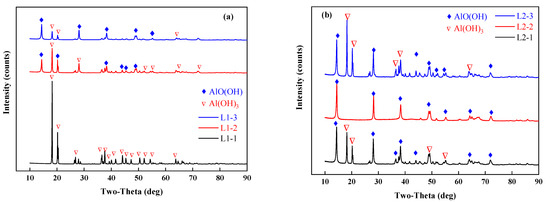
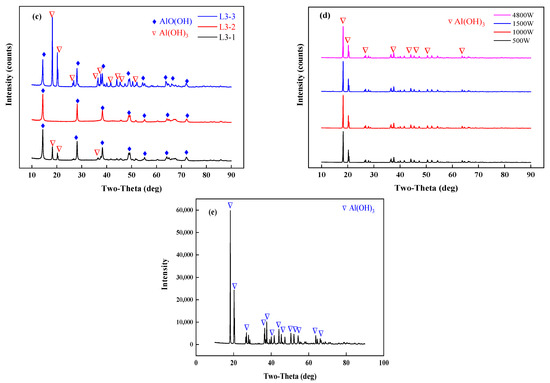
Figure 7.
XRD results analysis, (a) L1-1,2,3; (b) L2-1,2,3; (c) L3-1,2,3; (d) X-1,2,3,4; (e) dry the samples in a blast drying oven at 110 °C.
3.2.2. Appearance, Morphology, and Particle Size Analysis
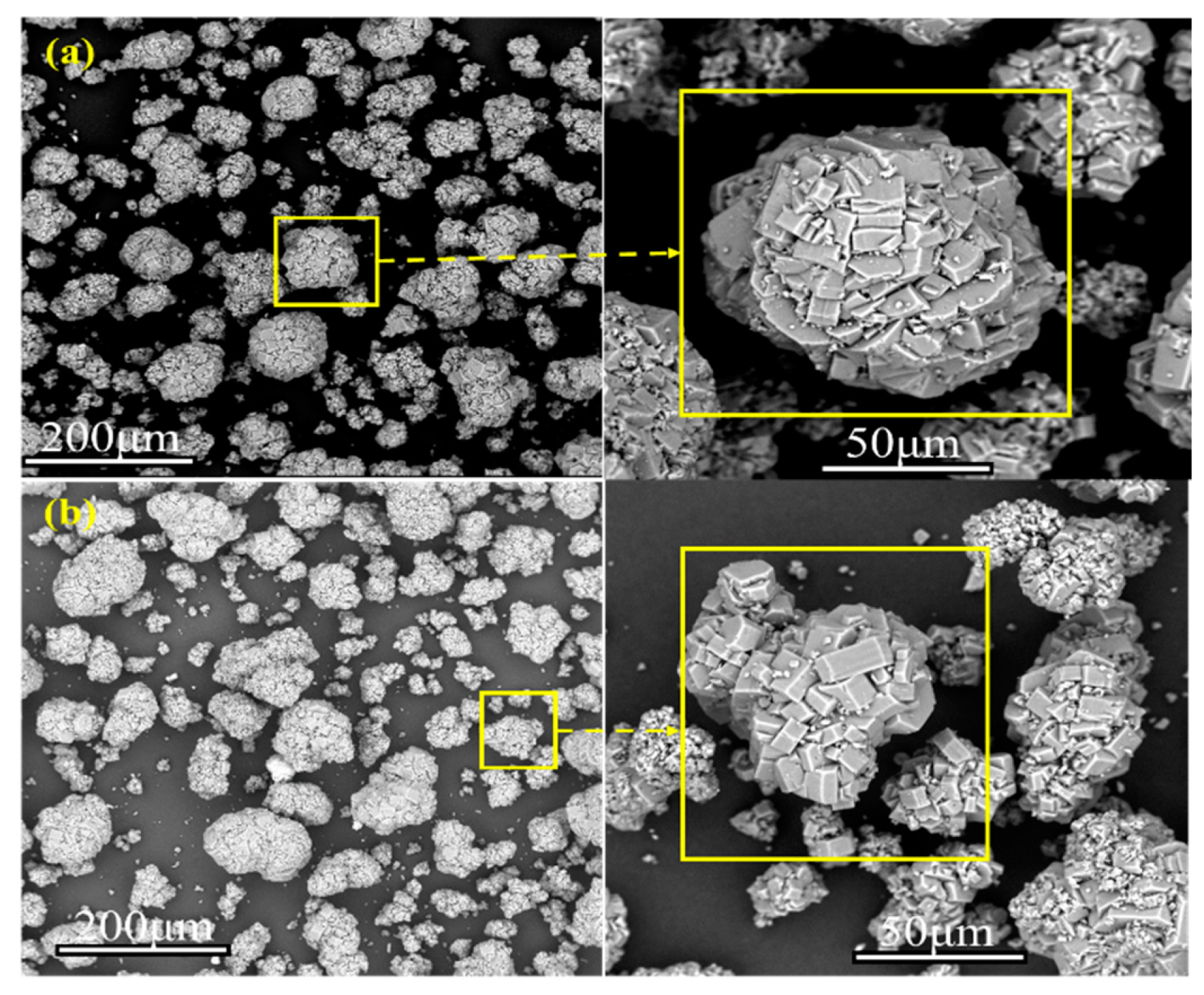
The appearance of the samples before and after drying is shown in Figure 8. The materials stuck together to form a ball shape before drying due to the bonding effect of water in the materials. After drying, the moisture was removed, and the materials were shaped as dispersed fine particles. Meanwhile, the material color changed from gray-white before drying to white after drying, which is attributed to the removal of moisture and impurities during microwave drying. The morphology of the samples before and after drying was analyzed with a Zeiss GeminiSEM 300 scanning electron microscope. Figure 9 summarizes the SEM results