Effects of Dynamic Flow Rates on the In Vitro Bio-Corrosion Behavior of Zn-Cu Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
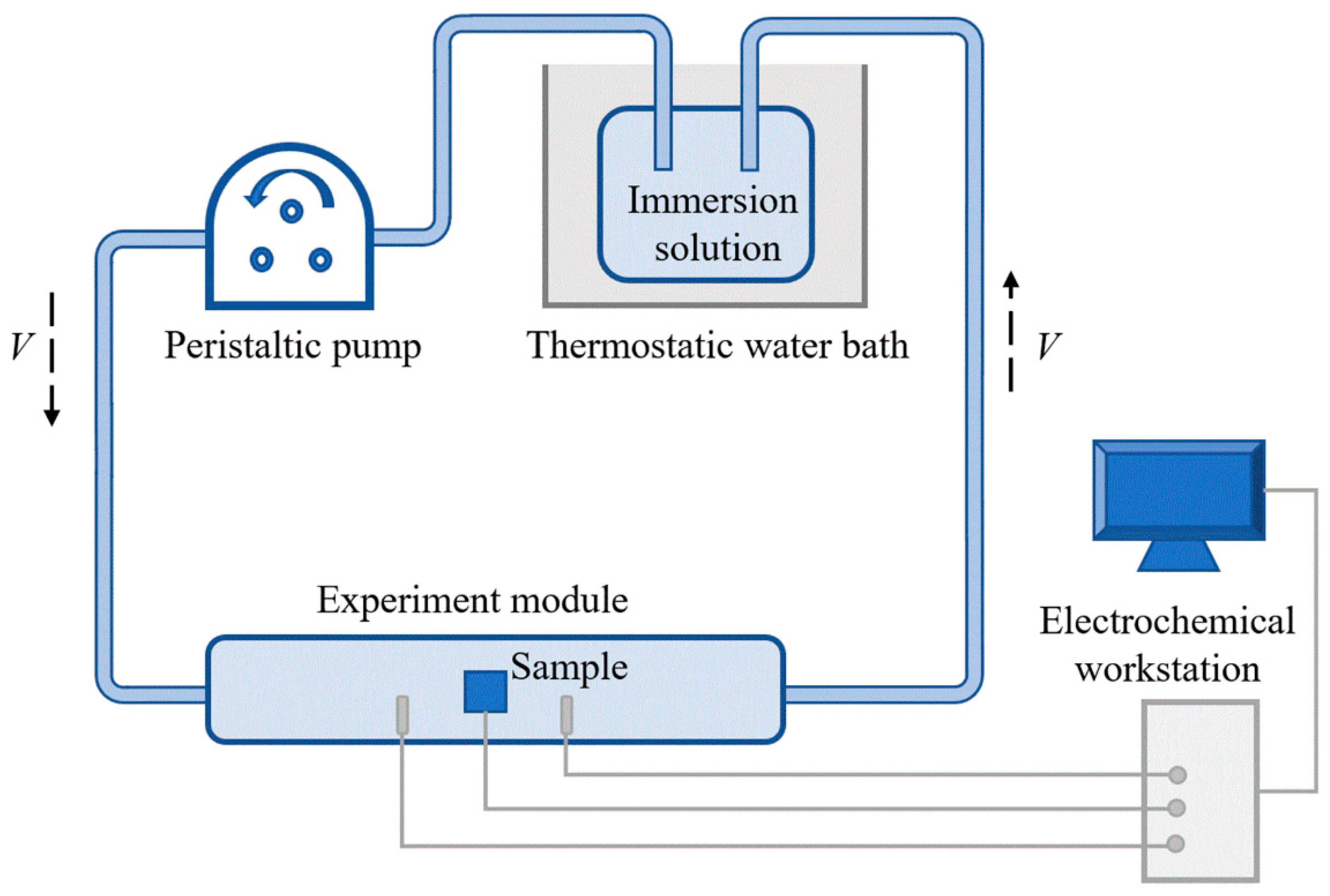
2.2. Immersion Tests
2.3. Materials Characterization
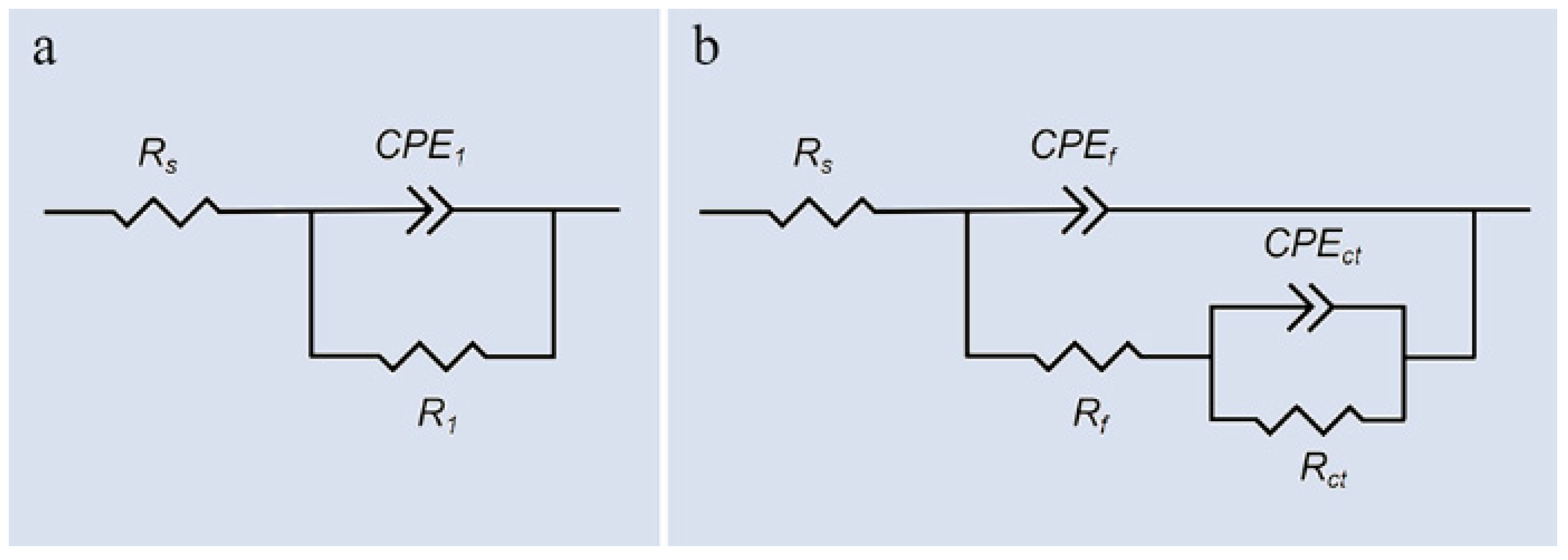
2.4. Electrochemical Evaluation
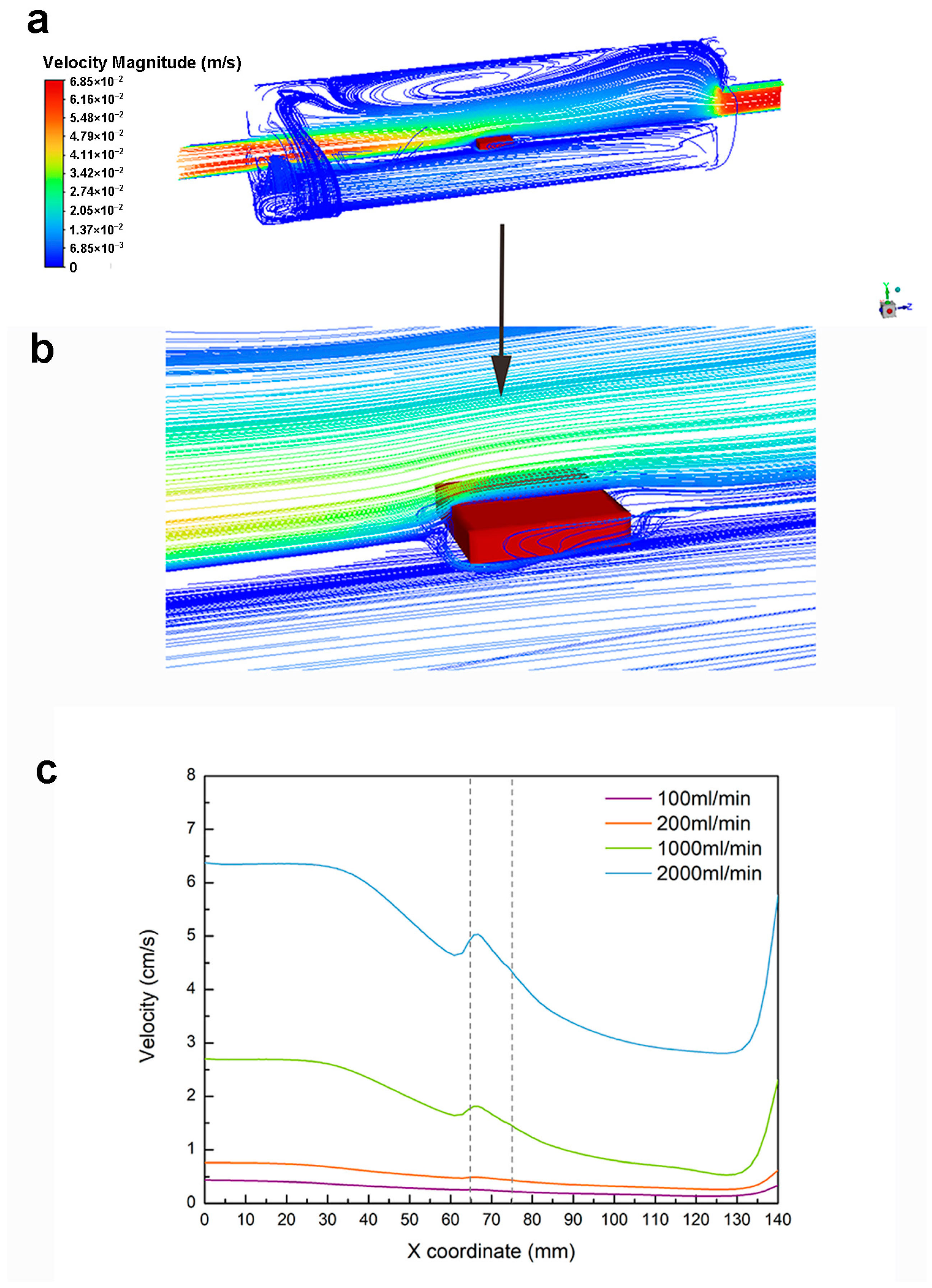
2.5. Statistical and Finite Element Analyses
3. Results
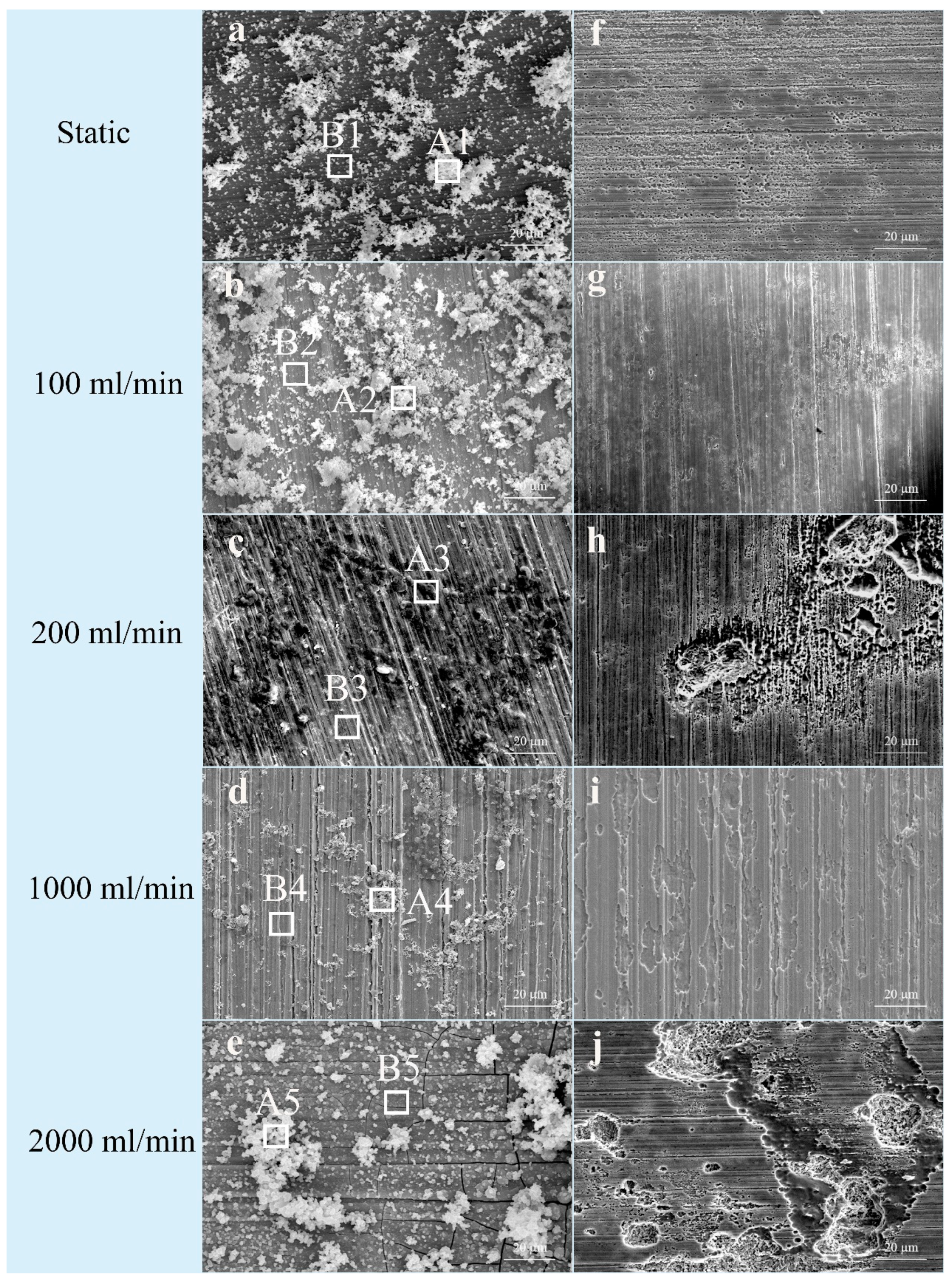
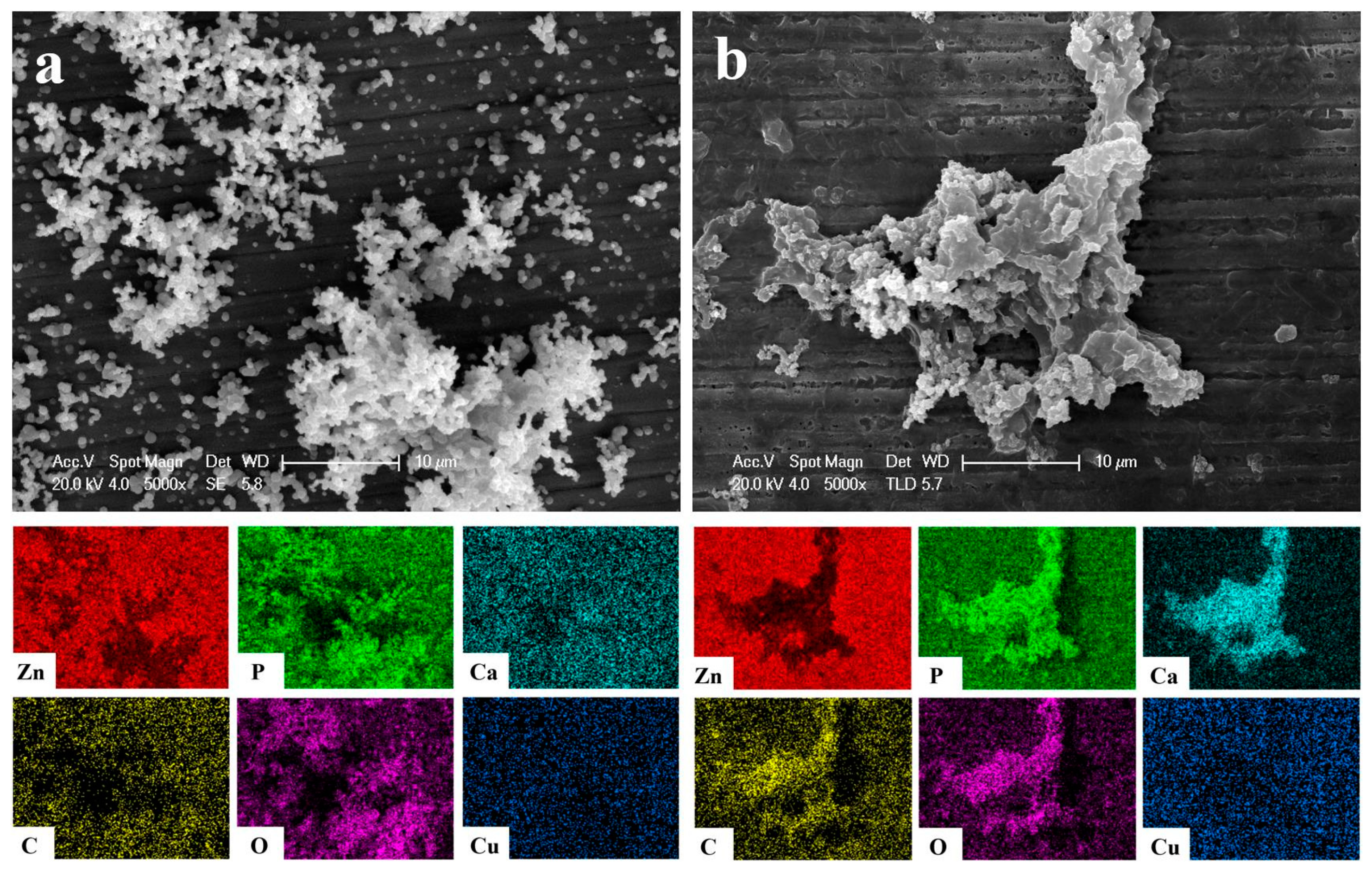
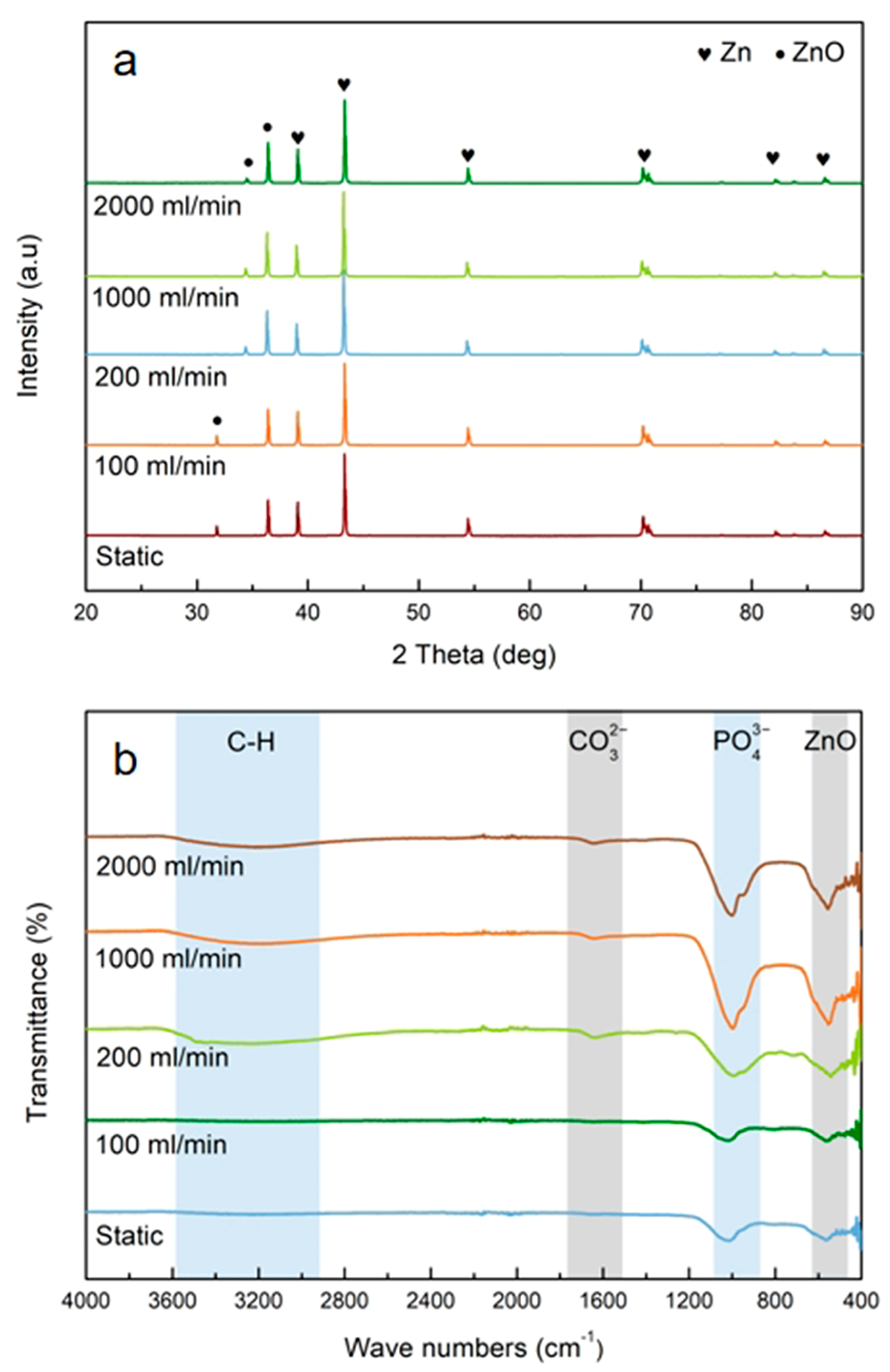
3.1. Morphology and Chemical Composition
3.2. pH and Tensile Strength
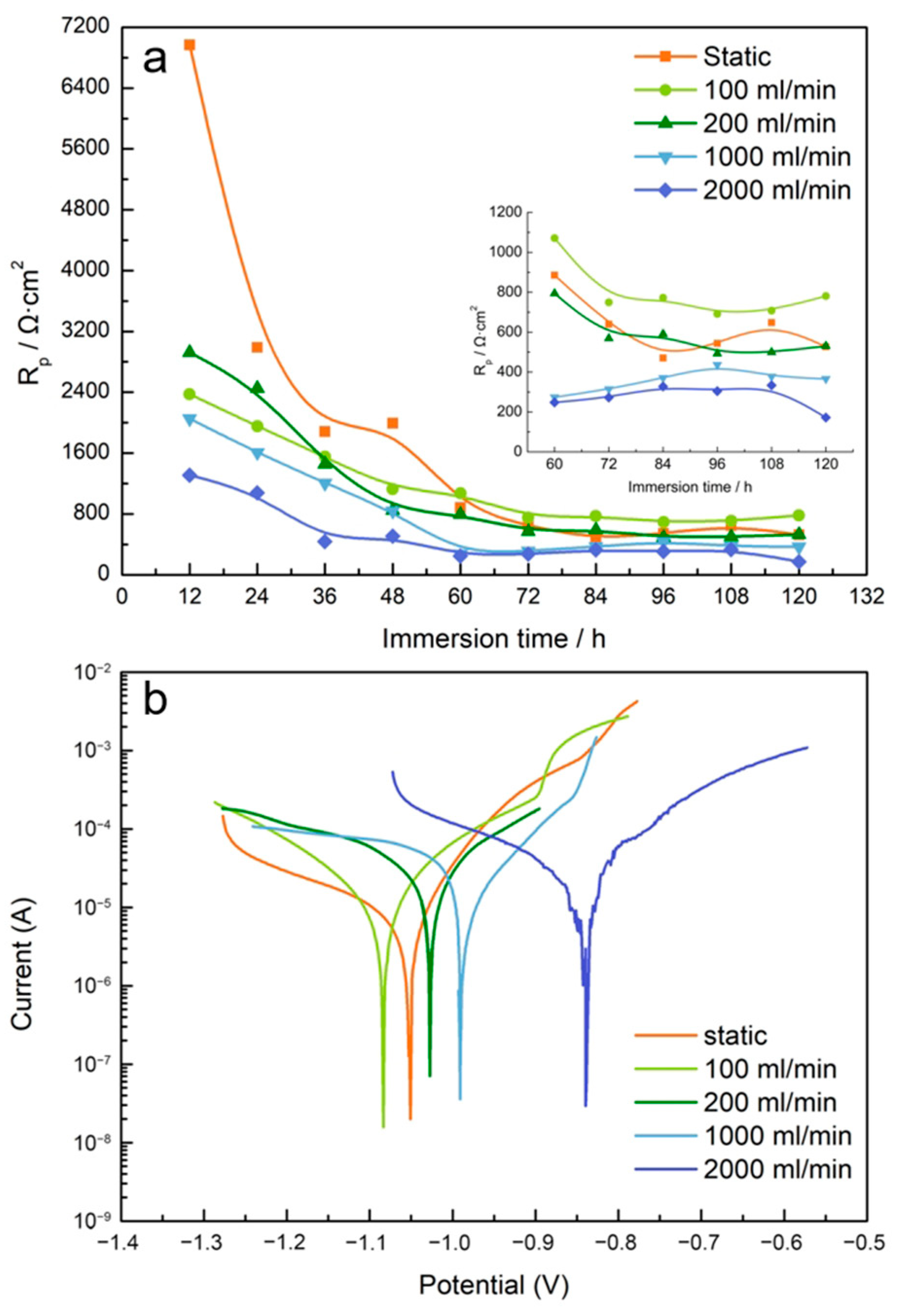
3.3. Electrochemical Assessment
4. Discussion
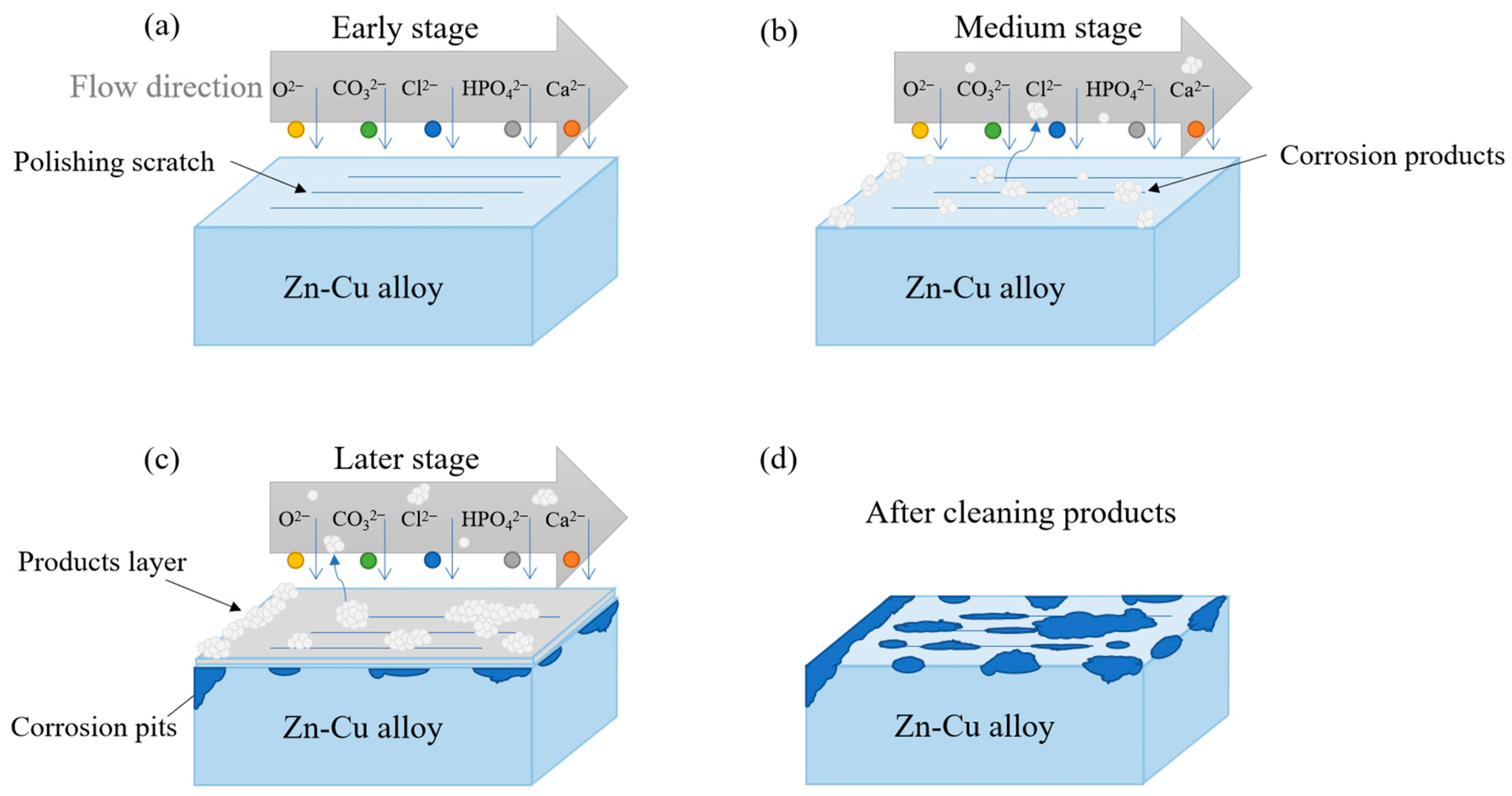
4.1. Corrosion Mechanism
4.2. Flow Rates
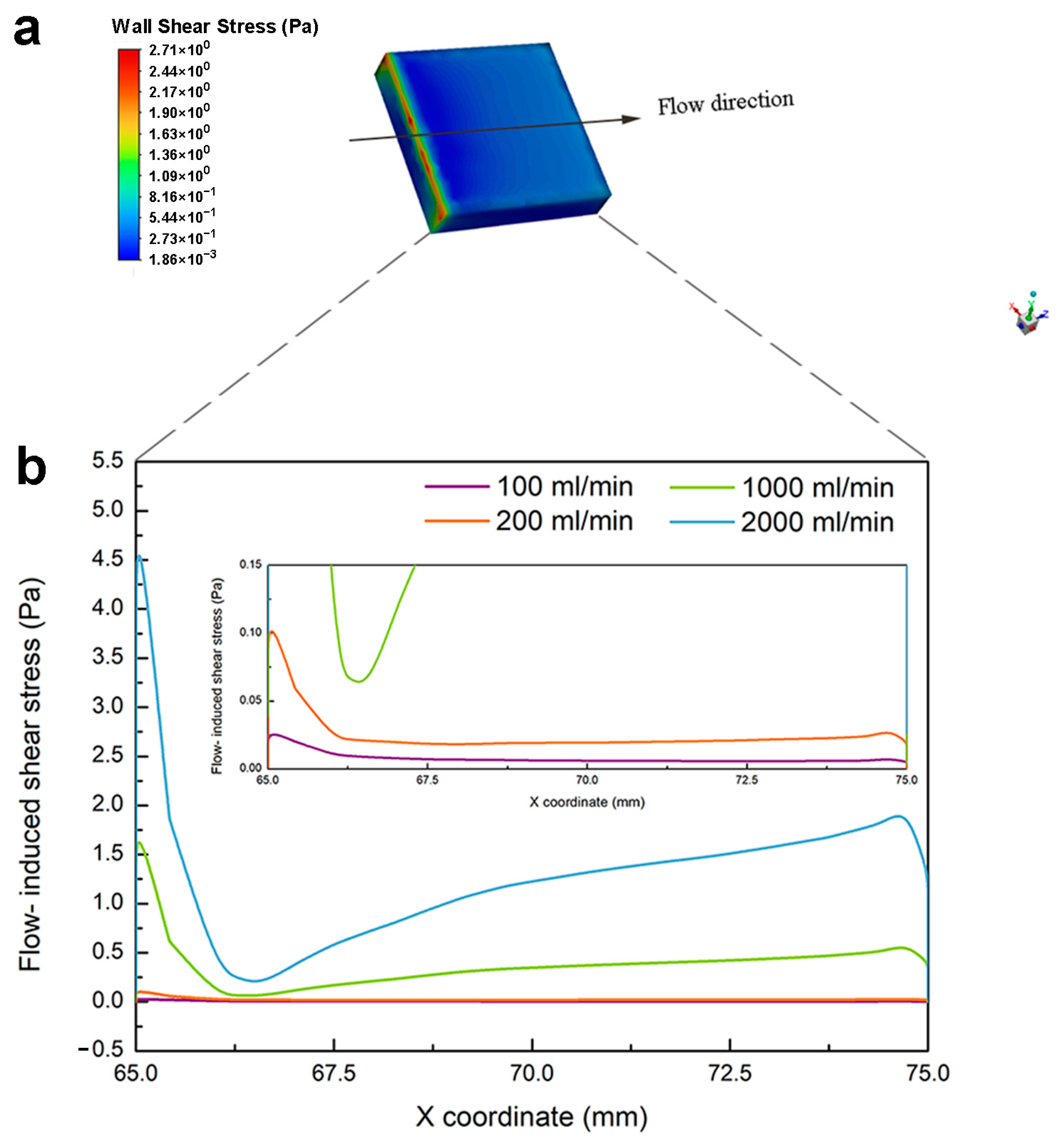
4.3. Flow-Induced Shear Stress
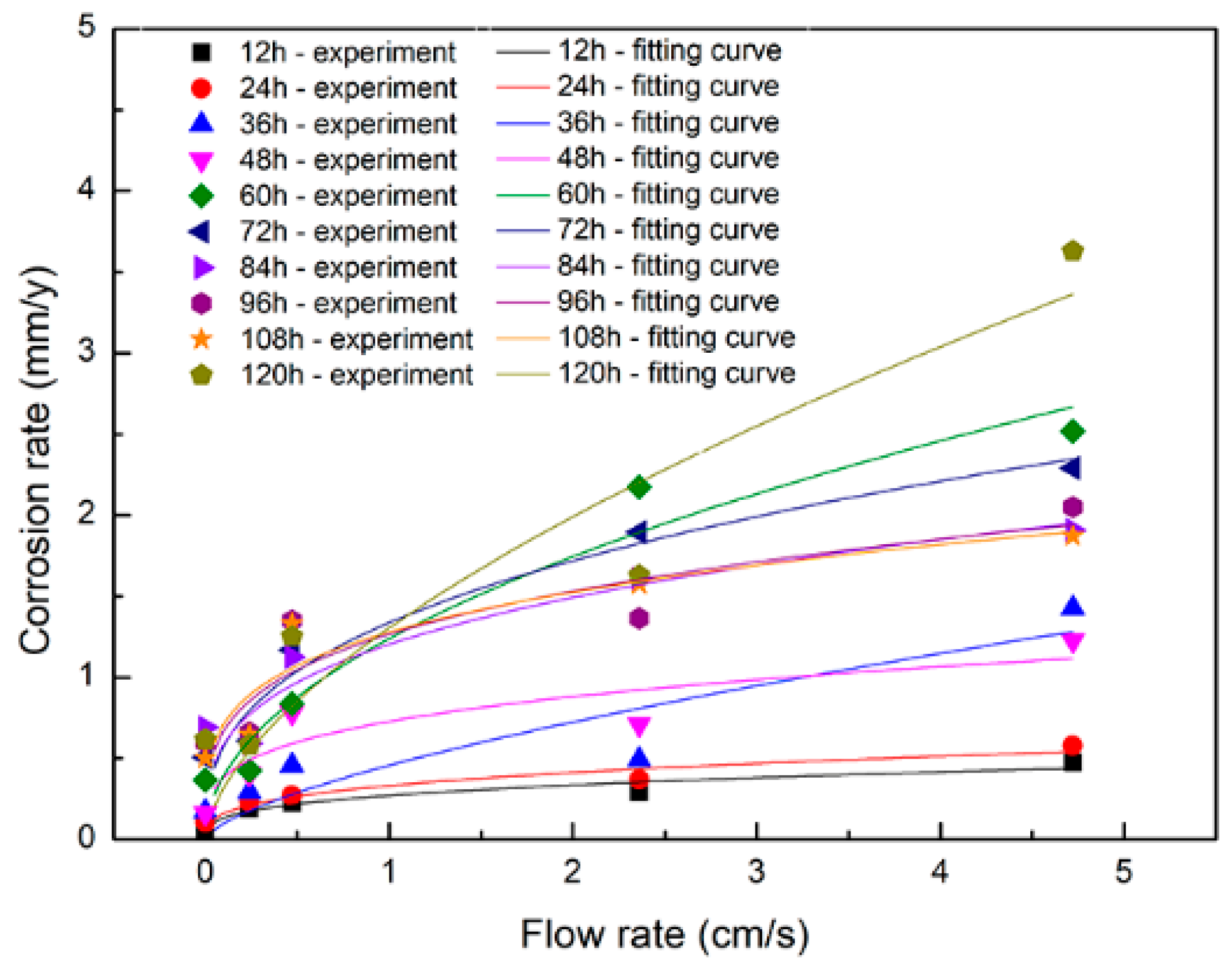
4.4. Corrosion Rates
4.5. Corrosion Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Shalawi, F.D.; Hanim, M.A.A.; Ariffin, M.K.A.; Kim, C.L.S.; Brabazon, D.; Calin, R.; Al-Osaimi, M.O. Biodegradable synthetic polymer in orthopaedic application: A review. Mater. Today Proc. 2023, 74, 540–546. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Huang, S.; Wu, W.; Su, Y.; Qiao, L.; Yan, Y. Insight into the Corrosion Behaviour and Degradation Mechanism of Pure Zinc in Simulated Body Fluid. Corros. Sci. 2021, 178, 109071. