Analyzing the Debinding Step of Ti64 Parts Fabricated by 3D Printing Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and 3D Print
2.2. Debinding and Sintering
2.3. Sample Characterization
2.4. Mechanical Properties Testing
2.5. Corrosion Tests
3. Results
3.1. Debinding and Sintering Analysis
3.2. Sample Characterization
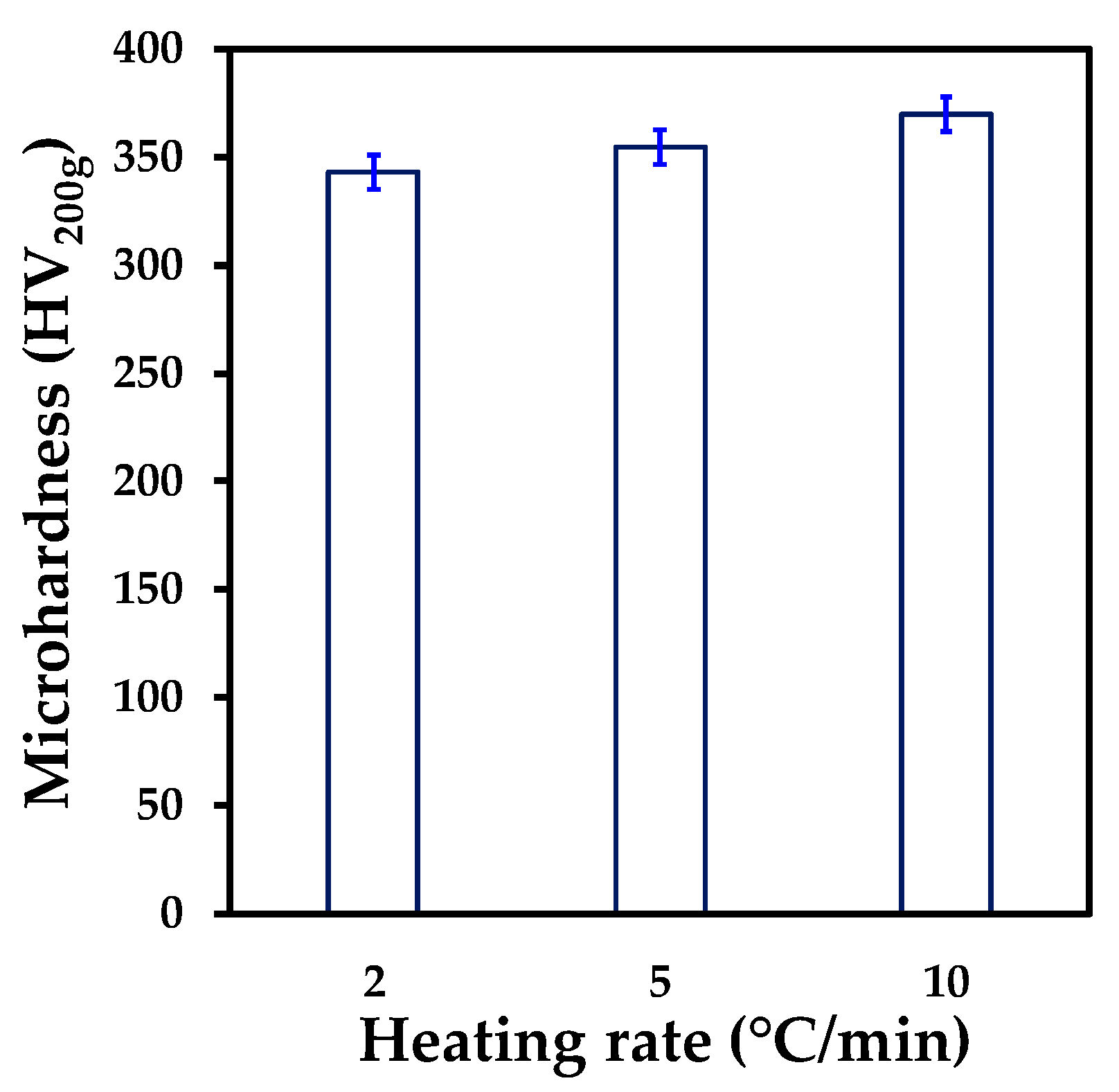
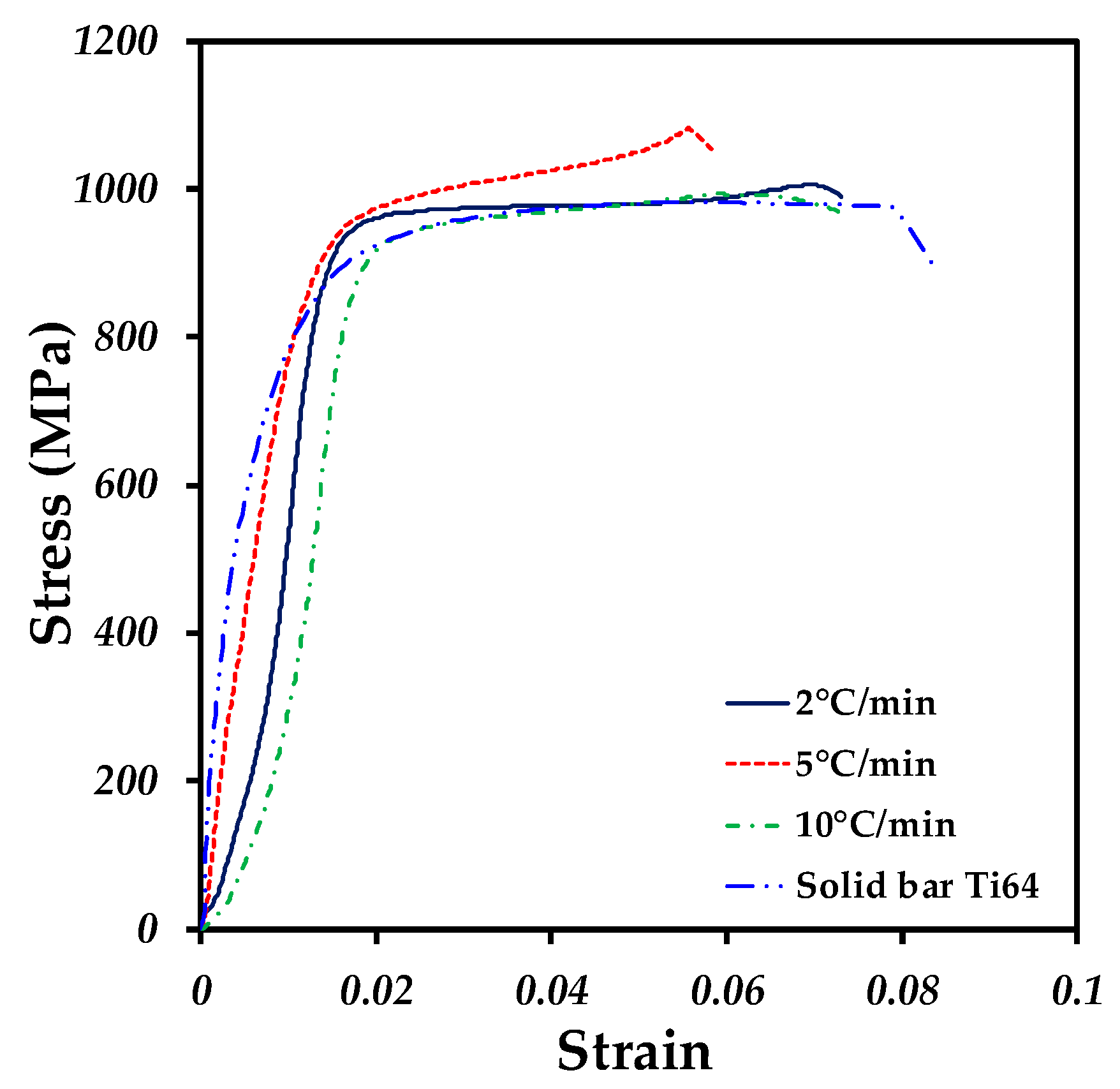
3.3. Mechanical Properties
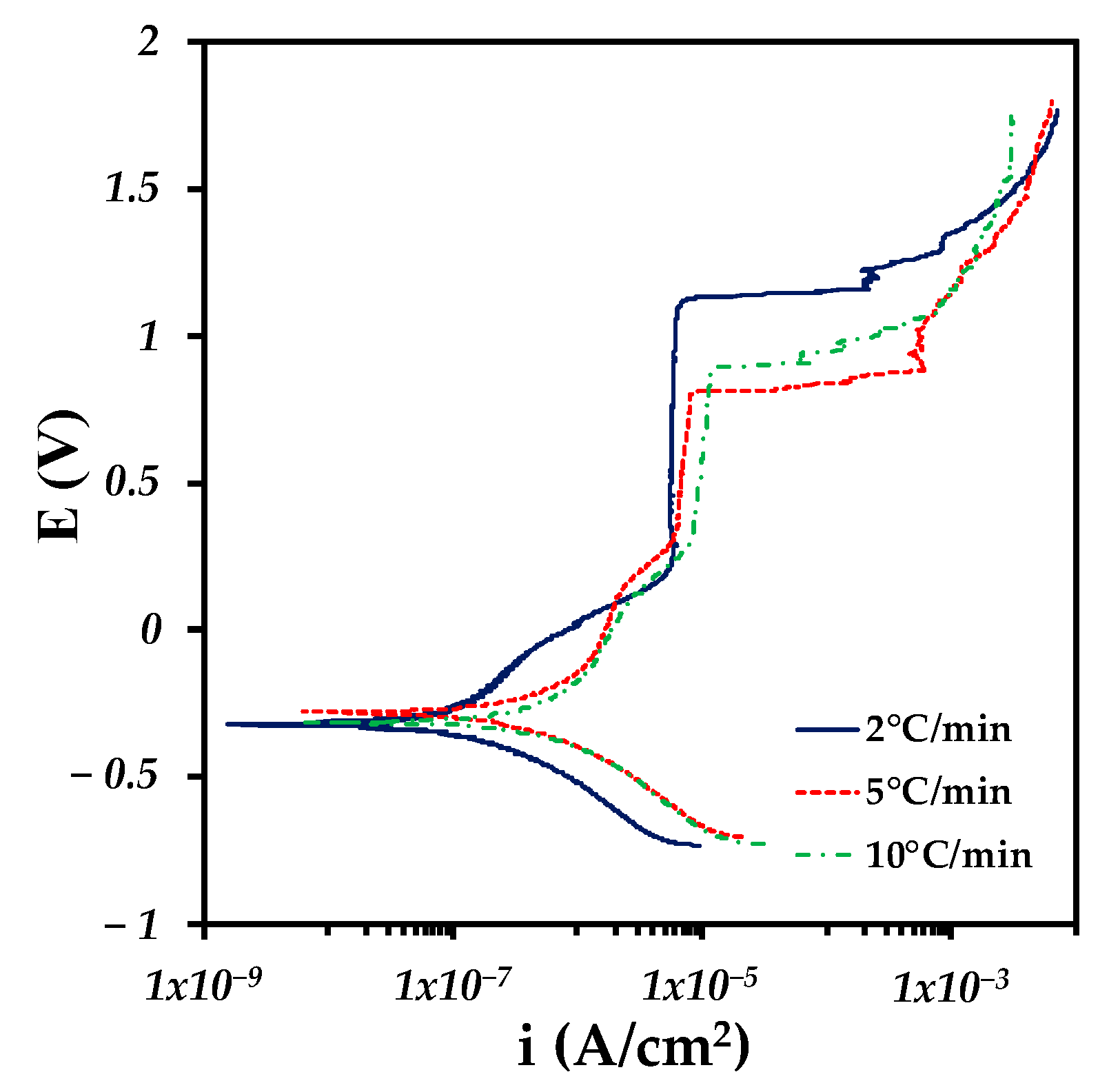
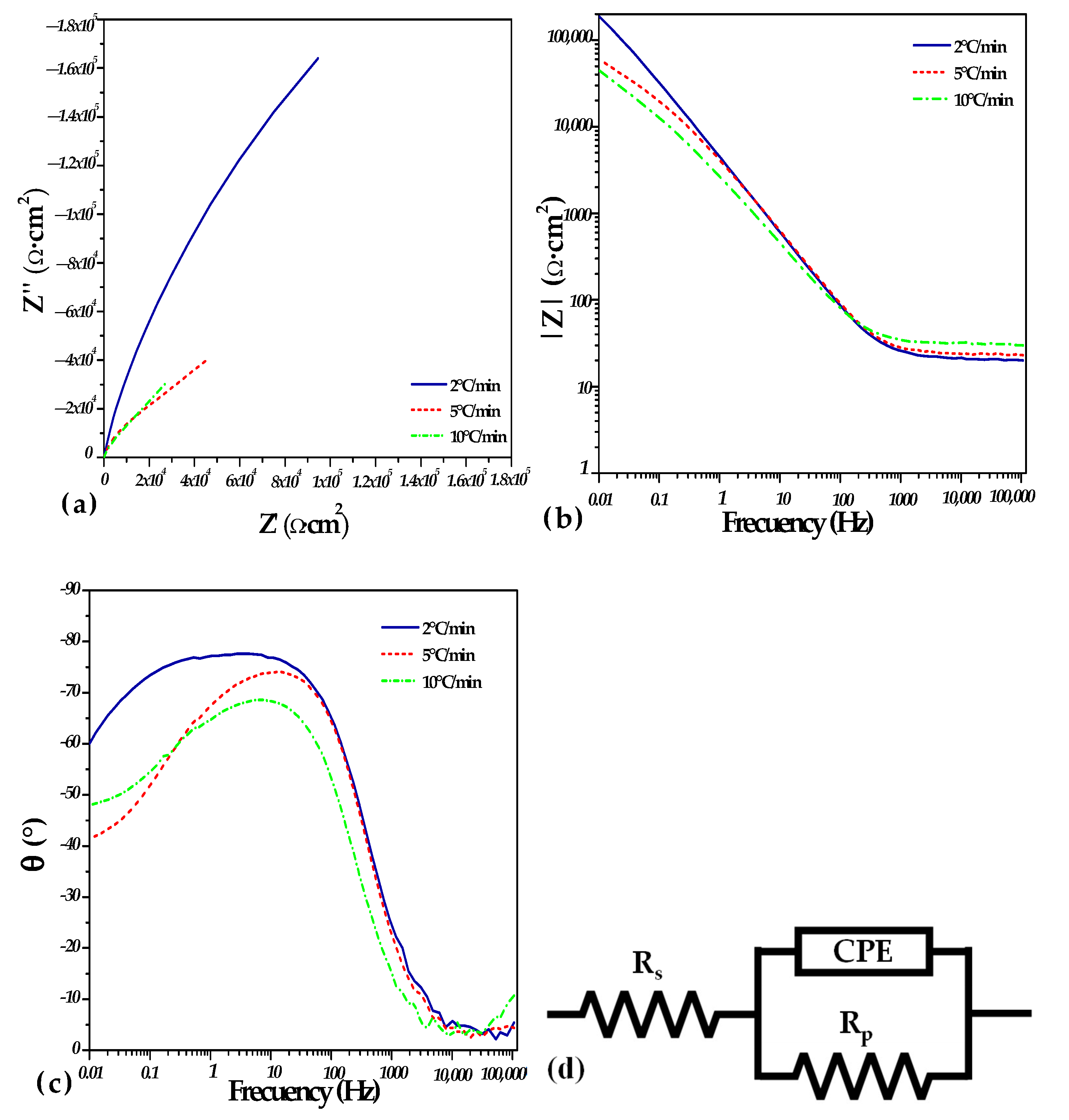
3.4. Corrosion Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koju, N.; Niraula, S.; Fotovvati, B. Additively manufactured porous Ti6Al4V for bone implants: A review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Alvarado-Hernández, F.; Mihalcea, E.; Jimenez, O.; Macías, R.; Olmos, L.; López-Baltazar, E.A.; Guevara-Martinez, S.; Lemus-Ruiz, J. Design of Ti64/Ta Hybrid Materials by Powder Metallurgy Mimicking Bone Structure. Materials 2023, 16, 4372. [Google Scholar] [CrossRef]
- Huang, S.; Wei, H.; Li, D. Additive manufacturing technologies in the oral implant clinic: A review of current applications and progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Song, Y.; Ghafari, Y.; Asefnejad, A.; Toghraie, D. An overview of selective laser sintering 3D printing technology for biomedical and sports device applications: Processes, materials, and applications. Opt. Laser Technol. 2024, 171, 110459. [Google Scholar] [CrossRef]