of the dried materials. There was no significant difference in the morphology of the materials dried by the blast drying oven and microwaves. The materials dried by microwaves were irregular balls of various sizes. In order to compare and analyze the particle size before and after drying, the particle size distribution was measured and analyzed by a Mastersizer 3000 laser particle size analyzer of Spectris Instrumentation and Systems Shanghai Ltd. Table 2 shows the particle size analysis results of the dried samples. The particle size of the obtained powder averaged approximately 80 μm A, with the maximum reaching 140 μm and the minimum ranging between 35 and 40 μm.

Figure 8.
Appearance of the sample before (a) and after drying (b).
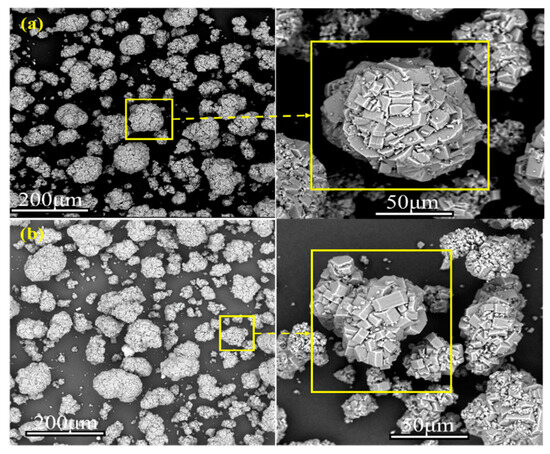
Figure 9.
SEM morphology analysis under different drying methods: (a) Microwave drying, (b) Blast drying oven drying.

Table 2.
Particle size distribution of samples.