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zheng, Y.; Zhou, F.; Qiu, K.; Wang, X. Design and Characterizations of Novel Biodegradable Ternary Zn-based Alloys with IIA Nutrient Alloying Elements Mg, Ca and Sr. Mater. Des. 2015, 83, 95–102. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Dong, C.; Yan, Y.; Volinsky, A.A.; Wang, L. Initial Formation of Corrosion Products On Pure Zinc in Simulated Body Fluid. J. Mater. Sci. Technol. 2018, 34, 2271–2282. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the Degradation Mechanism of Pure Zinc Stent in the One-Year Study of Rabbit Abdominal Aorta Model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Escobar, D.; Champagne, S.; Yilmazer, H.; Dikici, B.; Boehlert, C.J.; Hermawan, H. Current status and perspectives of zinc-based absorbable alloys for biomedical applications. Acta Biomater. 2019, 97, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Drelich, J.W.; Vedani, M. Zinc-Based Alloys for Degradable Vascular Stent Applications. Acta Biomater. 2018, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zheng, Y.; Chen, X.; Yang, J.; Zhu, D.; Ruan, L.; Takashima, K. Challenges in the Use of Zinc and its Alloys as Biodegradable Metals: Perspective from Biomechanical Compatibility. Acta Biomater. 2019, 97, 23–45. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Yang, Y.; Pu, Z.; Zheng, Y. In Vitro Investigation of Ultra-Pure Zn and its Mini-Tube as Potential Bioabsorbable Stent Material. Mater. Lett. 2015, 161, 53–56. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; Rehman, I.U. A Study on the Effect of Zinc Oxide and Zinc Peroxide Nanoparticles to Enhance Angiogenesis-Pro-Angiogenic Grafts for Tissue Regeneration Applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic Zinc Exhibits Optimal Biocompatibility for Bioabsorbable Endovascular Stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of Thermal Treatment on Microstructure, Mechanical and Degradation Properties of Zn–3Mg Alloy as Potential Biodegradable Implant Material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- García-Mintegui, C.; Córdoba, L.C.; Buxadera-Palomero, J.; Marquina, A.; Jiménez-Piqué, E.; Ginebra, M.; Cortina, J.L.; Pegueroles, M. Zn-Mg and Zn-Cu Alloys for Stenting Applications: From Nanoscale Mechanical Characterization to in Vitro Degradation and Biocompatibility. Bioact. Mater. 2021, 6, 4430–4446. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Huang, H.; Niu, J.; Jin, Z.; Dargusch, M.; Yuan, G. Characterization of Nano Precipitate Phase in an As-Extruded Zn-Cu Alloy. Scripta Mater. 2021, 200, 113907. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, J.; Liu, X.; Zhang, H.; Zhang, D.; Yin, Y.; Wang, L. Effects of Ag, Cu or Ca Addition on Microstructure and Comprehensive Properties of Biodegradable Zn-0.8Mn Alloy. Mater. Sci. Eng. C 2019, 99, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Huang, H.; Niu, J.; Zhang, L.; Zhang, H.; Pei, J.; Tan, J.; Yuan, G. Design and Characterizations of Novel Biodegradable Zn-Cu-Mg Alloys for Potential Biodegradable Implants. Mater. Des. 2017, 117, 84–94. [Google Scholar] [CrossRef]