- Stojković, J.R.; Stojković, M.; Turudija, R.; Aranđelović, J.; Marinkovic, D. Adjustable elasticity of anatomically shaped lattice bone scaffold built by electron beam melting Ti6Al4V powder. Metals 2023, 13, 1522. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Zhou, S.; Luo, Z. Powder bed fusion manufacturing of beta-type titanium alloys for biomedical implant applications: A review. J. Alloys Compd. 2023, 936, 168099. [Google Scholar] [CrossRef]
- Joshua, R.J.N.; Raj, S.A.; Hameed Sultan, M.T.; Łukaszewicz, A.; Józwik, J.; Oksiuta, Z.; Dziedzic, K.; Tofil, A.; Shahar, F.S. Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review. Materials 2024, 17, 769. [Google Scholar] [CrossRef]
- Pothala, S.; Raju, M.J. Recent advances of metallic bio-materials in additive manufacturing in biomedical implants—A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Kolli, S.; Beretta, M.; Selema, A.; Sergeant, P.; Kestens, L.A.; Rombouts, M.; Vleugels, J. Process optimization and characterization of dense pure copper parts produced by paste-based 3D micro-extrusion. Addit. Manuf. 2023, 73, 103670. [Google Scholar] [CrossRef]
- Sakib-Uz-Zaman, C.; Khondoker, M.A.H. A Review on Extrusion Additive Manufacturing of Pure Copper. Metals 2023, 13, 859. [Google Scholar] [CrossRef]