3.2.3. FTIR Spectrum Analysis
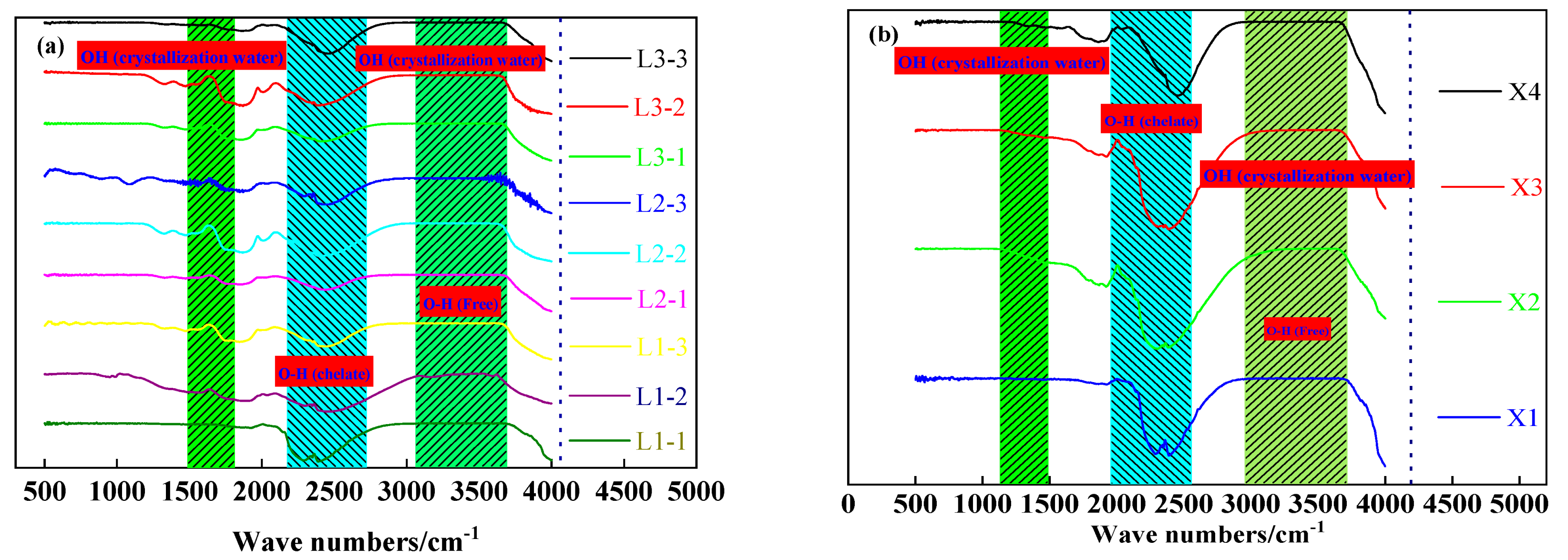
The FTIR spectrum (RAffinity-1, Shimadzu Corporation, Kyoto, Japan) analysis was made to prove the results of the water removal from aluminum hydroxide materials and to further clarify the microwave drying results of aluminum hydroxide. As shown in Figure 10, the stretching vibration peak of the water molecules at 3300 cm−1 disappears after microwave drying, indicating that the microwaves remove the free water and a part of the crystalline water from the materials. The bending vibration peak of the water molecules at 1625 cm−1 is observed in the spectrum of samples tested in the bench-scale experiment but disappears in the spectrum of samples tested in the pilot-scale experiment. Meanwhile, the O-H chelation peak at 2500 cm−1 still has a high strength after microwave drying, suggesting that there were chelated O-H bonds in the dried samples. The characteristic peak of free O-H at 3580 cm−1 almost disappears. Based on the phase analysis results in Figure 10, it is confirmed that microwave drying can remove free water from aluminum hydroxide.
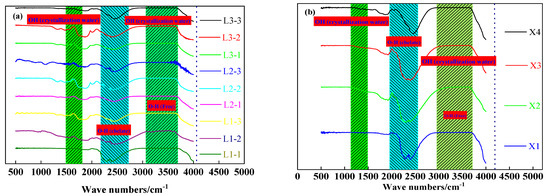
Figure 10.
Infrared spectral characteristics of the dried sample: (a) Small-scale experiments, (b) scale-up experiments.
