Cobalt Ion-Modified Titanium Oxide Nanorods: A Promising Approach for High-Performance Electrochromic Application
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of Ti-Co Thin Films
2.3. Electrochromic Device Fabrication
3. Material Characterization
4. Results and Discussion
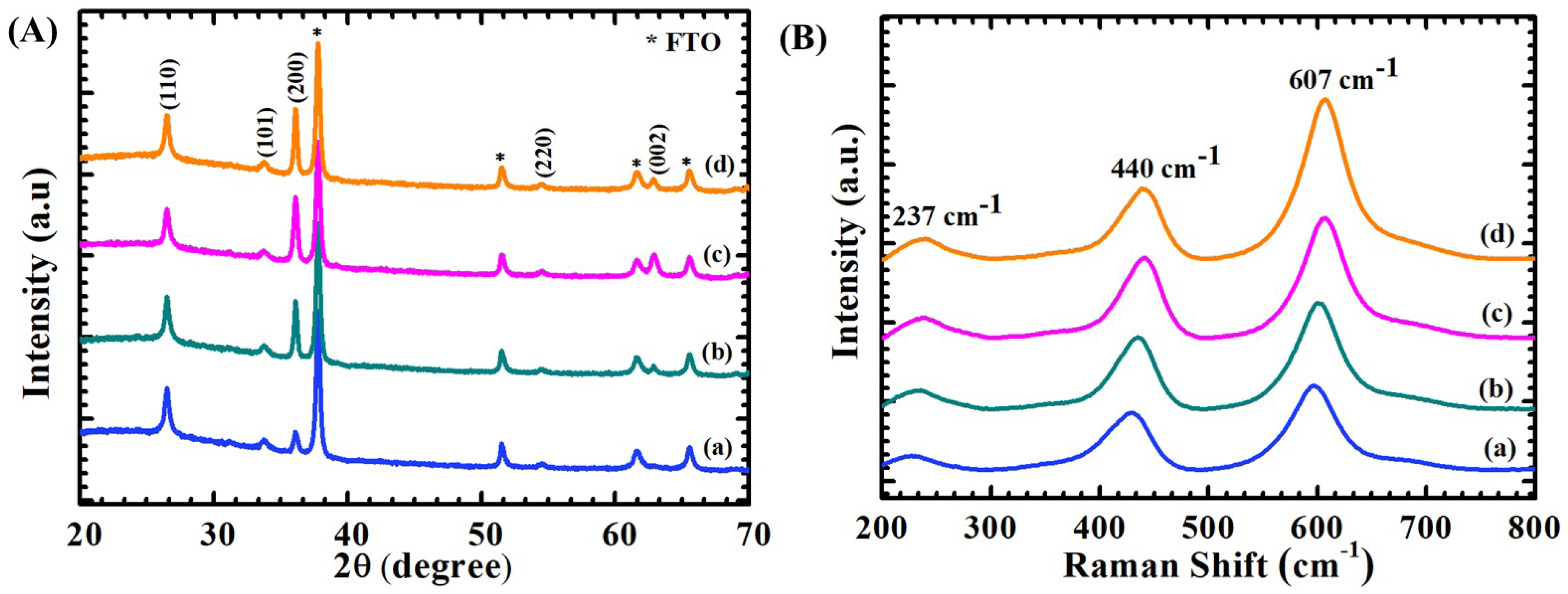
4.1. X-ray Diffraction Elucidation
4.2. Raman Spectroscopy Analysis
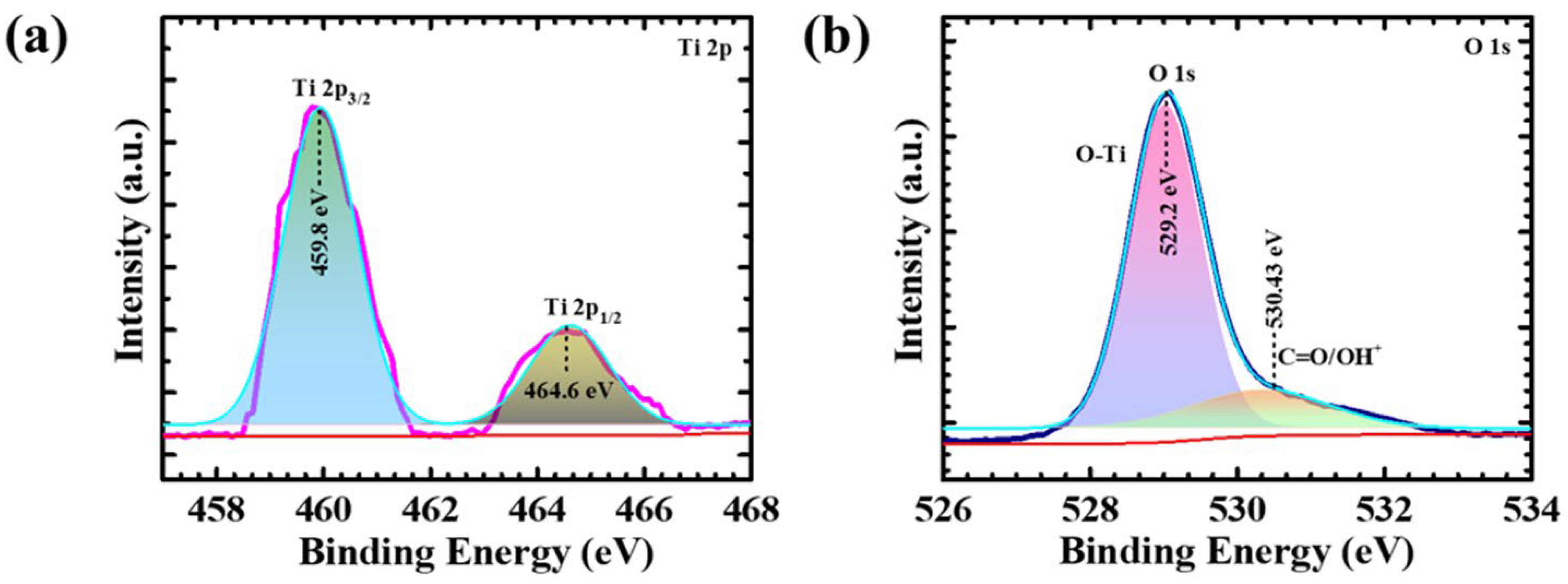
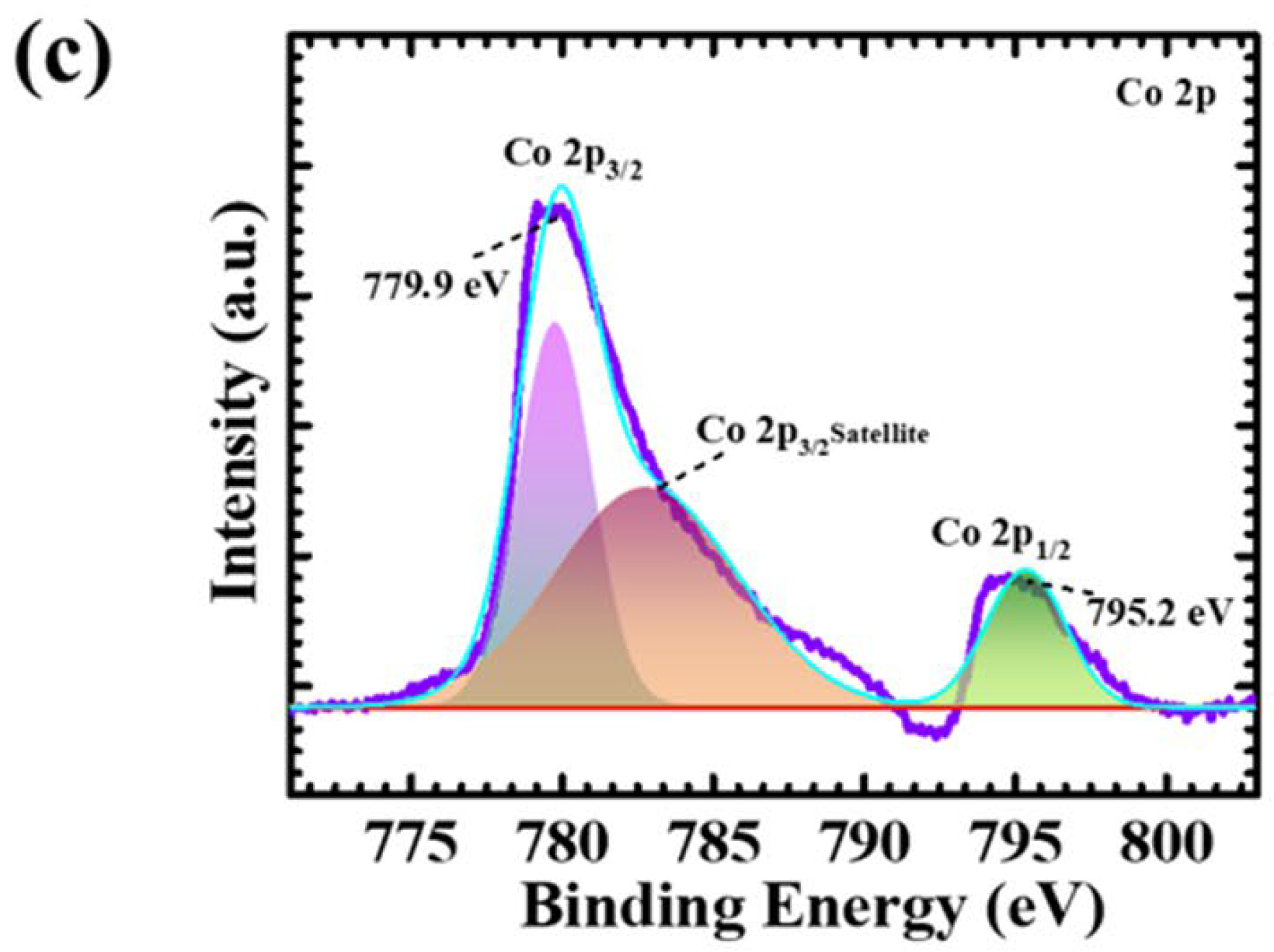
4.3. X-ray Photoelectron Spectroscopy Elucidation
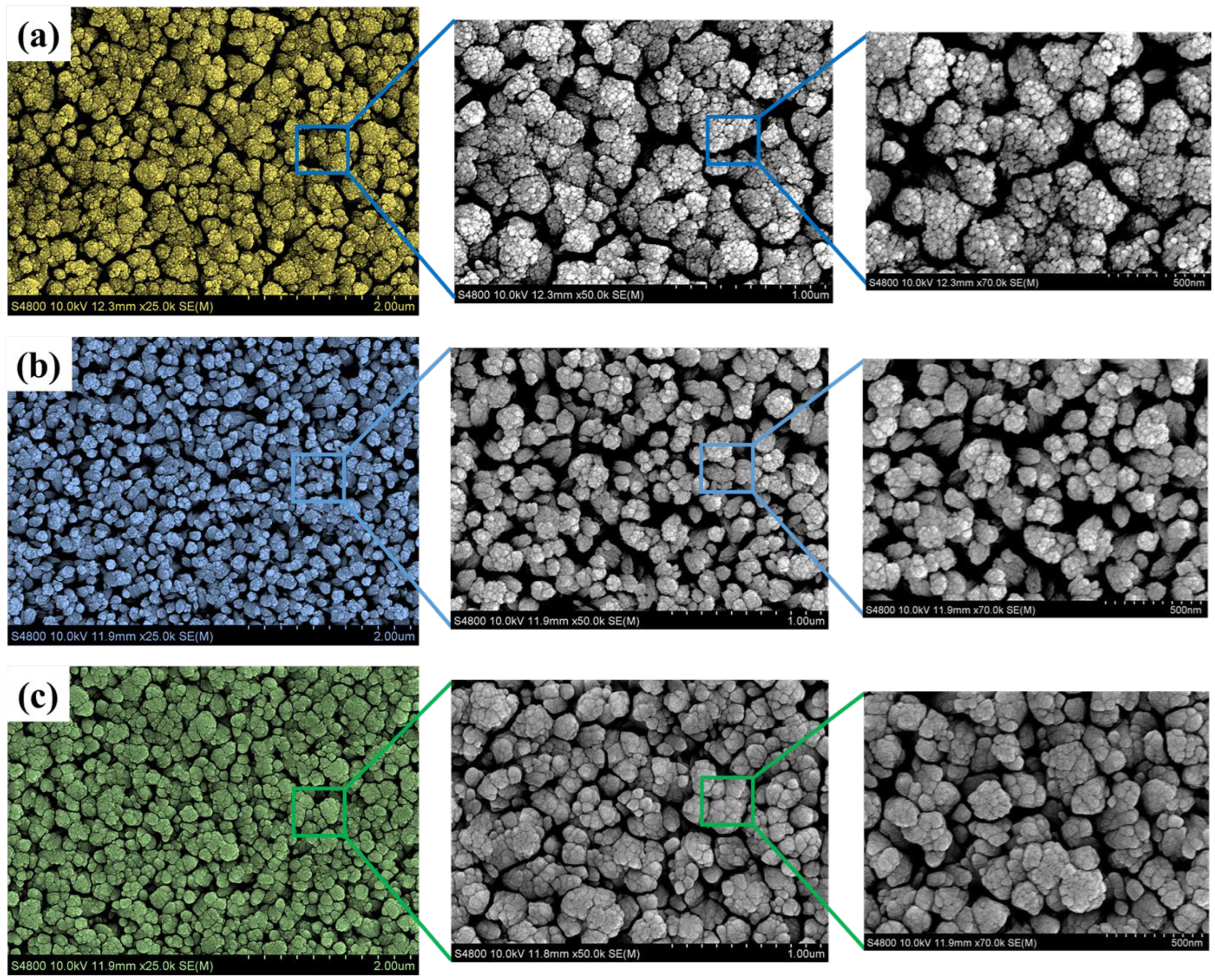
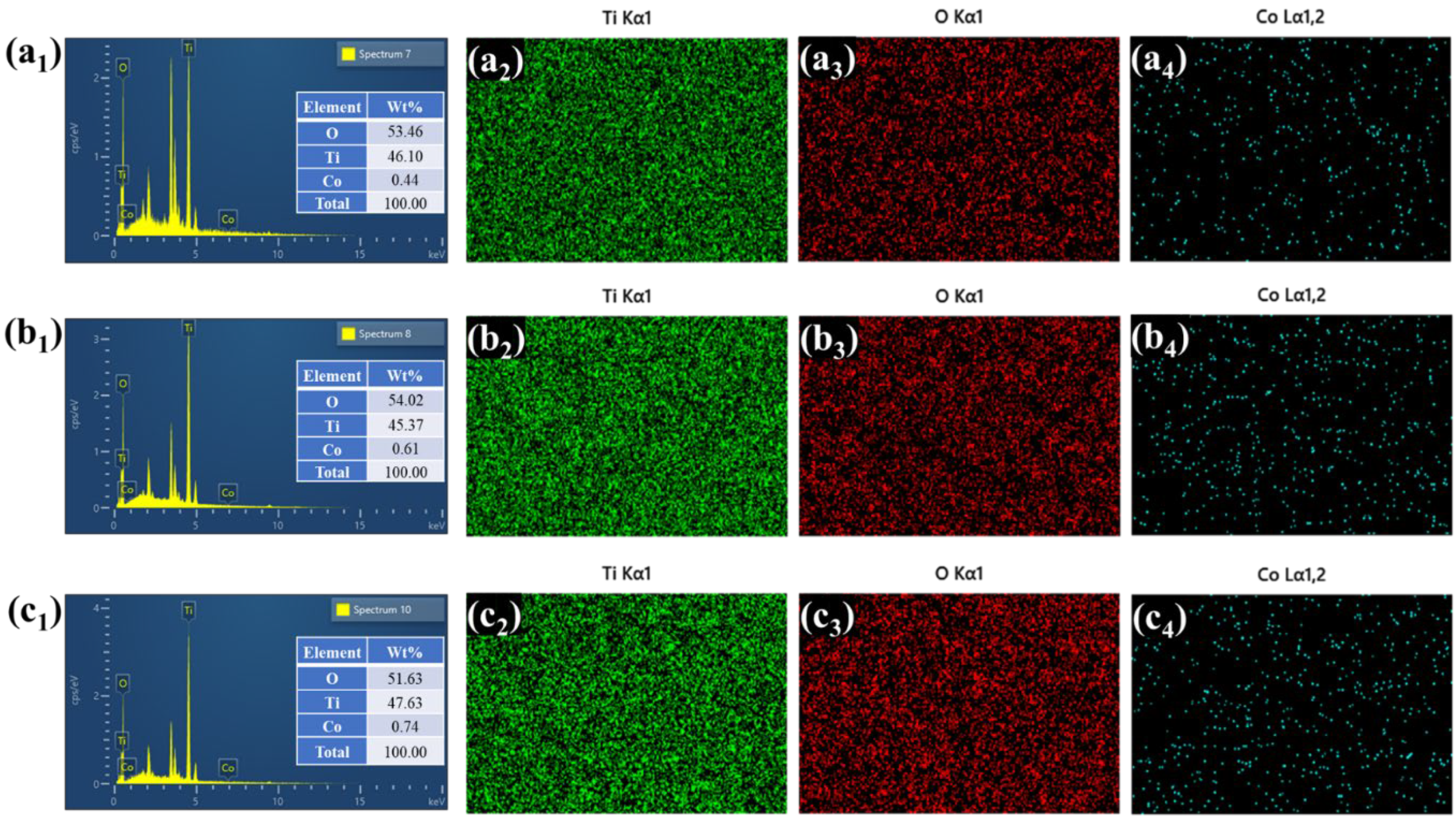
4.4. Morphological and Elemental Compositional Characteristics
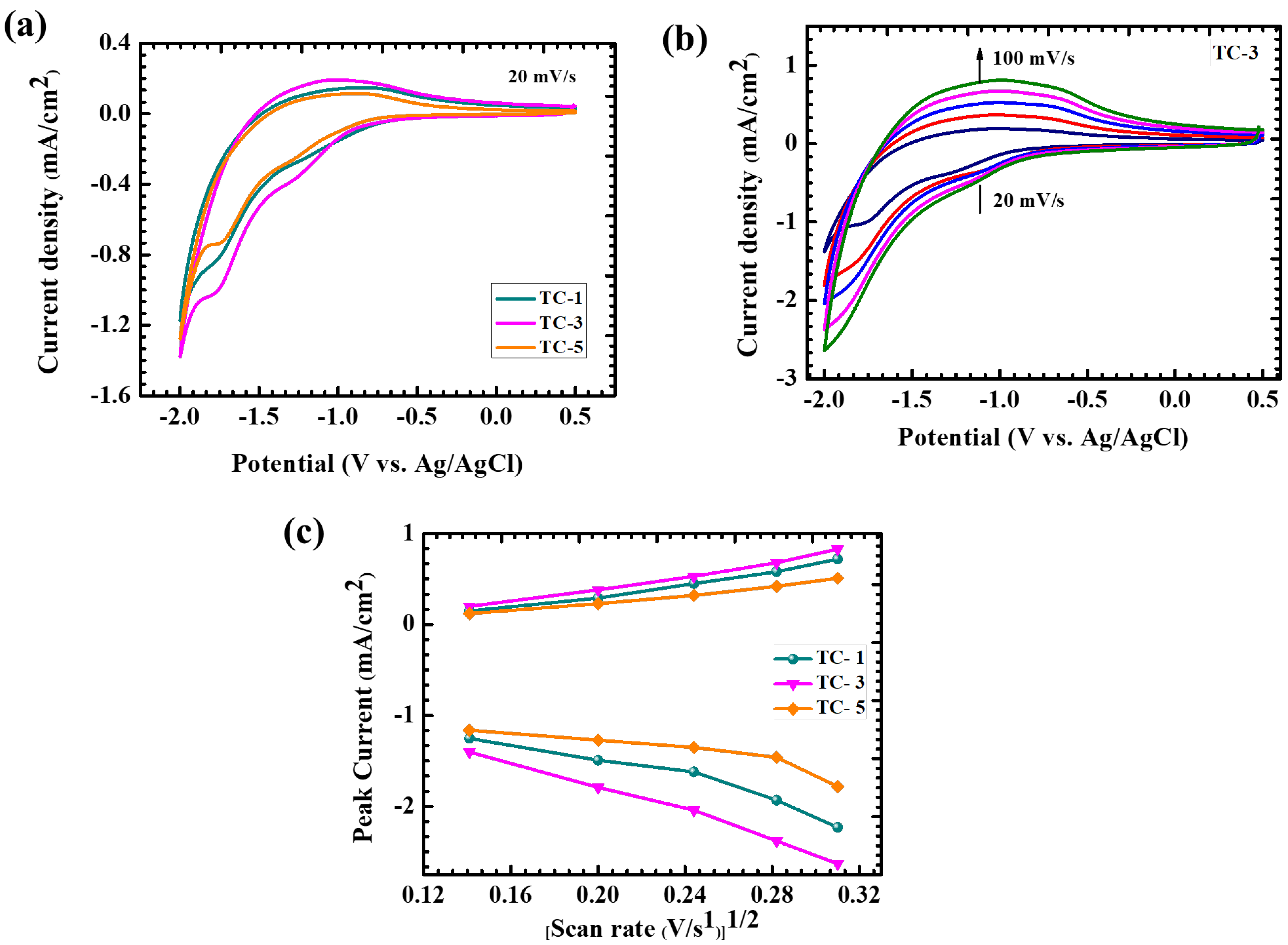
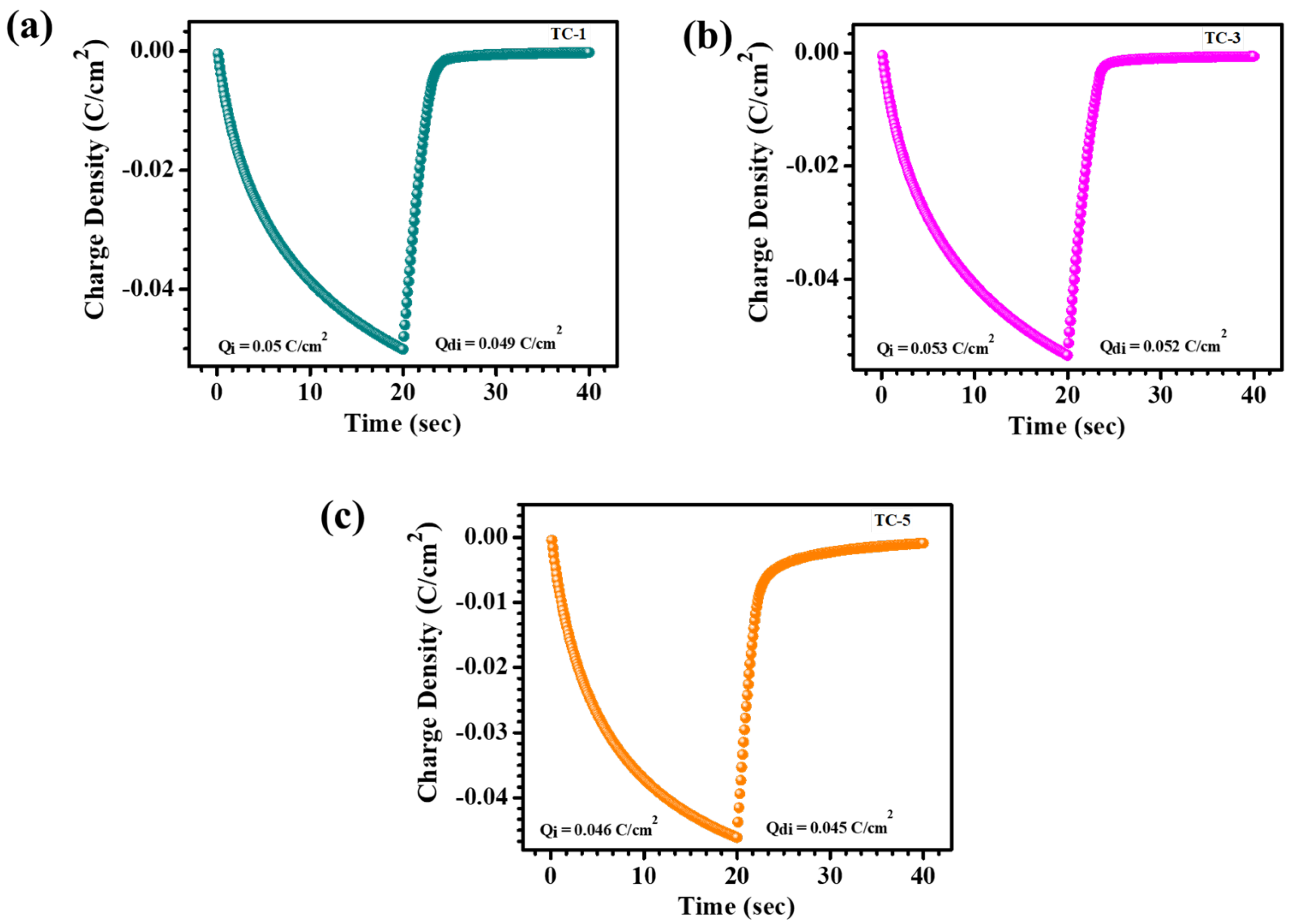
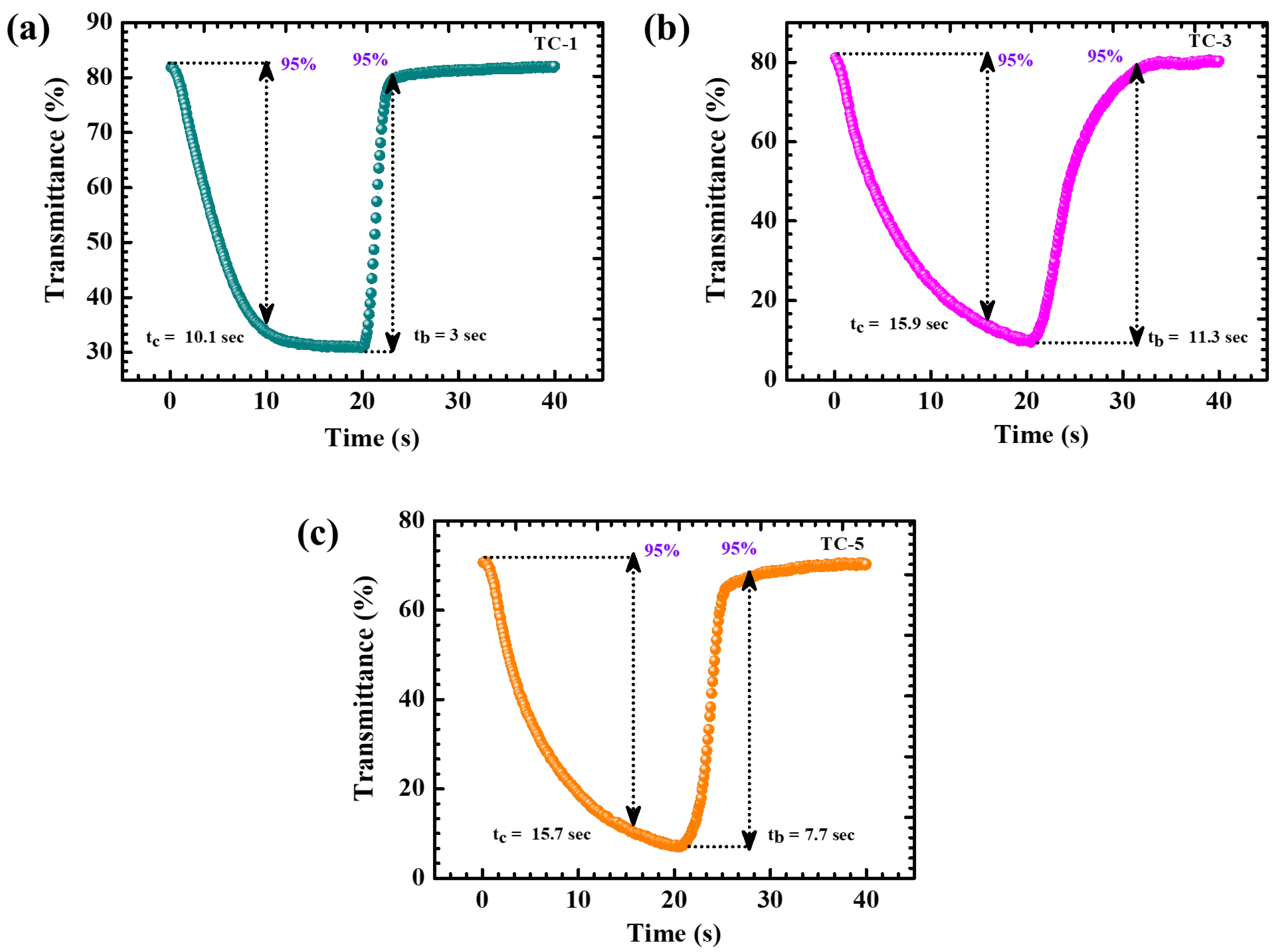
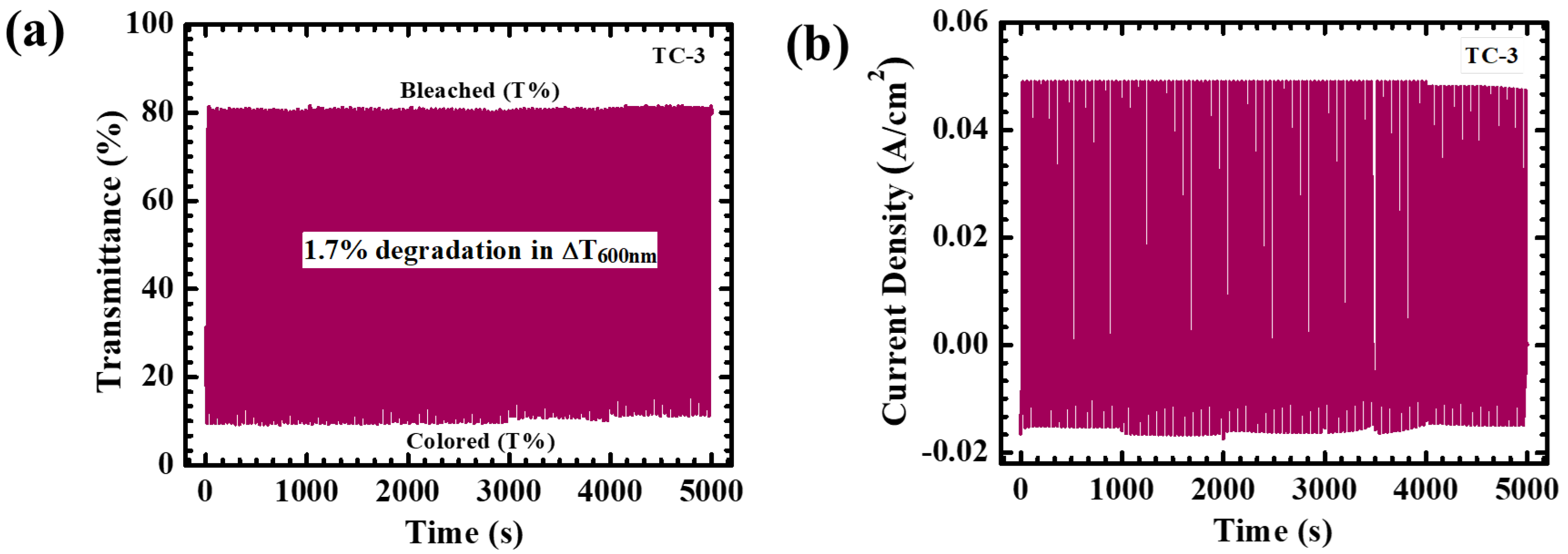
4.5. Electrochromic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A Critical Review of Metal-Doped TiO2 and Its Structure–Physical Properties–Photocatalytic Activity Relationship in Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 28553–28565. [Google Scholar] [CrossRef]
- Review, S.T.P.A.; Janus, M.; Szyma, K. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for Sensitized Solar Cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef]
- Zavatski, S.; Neilande, E.; Bandarenka, H.; Popov, A.; Piskunov, S.; Bocharov, D. Density Functional Theory for Doped TiO2: Current Research Strategies and Advancements. Nanotechnology 2024, 35, 192001. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Cai, Y.; Zhao, J.; Gao, Z.; Song, Y.Y. The Challenges and Opportunities for TiO2 Nanostructures in Gas Sensing. ACS Sens. 2024, 9, 1644–1655. [Google Scholar] [CrossRef]