- Coffigniez, M.; Gremillard, L.; Boulnat, X. Sinter-Based Additive Manufacturing of Graded Porous Titanium Scaffolds by Multi-Inks 3D Extrusion. Adv. Eng. Mater. 2023, 25, 2201159. [Google Scholar] [CrossRef]
- Waalkes, L.; Längerich, J.; Imgrund, P.; Emmelmann, C. Piston-based material extrusion of Ti-6Al-4V feedstock for complementary use in metal injection molding. Materials 2022, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Y.; Polzer, M.; Gonzalez-Gutierrez, J.; Kasian, O.; Heckl, J.P.; Dalbauer, V.; Kukla, C.; Felfer, P.J. Fused Filament Fabrication-Based Additive Manufacturing of Commercially Pure Titanium. Adv. Eng. Mater. 2021, 23, 2100380. [Google Scholar] [CrossRef]
- Singh, P.; Balla, V.K.; Tofangchi, A.; Atre, S.V.; Kate, K.H. Printability studies of Ti-6Al-4V by metal fused filament fabrication (MF3). Int. J. Refract. Met. Hard Mater. 2020, 91, 105249. [Google Scholar] [CrossRef]
- Singh, P.; Balla, V.K.; Atre, S.V.; German, R.M.; Kate, K.H. Factors affecting properties of Ti-6Al-4V alloy additive manufactured by metal fused filament fabrication. Powder Technol. 2021, 386, 9–19. [Google Scholar] [CrossRef]
- Costa, J.M.; Sequeiros, E.W.; Vieira, M.F. Fused Filament Fabrication for Metallic Materials: A Brief Review. Materials 2023, 16, 7505. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Gillon, F.; Najjar, D.; Benabou, A.; Witz, J.F.; Hecquet, M.; Quaegebeur, P.; Meersdam, M.; Auzene, D. MIM-like additive manufacturing of Fe3% Si magnetic materials. J. Magn. Magn. Mater. 2022, 564, 170104. [Google Scholar] [CrossRef]