3.2.4. Dielectric Properties Analysis
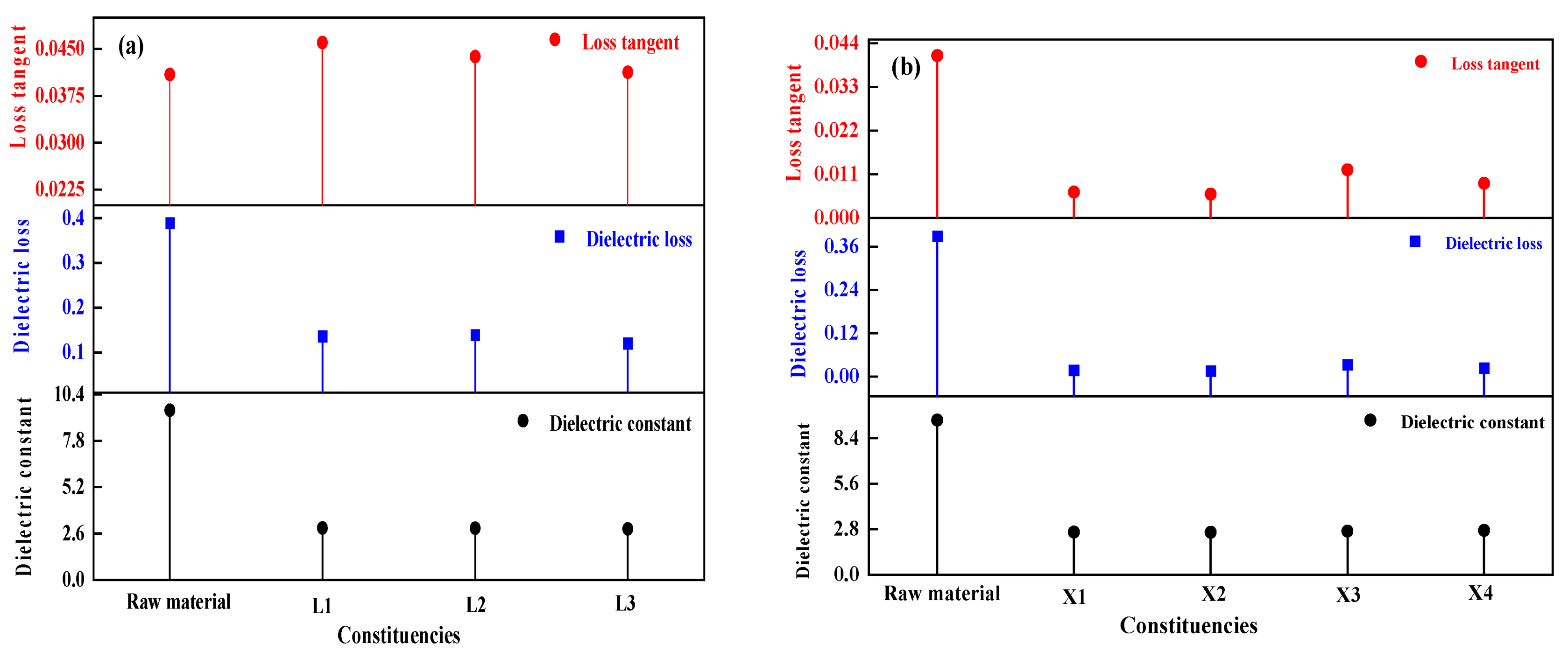
The dielectric properties of the materials before and after drying were measured at room temperature using a microwave dielectric temperature-varying test system, and the test results are shown in Figure 11. It is evident that both the dielectric constant and dielectric loss of the dried materials were lower than those of the raw materials. The raw materials have a high dielectric constant because the water molecules in them are polar molecules with strong polarization properties. After the removal of water molecules, the dielectric constant of the materials decreased, and the polarization performance weakened. Based on the above analysis, microwaves can effectively and quickly dry aluminum hydroxide materials, but at the same time change their dielectric properties. In addition, the dielectric constant and dielectric loss of the aluminum hydroxide materials decreased with the decrease of moisture content during the microwave drying process, which increased the effective depth of interaction of microwaves, but inhibited the microwave absorption and conversion by the materials. Therefore, it is necessary to increase microwave power properly to accelerate the drying process or extend the drying time to improve the drying effect when more materials need to be dried. If the quantity of the materials is fixed, the appropriate microwave power and drying time can help the materials to be dried evenly and prevent the wastage of microwave energy.
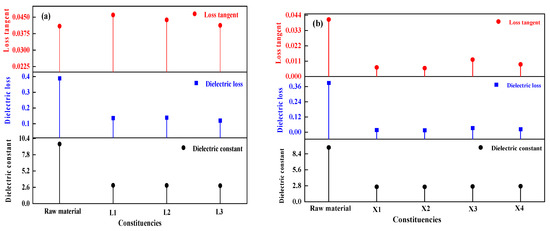
Figure 11.
Dielectric properties of dried samples: (a) Small-scale experiments, (b) scale-up experiments.
3.2.5. Simple Economic Analysis
In our research, the effect of microwave power and treatment time on the dehydration ratio and properties of aluminum hydroxide materials was researched. As we know, microwaves can quickly separate the free water from the material, according the experimental data from present works and with the condition of drying target temperature of 150 °C. The energy consumption under different microwave powers was shown in Table 3. As a result, we could infer that the mutual harmonization between microwave power, processing time, and material quality is very important for energy saving.