- Mahmoud, Z.H.; Ajaj, Y.; Kamil Ghadir, G.; Musaad Al-Tmimi, H.; Hameed Jasim, H.; Al-Salih, M.; Hasen shuhata Alubiady, M.; Muzahem Al-Ani, A.; Salih Jumaa, S.; Azat, S.; et al. Carbon-Doped Titanium Dioxide (TiO2) as Li-Ion Battery Electrode: Synthesis, Characterization, and Performance. Results Chem. 2024, 7, 101422. [Google Scholar] [CrossRef]
- Sun, X.; Wang, D.; Wu, W.; Zhao, X.; Zhang, X.; Wang, B.; Rong, X.; Wu, G.; Wang, X. Amorphous and Crystalline Ti-Doped WO3·2H2O for Dual-Band Electrochromic Smart Windows. ACS Sustain. Chem. Eng. 2024, 12, 5459–5467. [Google Scholar] [CrossRef]
- Yin, Y.; Zhu, Y.; Liao, P.; Yuan, X.; Jia, J.; Lan, C.; Li, C. Co-Sputtering Construction of Gd-Doped WO3 Nano-Stalagmites Film for Bi-Funcional Electrochromic and Energy Storage Applications. Chem. Eng. J. 2024, 487, 150615. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Y.; Zhang, W.; Wang, Z.; Wang, F.; Zhang, L.; Ma, H. Experimental Study and Simulation of Electrochromic Properties of TiO2-Doped NiO Films. Ceram. Int. 2024, 50, 15065–15075. [Google Scholar] [CrossRef]
- Tong, Z.; Zhu, X.; Xu, H.; Li, Z.; Li, S.; Xi, F.; Kang, T.; Ma, W.; Lee, C.S. Multivalent-Ion Electrochromic Energy Saving and Storage Devices. Adv. Funct. Mater. 2024, 2308989. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Preparation and Investigation of Sol–Gel TiO2-NiO Films: Structural, Optical and Electrochromic Properties. Crystals 2024, 14, 192. [Google Scholar] [CrossRef]