- Xu, T.; Long, F.; Liang, Y.; Zhang, H.; Shi, S.; Cheng, Y.; Xu, G.; Li, Z.; Ge, Y. 3D extrusion printing of 304 stainless steel/polypropylene composites and sintering process optimization. Appl. Phys. A 2023, 129, 285. [Google Scholar] [CrossRef]
- Ramazani, H.; Kami, A. Metal FDM, a new extrusion-based additive manufacturing technology for manufacturing of metallic parts: A review. Prog. Addit. Manuf. 2022, 7, 609–626. [Google Scholar] [CrossRef]
- Bakan, H.I.; Jumadi, Y.; Messer, P.F.; Davies, H.A.; Ellis, B. Study of processing parameters for MIM feedstock based on composite PEG-PMMA binder. Powder Metall. 1998, 41, 289–291. [Google Scholar] [CrossRef]
- Páez-Pavón, A.; Jiménez-Morales, A.; Santos, T.G.; Quintino, L.; Torralba, J.M. Influence of thermal debinding on the final properties of Fe–Si soft magnetic alloys for metal injection molding (MIM). J. Magn. Magn. Mater. 2016, 416, 342–347. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef]
- Seerane, M.; Chikwanda, H.K.; Machaka, R. Determination of optimum process for thermal debinding and sintering using Taguchi method. Mater. Sci. Forum 2015, 828–829, 138–144. [Google Scholar] [CrossRef]