Table 3.
Energy consumption calculation.
4. Conclusions
- (1)
- Microwave drying can remove free water from aluminum hydroxide materials at a fast speed. When a fixed quantity of the materials is dried, the drying time is shorter at a higher microwave power and a faster heating rate.
- (2)
- The dehydration ratio and the proportion of dry matter of aluminum hydroxide are related to the final temperature of the material. At temperatures above 220 °C, the crystalline water in aluminum hydroxide is removed, and a low-temperature phase transition to boehmite occurs. Within the range of experimental parameters, the optimal temperature is 150 °C. The XRD spectra indicate that at 150 °C, aluminum hydroxide does not undergo low-temperature phase transition and has a stable composition.
- (3)
- According to the morphology and particle size analyses of aluminum hydroxide samples dried by the traditional and microwave processes, the particle size and morphology of the two types of samples have a slight difference. The test results of the dielectric properties of the samples dried by microwaves show that the dielectric constant and dielectric loss are tremendously reduced due to the removal of moisture.
Author Contributions
X.Z.: Writing—Original Draft, Conceptualization, and Methodology, Investigation, Validation; F.Y.: Investigation; A.M.: Visualization, Writing—Review & Editing, funding acquisition; S.T.: Validation, Supervision, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research and Cultivation Project of Liupanshui Normal University (LPSSY2023KJYBPY06), the Science and technology development project of Liupanshui City (52020-2023-0-2-6), the discipline team of Liupanshui Normal University (LPSSY2023XKTD07), the Guizhou Provincial First-class Professional (GZSylzy202103), the key cultivation disciplines of Liupanshui Normal University (LPSSYZDXK202001), Carbon Neutral Engineering Research Center of Guizhou colleges and universities in Coal Industry (Qian Jiao Ji [2023] No. 044), and the College Students’ Innovation and Entrepreneurship training program of Guizhou Province (S202310977119).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to technical or time limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Jiang, K.-X.; Zhang, T.-A. Cleaner extraction of alumina from coal fly ash: Baking-electrolysis method. Fuel 2020, 273, 117697. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, K.-X.; Zhang, T.-A.; Zhu, X.-F. Simultaneous and clean separation of titanium, iron, and alumina from coal fly ash in one spot: Electrolysis-hydrolysis method. Sep. Purif. Technol. 2022, 294, 121247. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Kang, S.-J.L.; Eun, K.Y.; Kim, Y.-S. Synthesis of Aluminum Monohydroxide Nanofiber by Electrolysis of Aluminum Plates. J. Korean Powder Metall. Inst. 2006, 13, 108–111. [Google Scholar] [CrossRef]
- Orasa, P.; Somjai, C. Improvements of Natural Rubber for Flame Resistance. Songklanakarin J. Sci. Technol. 2010, 32, 299–305. [Google Scholar]
- Sarawut, K.; Pohnpawee, N.; Kritapas, L.; Nisanart, T.; Rakchart, T. Reversible thermochromic polydiacetylene/zinc-aluminium layered double hydroxides nanocomposites for smart paints and colorimetric sensors: The crucial role of zinc ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125733. [Google Scholar] [CrossRef]
- Chen, P.; Ma, B.; Tan, H.; Liu, X.; Zhang, T.; Qi, H.; Peng, Y.; Yang, Q.; Wang, J. Effects of amorphous aluminum hydroxide on chloride immobilization in cement-based materials. Constr. Build. Mater. 2020, 231, 117171. [Google Scholar] [CrossRef]
- Wang, L.; Mei, L.; Zang, Z.; Cai, Y.; Jiang, P.; Zhou, L.; Du, Z.; Yang, L.; Gu, Z.; Liu, T.; et al. Aluminum hydroxide exposure induces neurodevelopmental impairment in hESC-derived cerebral organoids. Ecotoxicol. Environ. Saf. 2023, 256, 114863. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Light Metal Metallurgy; Metallurgical Industry Press (MIP): Beijing, China, 1991; pp. 61–65. (In Chinese) [Google Scholar]