- Zhang, E.; Fu, S.; Wang, R.; Li, H.; Liu, Y.; Ma, Z.; Liu, G.; Zhu, C.; Qin, G.; Chen, D. Role of Cu Element in Biomedical Metal Alloy Design. Rare Met. 2019, 38, 476–494. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-Induced Vascular Endothelial Growth Factor Expression and Wound Healing. Am. J. Physiol.-Heart C. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef]
- Tang, Z.; Niu, J.; Huang, H.; Zhang, H.; Pei, J.; Ou, J.; Yuan, G. Potential Biodegradable Zn-Cu Binary Alloys Developed for Cardiovascular Implant Applications. J. Mech. Behav. Biomed. 2017, 72, 182–191. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-Containing Mesoporous Bioactive Glass Scaffolds with Multifunctional Properties of Angiogenesis Capacity, Osteostimulation and Antibacterial Activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Tang, Z.; Huang, H.; Pei, J.; Zhang, H.; Yuan, G.; Ding, W. Research on a Zn-Cu Alloy as a Biodegradable Material for Potential Vascular Stents Application. Mater. Sci. Eng. C 2016, 69, 407–413. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Dai, J.; Xepapadeas, A.B.; Schweizer, E.; Alexander, D.; Scheideler, L.; Zhou, C.; Zhang, H.; Wan, G.; et al. Investigation of Zinc-Copper Alloys as Potential Materials for Craniomaxillofacial Osteosynthesis Implants. Mater. Sci. Eng. C 2019, 103, 109826. [Google Scholar] [CrossRef]