- Ma, D.; Yang, T.; Feng, X.; Wang, P.; Huang, J.; Wang, J.; Li, H. Quadruple Control Electrochromic Devices Utilizing Ce4W9O33 Electrodes for Visible and Near-Infrared Transmission Intelligent Modulation. Adv. Sci. 2024, 11, 2307223. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Y.; Long, X.; Liao, W.; Hu, B.; Miao, R.; Zhang, G.; Sun, G.; Xie, Y.; Miao, L. Manganese Doped Nickel Oxide Thin Film with Improved Electrochromic Performance towards Smart Window Application. Ceram. Int. 2024, 50, 12810–12817. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Mohd Abdah, M.A.A.; Mohamad Saidi, N.; Wong, W.P.; Tan, Y.S.; Numan, A.; Sulaiman, Y.; Walvekar, R.; Mohammad Azlan, F.N.; Khalid, M. High-Performance Electrochromic Supercapacitor with Bimetallic Phosphate and Vanadium Carbide MXene. J. Power Sources 2024, 595, 234079. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Beknalkar, S.A.; Chavan, G.T.; Ahir, N.A.; Dalavi, D.S.; Jeon, C.W. Versatile Electrochromic Energy Storage Smart Window Utilizing Surfactant-Assisted Niobium Oxide Thin Films. Chem. Eng. J. 2024, 484, 149556. [Google Scholar] [CrossRef]
- García-Tecedor, M.; Villar-Garcia, I.J.; Gorni, G.; Liras, M.; de la Peña O’Shea, V.A.; Barawi Moran, M. Unveiling the Non-Innocence of Vanadium Dopant in TiO2 Nanocrystals for Advanced Energy Storage and Smart Windows. J. Phys. Energy 2024, 6, 025024. [Google Scholar] [CrossRef]
- Öztetïk, B.; Pat, S.; Korkmaz, Ş. Investigation on the Optical and Electrochromic Characteristics of Thin Films of WO3 Doped with Graphene and MXene (Ti2AlC). Ceram. Int. 2024, 50, 13113–13124. [Google Scholar] [CrossRef]