- Singh, P.; Balla, V.K.; Gokce, A.; Atre, S.V.; Kate, K.H. Additive manufacturing of Ti-6Al-4V alloy by metal fused filament fabrication (MF 3): Producing parts comparable to that of metal injection molding. Prog. Addit. Manuf. 2021, 6, 593–606. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Ecker, J.V.; Kitzmantel, M.; Arbeiter, F.; Kukla, C.; Holzer, C. Bending properties of lightweight copper specimens with different infill patterns produced by material extrusion additive manufacturing, solvent debinding and sintering. Appl. Sci. 2021, 11, 7262. [Google Scholar] [CrossRef]
- Singh, G.; Missiaen, J.M.; Bouvard, D.; Chaix, J.M. Copper additive manufacturing using MIM feedstock: Adjustment of printing, debinding, and sintering parameters for processing dense and defectless parts. Int. J. Adv. Manuf. Technol. 2021, 115, 449–462. [Google Scholar] [CrossRef]
- Singh, G.; Missiaen, J.M.; Bouvard, D.; Chaix, J.M. Additive manufacturing of 17–4 PH steel using metal injection molding feedstock: Analysis of 3D extrusion printing, debinding and sintering. Addit. Manuf. 2021, 47, 102287. [Google Scholar] [CrossRef]
- Enneti, R.K.; Park, S.J.; German, R.M.; Atre, S.V. Thermal debinding process in particulate materials processing. Mater. Manuf. Process. 2012, 27, 103–118. [Google Scholar] [CrossRef]
- Singh, G.; Missiaen, J.M.; Bouvard, D.; Chaix, J.M. Copper extrusion 3D printing using metal injection moulding feedstock: Analysis of process parameters for green density and surface roughness optimization. Addit. Manuf. 2021, 38, 101778. [Google Scholar] [CrossRef]