- Hybasek, V.; Kubasek, J.; Capek, J.; Alferi, D.; Pinc, J.; Jiru, J.; Fojt, J. Influence of Model Environment Complexity On Corrosion Mechanism of Biodegradable Zinc Alloys. Corros. Sci. 2021, 187, 109520. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; Jia, Q.; Bian, D.; Guan, S.; Kulyasova, O.; Valiev, R.Z.; Rau, J.V.; Zheng, Y. Recent Advances On the Mechanical Behavior of Zinc Based Biodegradable Metals Focusing On the Strain Softening Phenomenon. Acta Biomater. 2022, 152, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Králová, Z.O.; Gorejová, R.; Oriňaková, R.; Petráková, M.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Sopčák, T.; Baláž, M.; Maskaľová, I.; et al. Biodegradable Zinc-Iron Alloys: Complex Study of Corrosion Behavior, Mechanical Properties and Hemocompatibility. Prog. Nat. Sci. Mater. Int. 2021, 31, 279–287. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In Vitro and in Vivo Studies on Zinc-Hydroxyapatite Composites as Novel Biodegradable Metal Matrix Composite for Orthopedic Applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Han, L.; Li, X.; Xue, F.; Chu, C.; Bai, J. Biocorrosion Behavior of Micro-Arc-Oxidized AZ31 Magnesium Alloy in Different Simulated Dynamic Physiological Environments. Surf. Coat. Technol. 2019, 361, 240–248. [Google Scholar] [CrossRef]