- Qin, L.; Liu, Y.; Long, M.; Zou, B.; Cao, S. Advancements in Dual-Band Electrochromic Smart Windows: Exploring Single-Component Materials for Sustainable Building Solutions. Ceram. Int. 2024, 50, 22174–22183. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Lv, Y.; Cai, Z.; Shen, Y.; Ruhlmann, L.; Gan, L.; Liu, M. Electrode Materials for Electrochromic Supercapacitors. Nanotechnology 2024, 35, 152001. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Meng, D.; Yang, F. Photoelectrocatalytic Activation of Sulfate for Sulfamethoxazole Degradation and Simultaneous H2 Production by Bifunctional N,P Co-Doped Black-Blue TiO2 Nanotube Array Electrode. Chem. Eng. J. 2024, 485, 149828. [Google Scholar] [CrossRef]
- Huang, L.; He, G.; Yuan, Y.; Zhang, T.C.; Wang, Y.; Yuan, S. Trivalent Metal Ions (Al, Ga, In)-Doped TiO2 for Enhanced Photocatalytic Desulfurization of H2S: Band Structure Regulation, Performance, and Mechanism. Ind. Eng. Chem. Res. 2024, 63, 7154–7165. [Google Scholar] [CrossRef]
- Jian, L.; Wu, X.; Li, R.; Zhao, F.; Liu, P.; Wang, F.; Liu, D.; Yao, Q.; Deng, J. Enhanced Aluminum-Ion Storage Properties of N-Doped Titanium Dioxide Electrode in Aqueous Aluminum-Ion Batteries. Nanomaterials 2024, 14, 472. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Teli, A.M.; Chavan, G.T.; Beknalkar, S.A.; Dalavi, D.S.; Ahir, N.A.; Jeon, C.W. Surfactant Integrated Nanoarchitectonics for Controlled Morphology and Enhanced Functionality of Tungsten Oxide Thin Films in Electrochromic Supercapacitors. J. Energy Storage 2023, 73, 109095. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, H.; Chen, C.; Tu, X.; Fang, L.; Zhang, M.; He, W.; Wang, P. Self-Powered Dual-Band Electrochromic Supercapacitor Devices for Smart Window Based on Ternary Dielectric Triboelectric Nanogenerator. Nanomaterials 2024, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, S.; Chang, S.; Li, C.; Fang, C.; Xia, X.; Shen, L.; Yang Lee, J.; Cao, C.; Zhang, X.; et al. A Flexible Electrochromic Device with Variable Infrared Emissivity Based on W18O49 Nanowire Cathode and MXene Infrared Transparent Conducting Electrode. Chem. Eng. J. 2024, 480, 148010. [Google Scholar] [CrossRef]
- Songara, S.; Saini, L.; Gowd, G.S.; Rajpurohit, J.S.; Gupta, V.; Patra, M.K.; Vadera, S.R.; Kumar, N. Improved Photochromic Properties of W6+ Doped Nanostructured TiO2 Coatings. Results Surf. Interfaces 2024, 14, 100201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhang, M.; Huo, X.; Guo, M. A Semi-Solid, Polychromatic Dual-Band Electrochromic Smart Window: Visualizing Sunlight and Solar Heat Transmission. Chem. Eng. J. 2024, 484, 149628. [Google Scholar] [CrossRef]
- Qiao, P.; Zhang, Y.; Wang, J.; Peng, Z.; Wu, D.; Chao, X.; Yang, Z.; Liang, P. Enhanced Dielectric Properties and Improved Thermal Stability in TiO2-Based Ceramics by Cu and Nb Co-Doping. Ceram. Int. 2024, 50, 2242–2248. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Zhao, S.; Li, R.; Gao, D.; Jiang, H.; Zhang, R. Novel Self-Assembled Porous Yolk-Shell NiO Nanospheres with Excellent Electrochromic Performance for Smart Windows. Particuology 2024, 84, 72–80. [Google Scholar] [CrossRef]