- Obasi, G.C.; Ferri, O.M.; Ebel, T.; Bormann, R. Influence of processing parameters on mechanical properties of Ti–6Al–4V alloy fabricated by MIM. Mater. Sci. Eng. A 2010, 527, 3929–3935. [Google Scholar] [CrossRef]
- Olmos, L.; Bouvard, D.; Cabezas-Villa, J.L.; Lemus-Ruiz, J.; Jiménez, O.; Arteaga, D. Analysis of compression and permeability behavior of porous Ti6Al4V by computed microtomography. Met. Mater. Int. 2019, 25, 669–682. [Google Scholar] [CrossRef]
- Elmoutaouakkil, A.; Salvo, L.; Maire, E.; Peix, G. 2D and 3D Characterization of Metal Foams Using X-ray Tomography. Adv. Eng. Mater. 2002, 4, 803–807. [Google Scholar]
- Tischel, F.; Reineke, L.; Alrashdan, J.; Ploshikhin, V. Modelling Sintering Densification of Binder Jetted Ti-6al-4v Samples. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4572381 (accessed on 10 May 2024).
- Kim, Y.; Lee, J.; Lee, B.; Ryu, H.J.; Hong, S.H. Dilatometric analysis and microstructural investigation of the sintering mechanisms of blended elemental Ti-6Al-4V powders. Metall. Mater. Trans. A 2016, 47, 4616–4624. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Lemus-Ruiz, J.; Bouvard, D.; Jiménez, O.; Vergara-Hernández, H.J.; Olmos, L. Sintering study of Ti6Al4V powders with different particle sizes and their mechanical properties. Int. J. Miner. Metall. Mater. 2018, 25, 1389–1401. [Google Scholar] [CrossRef]