- Tian, J.; Huang, H.; Pan, Z.; Zhou, H. Effect of Flow Velocity on Corrosion Behavior of AZ91D Magnesium Alloy at Elbow of Loop System. T. Nonferr. Metal. Soc. 2016, 26, 2857–2867. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Wang, J.; Tang, X. Effect of High Flow Velocity on Corrosion Behavior of Ni Based and Ni-Fe Based Alloys in Supercritical Water Oxidation Environment. J. Supercrit. Fluids 2021, 170, 105126. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xing, X.; He, S.; Zhang, L.; Lu, M. Effects of Flow Velocity on the Corrosion Behaviour of Super 13Cr Stainless Steel in ultra-HTHP CO2–H2S Coexistence Environment. Corros. Sci. 2022, 200, 110235. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Maitz, M.F.; Chen, M.; Zhang, H.; Mao, J.; Zhao, Y.; Huang, N.; Wan, G. Comparative Corrosion Behavior of Zn with Fe and Mg in the Course of Immersion Degradation in Phosphate Buffered Saline. Corros. Sci. 2016, 111, 541–555. [Google Scholar] [CrossRef]
- Li, L.; Jiao, H.; Liu, C.; Yang, L.; Suo, Y.; Zhang, R.; Liu, T.; Cui, J. Microstructures, Mechanical Properties and in Vitro Corrosion Behavior of Biodegradable Zn Alloys Microalloyed with Al, Mn, Cu, Ag and Li Elements. J. Mater. Sci. Technol. 2022, 103, 244–260. [Google Scholar] [CrossRef]
- Liu, L.; Meng, Y.; Volinsky, A.A.; Zhang, H.; Wang, L. Influences of Albumin On in Vitro Corrosion of Pure Zn in Artificial Plasma. Corros. Sci. 2019, 153, 341–356. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of Zinc in Saline Solutions, Plasma, and Whole Blood. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wu, Y.; Maitz, M.F.; Wang, Z.; Koo, Y.; Zhao, A.; Sankar, J.; Kong, D.; Huang, N.; et al. Ex Vivo Blood Vessel Bioreactor for Analysis of the Biodegradation of Magnesium Stent Models with and without Vessel Wall Integration. Acta Biomater. 2017, 50, 546–555. [Google Scholar] [CrossRef]
- Wang, J.; Smith, C.E.; Sankar, J.; Yun, Y.; Huang, N. Absorbable Magnesium-Based Stent: Physiological Factors to Consider for in Vitro Degradation Assessments. Regen. Biomater. 2015, 2, 59–69. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and their Limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Chen, J.; Dai, J.; Bai, J.; Huang, Z.; Guo, C.; Xue, F.; Han, L.; Chu, C. Effects of Different Concentrations of BSA on in Vitro Corrosion Behavior of Pure Zinc in Artificial Plasma. ACS Biomater. Sci. Eng. 2022, 8, 4365–4376. [Google Scholar] [CrossRef] [PubMed]


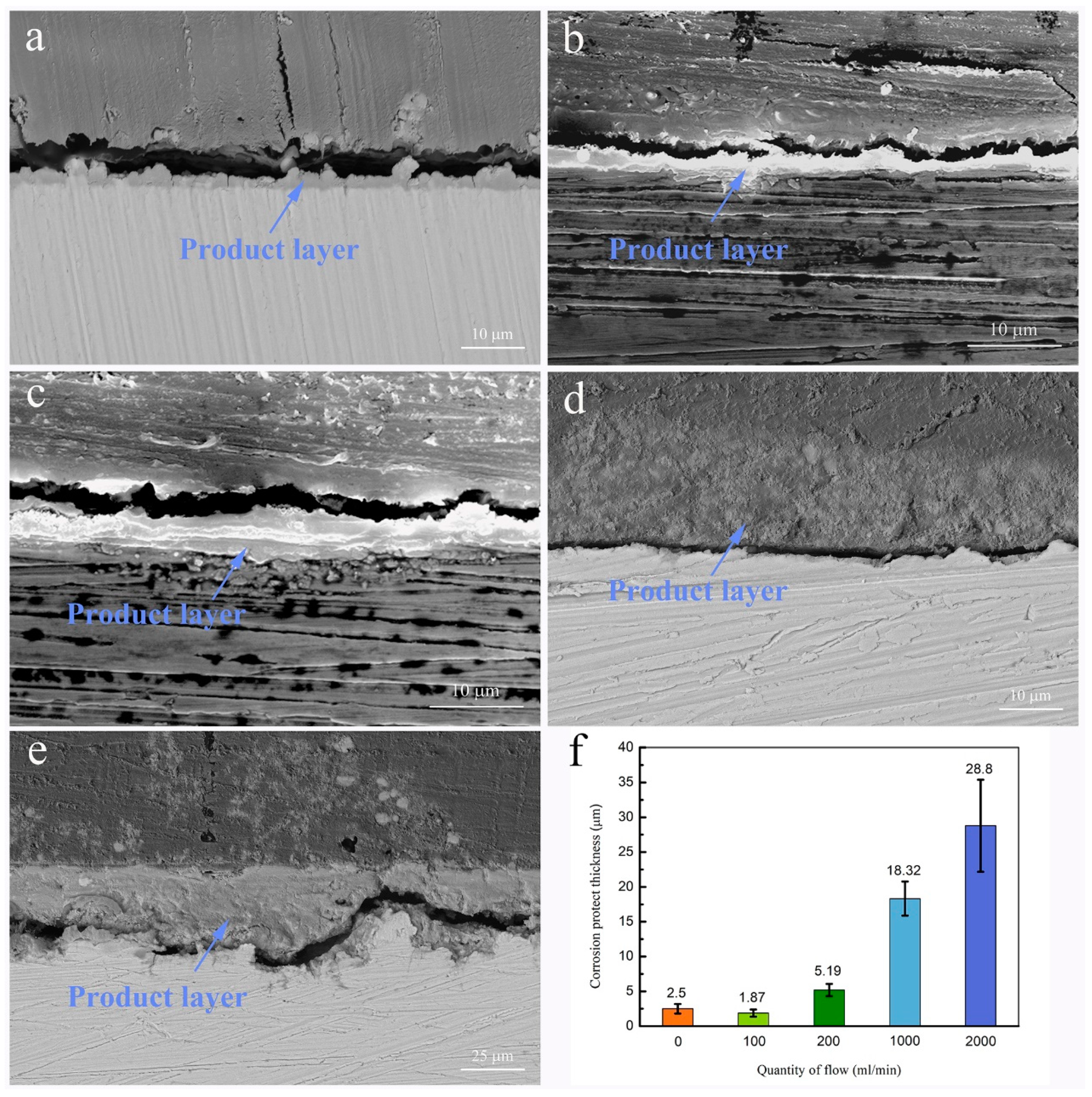
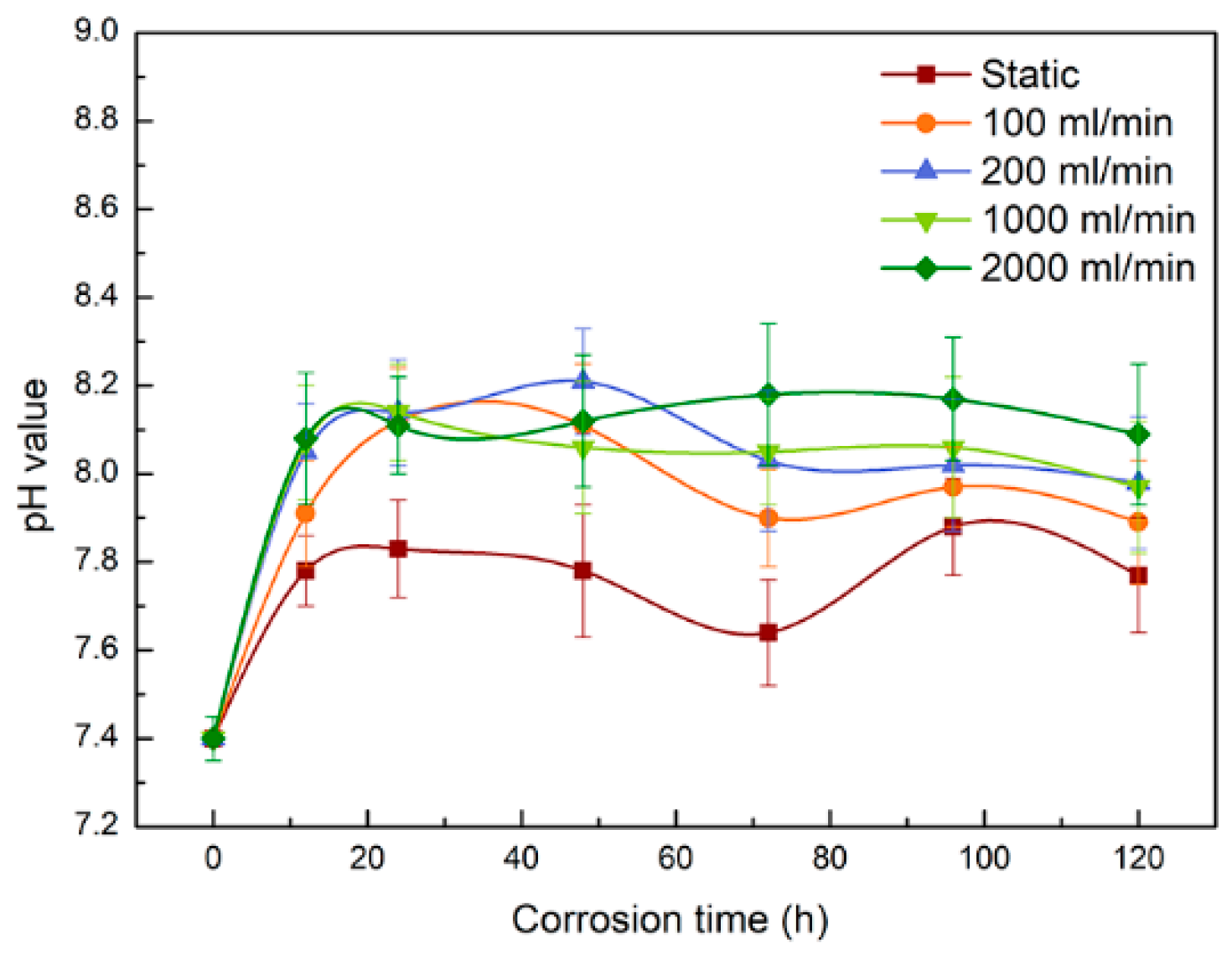
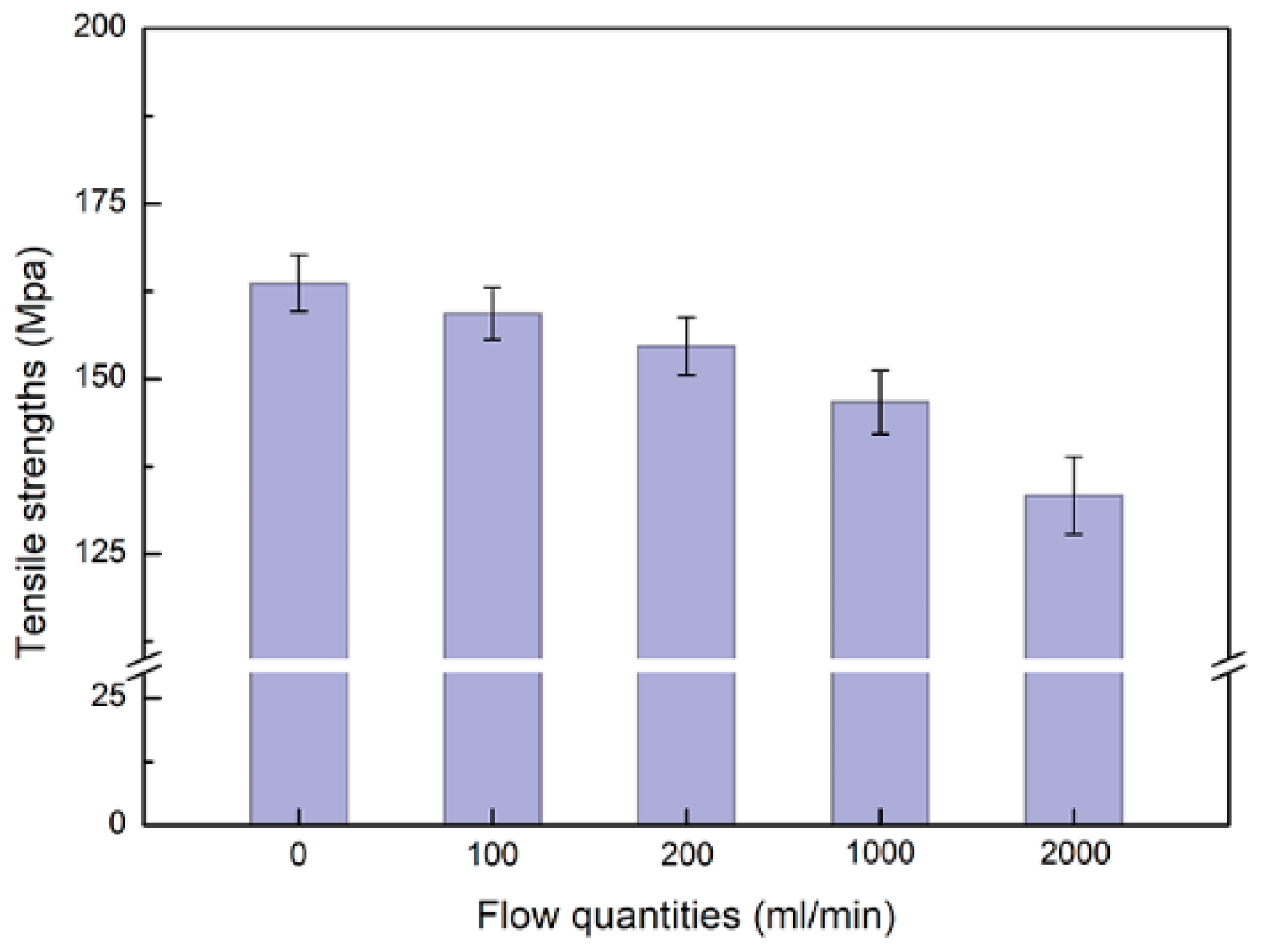
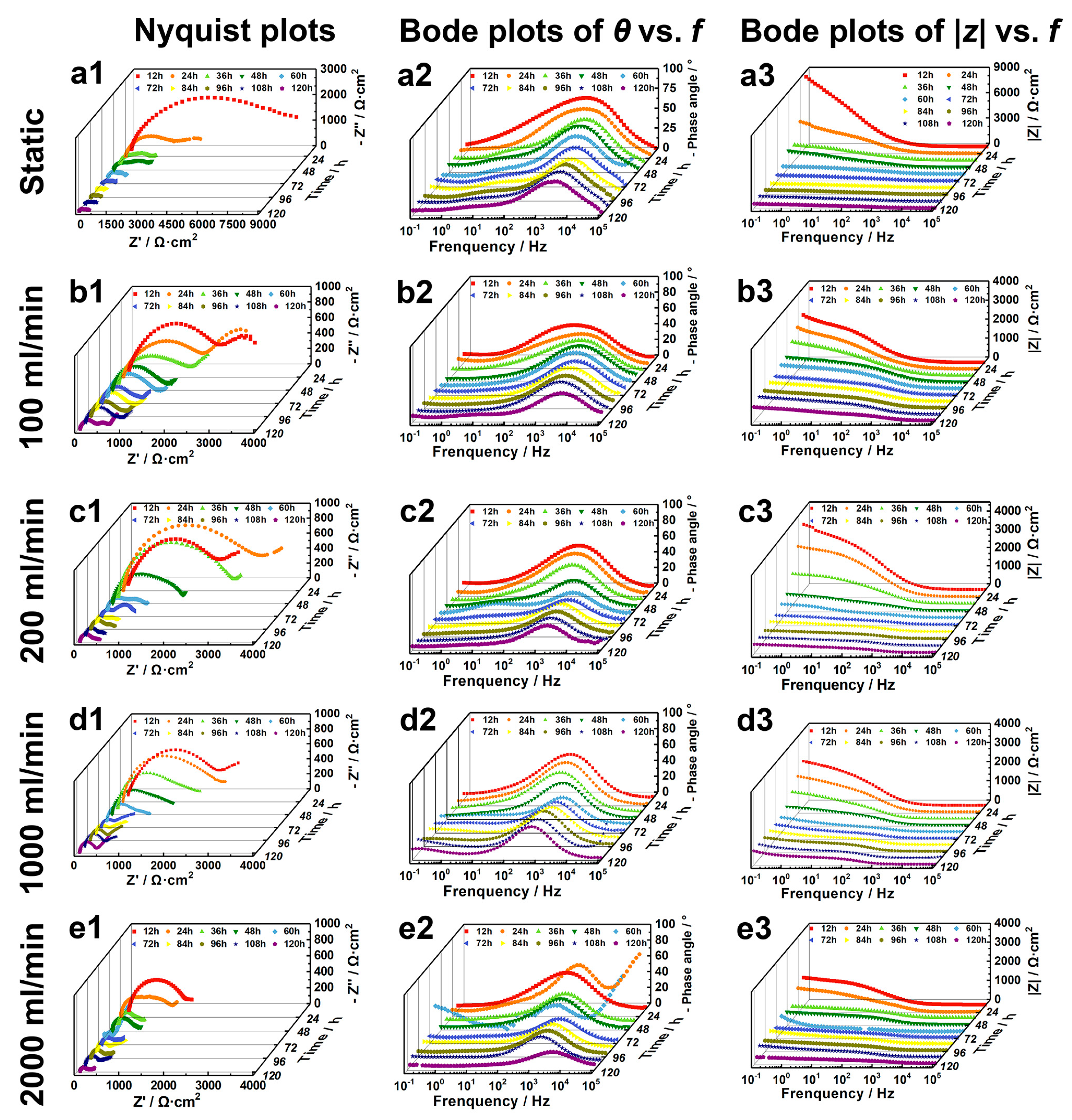

| Reagents | Composition (g·L−1) |
|---|---|
| NaCl | 6.85 |
| KCl | 0.27 |
| MgSO4·7H2O | 0.04 |
| KH2PO4 | 0.02 |
| NaHCO3 | 0.35 |
| Na2HPO4·H2O | 0.04 |
| CaCl2 | 0.65 |
| Glucose | 0.28 |
| Flow Quantities (mL/min) | Ecorr (V) | icorr (μA/cm) | ba (mV) | bc (mV) | CR (mm/y) |
|---|---|---|---|---|---|