- Morankar, P.J.; Amate, R.U.; Chavan, G.T.; Teli, A.M.; Dalavi, D.S.; Jeon, C.W. Improved Electrochromic Performance of Potentiostatically Electrodeposited Nanogranular WO3 Thin Films. J. Alloys Compd. 2023, 945, 169363. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, S.; Zhang, T.; Lee, J.Y. Fluoride-Assisted Synthesis of Plasmonic Colloidal Ta-Doped TiO2 Nanocrystals for Near-Infrared and Visible-Light Selective Electrochromic Modulation. Chem. Mater. 2018, 30, 4838–4846. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Chen, K.; Mao, J.; Diao, X. Investigation on the Properties and Mechanism of W-Doped TiO2 Electrochromic Films Deposited by Cosputtering. ACS Appl. Electron. Mater. 2023, 5, 6778–6786. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Tan, S.; Liu, C.; Zhang, J. Study on Electrochromic Properties of TiO2 Films under Co Ion Content Fluctuations. Opt. Mater. 2020, 100, 109659. [Google Scholar] [CrossRef]
- Khot, A.C.; Desai, N.D.; Khot, K.V.; Salunkhe, M.M.; Chougule, M.A.; Bhave, T.M.; Kamat, R.K.; Musselman, K.P.; Dongale, T.D. Bipolar Resistive Switching and Memristive Properties of Hydrothermally Synthesized TiO2 Nanorod Array: Effect of Growth Temperature. Mater. Des. 2018, 151, 37–47. [Google Scholar] [CrossRef]
- Chanda, A.; Rout, K.; Vasundhara, M.; Joshi, S.R.; Singh, J. Structural and Magnetic Study of Undoped and Cobalt Doped TiO2 Nanoparticles. RSC Adv. 2018, 8, 10939–10947. [Google Scholar] [CrossRef] [PubMed]
- Perevedentseva, E.; Lin, Y.C.; Karmenyan, A.; Wu, K.T.; Lugovtsov, A.; Shirshin, E.; Priezzhev, A.; Cheng, C.L. Raman Spectroscopic Study of TiO2 Nanoparticles’ Effects on the Hemoglobin State in Individual Red Blood Cells. Materials 2021, 14, 5920. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, C.; Ji, D.; Yin, G.; Wang, W.; Chen, Z. Cobalt-Doped TiO2 Nanowire Arrays Coated with NiFe Layered-Double-Hydroxide Nanoplatelets as Photoanodes for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2020, 3, 6598–6608. [Google Scholar] [CrossRef]