- Peng, X.; Wu, Y.; Li, S.; Zhang, J.; Song, Y. Energy consumption analysis for evaporation process in alumina refinery. J. Cent. South Univ. 2013, 44, 367–371. (In Chinese) [Google Scholar]
- Hua, Y.; Cai, C.; Cui, Y. Microwave-enhanced roasting of copper sulfide concentrate in the presence of CaCO3. Sep. Purif. Technol. 2006, 50, 22–29. [Google Scholar] [CrossRef]
- Sharma, P.; Osama, K.; Varjani, S.; Farooqui, A.; Younis, K. Microwave-assisted valorization and characterization of Citrus limetta peel waste into pectin as a perspective food additive. J. Food Sci. Technol. 2023, 60, 1284–1293. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M.; Mujumdar, A.S. Recent developments in high efficient freeze-drying of fruits and vegetables assisted by microwave: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Kosai, S.; Yamasue, E. Microwave-based extractive metallurgy to obtain pure metals: A review. Clean. Eng. Technol. 2021, 5, 100306. [Google Scholar] [CrossRef]
- Ma, A.; Peng, J.; Xia, H.; Zuo, Y. Dielectric properties and temperature increase of zinc oxide dust derived from volatilization in rotary kilns. J. Microw. Power Electromagn. Energy 2014, 48, 25–34. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, L.; Liu, B.; He, G.; Peng, J.; Huang, M. Dielectric Properties Measurement for Microwave Synthesis of Titanium Carbide with Ti-bearing Blast Furnace Slag. Int. J. Miner. Metall. Mater. 2021, 28, 88–97. [Google Scholar] [CrossRef]
- Pawelski, D.; Plonska-Brzezinska, M.E. Microwave-Assisted Synthesis as a Promising Tool for the Preparation of Materials Containing Defective Carbon Nanostructures: Implications on Properties and Applications. Materials 2023, 16, 6549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, S.; Wang, Z.; Ji, N.; Li, D.; Wu, Y. Preparation of bamboo-epoxy resin materials with microwave assistance. J. Mater. Res. Technol. 2022, 18, 3266–3272. [Google Scholar] [CrossRef]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-Saving Pathways for Thermoelectric Nanomaterial Synthesis: Hydrothermal/Solvothermal, Microwave-Assisted, Solution-Based, and Powder Processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2021, 49, 885–900. [Google Scholar] [CrossRef]
- Yan, Y.; Jing, C.; Song, Z.; Zhao, X.; Wang, W.; Mao, Y.; Sun, J. Effect of hot air and microwave drying on the quality of coal slime. Chem. Ind. Eng. Prog. 2019, 38, 122–127. (In Chinese) [Google Scholar]
- Ru, S.; Ceng, H.; Yanxiong, F.; Ji, H. Effect of microwave drying and hot air drying on quality of Jin Xuan tea. Chem. Ind. Eng. Prog. 2012, 31, 76–79. (In Chinese) [Google Scholar]
- Guo, L.; Tu, H.; Jinhui, P.; Yin, S.; Zhang, L.; Li, Z. Experimental study on the microwave drying of hydrometallurgy mud. Chem. Ind. Eng. Prog. 2015, 34, 3472–3475. (In Chinese) [Google Scholar] [CrossRef]
- Guo, L.; Zhang, L.; Peng, J.; Duan, X.; Wang, X. An optimization study of microwave drying process for scheelite concentrate with RSM. Min. Metall. 2012, 21, 54–57. (In Chinese) [Google Scholar] [CrossRef]
- Bin, Z. Study on Calcination Process and Crystal Transformation of Aluminum Hydroxide in Microwave Field; Kunming University of Technology: Kunming, China, 2012. (In Chinese) [Google Scholar]
- Tian, C.; Zhou, J.; Ren, C.; Omran, M.; Zhang, F.; Tang, J. Drying kinetics of microwave-assisted drying of leaching Rresidues from hydrometallurgy of Zinc. Materials 2023, 16, 5546. [Google Scholar] [CrossRef]
- Koua, B.K.; Kof, P.M.E.; Gbah, P. Evolution of shrinkage, real density, porosity, heat and mass transfer coefficients during indirect solar drying of cocoa beans. J. Saudi Soc. Agric. Sci. 2017, 18, 72–82. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Fan, W.; Li, W.; Han, D.; Zheng, C. Influence of particle size on kinetics of low temperature phase transformation of aluminum hydroxide. Chem. Ind. Eng. Prog. 2020, 39, 1101–1107. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).