| 0 | −1.05 ± 0.01 | 6.49 ± 1.38 | 149.45 ± 22.15 | 75.21 ± 19.11 | 0.097 ± 0.02 |
| 100 | −1.08 ± 0.02 | 14.87 ± 1.22 | 160.78 ± 36.23 | 125.11 ± 30.74 | 0.22 ± 0.02 |
| 200 | −1.03 ± 0.01 | 31.00 ± 0.93 | 167.35 ± 29.16 | 263.55 ± 35.87 | 0.46 ± 0.01 |
| 1000 | −0.99 ± 0.02 | 35.04 ± 1.55 | 292.72 ± 42.40 | 93.42 ± 33.21 | 0.52 ± 0.02 |
| 2000 | −0.84 ± 0.02 | 36.09 ± 0.91 | 302.75 ± 38.36 | 140.97 ± 28.39 | 0.54 ± 0.01 |
| Corrosion Time (h) | α | β |
|---|---|---|
| 12 | 0.27 | 0.31 |
| 24 | 0.33 | 0.31 |
| 36 | 0.64 | 0.51 |
| 48 | 0.58 | 0.43 |
| 60 | 1.24 | 0.50 |
| 72 | 1.37 | 0.36 |
| 84 | 1.20 | 0.31 |
| 96 | 1.05 | 0.41 |
| 108 | 1.12 | 0.35 |
| 120 | 1.62 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, L.; Han, L.; Bai, J.; Huang, Z.; Guo, C.; Xue, F.; Chu, P.K.; Chu, C. Effects of Dynamic Flow Rates on the In Vitro Bio-Corrosion Behavior of Zn-Cu Alloy. Coatings 2024, 14, 711. https://doi.org/10.3390/coatings14060711
Zhang X, Zhang L, Han L, Bai J, Huang Z, Guo C, Xue F, Chu PK, Chu C. Effects of Dynamic Flow Rates on the In Vitro Bio-Corrosion Behavior of Zn-Cu Alloy. Coatings. 2024; 14(6):711. https://doi.org/10.3390/coatings14060711
Chicago/Turabian StyleZhang, Xin, Lu Zhang, Linyuan Han, Jing Bai, Zhihai Huang, Chao Guo, Feng Xue, Paul K. Chu, and Chenglin Chu. 2024. "Effects of Dynamic Flow Rates on the In Vitro Bio-Corrosion Behavior of Zn-Cu Alloy" Coatings 14, no. 6: 711. https://doi.org/10.3390/coatings14060711