- Siddiqa, A.; Masih, D.; Anjum, D.; Siddiq, M. Cobalt and Sulfur Co-Doped Nano-Size TiO2 for Photodegradation of Various Dyes and Phenol. J. Environ. Sci. 2015, 37, 100–109. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Teli, A.M.; Chavan, G.T.; Beknalkar, S.A.; Ahir, N.A.; Jeon, C.W. Exploring the Electrochemical Performance of Niobium Phosphate Electrode for Supercapacitor Application. Surf. Interfaces 2023, 41, 103265. [Google Scholar] [CrossRef]
- Xiang, S.; Xu, G.; Liu, C.; Bu, H.; Zhang, Y. Study on the Variable Color and Emissivity Properties of Co Doped TiO2 under Temperature Fluctuations. Opt. Mater. 2018, 85, 254–260. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Chavan, G.T.; Teli, A.M.; Desai, R.S.; Dalavi, D.S.; Jeon, C.W. Bi-Functional Electrochromic Supercapacitor Based on Hydrothermal-Grown 3D Nb2O5 Nanospheres. Electrochim. Acta 2023, 459, 142522. [Google Scholar] [CrossRef]
- Teli, A.M.; Beknalkar, S.A.; Amte, R.U.; Morankar, P.J.; Yewale, M.A.; Burungale, V.V.; Jeon, C.W.; Efstathiadis, H.; Shin, J.C. Investigating into the Intricacies of Charge Storage Kinetics in NbMn-Oxide Composite Electrodes for Asymmetric Supercapacitor and HER Applications. J. Alloys Compd. 2023, 965, 171305. [Google Scholar] [CrossRef]


| Sample Code | Diffusion Coefficient (cm2/s × 10−10) | |
|---|---|---|
| Reduction | Oxidation | |
| TC-1 | 2.7 | 0.03 |
| TC-3 | 3.4 | 0.06 |
| TC-5 | 2.3 | 0.02 |
| Sample Name | Charge Intercalation ) (C/cm2) | Charge Deintercalation ) (C/cm2) | Reversibility (%) | Coloration Time (s) (TC) | Bleaching Time (s) (Tb) | Tb % | TC % | Optical Modulation (ΔT600nm%) | Optical Density (ΔOD) | Coloration Efficiency (cm2/C) |
|---|---|---|---|---|---|---|---|---|---|---|
| TC-1 | 0.050 | 0.049 | 98% | 10.1 | 3 | 73.2 | 10.5 | 62.7 | 1.94 | 77.6 |
| TC-3 | 0.053 | 0.052 | ~99% | 15.9 | 11.3 | 83.1 | 9.5 | 73.6 | 2.16 | 81.50 |
| TC-5 | 0.046 | 0.045 | 97.82% | 15.7 | 7.7 | 82.5 | 30.7 | 51.8 | 0.98 | 42.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morankar, P.J.; Amate, R.U.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.-W. Cobalt Ion-Modified Titanium Oxide Nanorods: A Promising Approach for High-Performance Electrochromic Application. Coatings 2024, 14, 707. https://doi.org/10.3390/coatings14060707
Morankar PJ, Amate RU, Teli AM, Beknalkar SA, Jeon C-W. Cobalt Ion-Modified Titanium Oxide Nanorods: A Promising Approach for High-Performance Electrochromic Application. Coatings. 2024; 14(6):707. https://doi.org/10.3390/coatings14060707
Chicago/Turabian StyleMorankar, Pritam J., Rutuja U. Amate, Aviraj M. Teli, Sonali A. Beknalkar, and Chan-Wook Jeon. 2024. "Cobalt Ion-Modified Titanium Oxide Nanorods: A Promising Approach for High-Performance Electrochromic Application" Coatings 14, no. 6: 707. https://doi.org/10.3390/coatings14060707
APA StyleMorankar, P. J., Amate, R. U., Teli, A. M., Beknalkar, S. A., & Jeon, C.-W. (2024). Cobalt Ion-Modified Titanium Oxide Nanorods: A Promising Approach for High-Performance Electrochromic Application. Coatings, 14(6), 707. https://doi.org/10.3390/coatings14060707