- Cherkaoui, M.; Capolungo, L. Atomistic and Continuum Modeling of Nanocrystalline Materials; Springer Series in Materials Science; Springer: New York, NY, USA, 2009; Volume 112, pp. 1–27. [Google Scholar]
- Gibson, L.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Ye, S.; Mo, W.; Lv, Y.; Wang, Z.; Kwok, C.T.; Yu, P. The technological design of geometrically complex Ti-6Al-4V parts by metal injection molding. Appl. Sci. 2019, 9, 1339. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, S.; Riede, M.; Garratt, E.; Roch, A. A comprehensive study on fused filament fabrication of Ti-6Al-4V structures. Addit. Manuf. 2020, 34, 101256. [Google Scholar] [CrossRef]
- Chen, L.Y.; Zhang, H.Y.; Zheng, C.; Yang, H.Y.; Qin, P.; Zhao, C.; Lu, S.; Liang, S.X.; Chai, L.; Zhang, L.C. Corrosion behavior and characteristics of passive films of laser powder bed fusion produced Ti–6Al–4V in dynamic Hank’s solution. Mater. Des. 2021, 208, 109907. [Google Scholar] [CrossRef]
- Li, X.; Du, S.; Ma, C.; Shi, T.; Qi, W.; Yang, H. Nano-SiO2 based anti-corrosion superhydrophobic coating on Al alloy with mechanical stability, anti-pollution and self-cleaning properties. Ceram. Int. 2024, 50, 9469–9478. [Google Scholar] [CrossRef]
- Cheng, J.; Li, J.; Yu, S.; Du, Z.; Dong, F.; Zhang, J.; Zhang, X. Corrosion behavior of as-cast Ti–10Mo–6Zr–4Sn–3Nb and Ti–6Al–4V in Hank’s solution: A comparison investigation. Metals 2020, 11, 11. [Google Scholar] [CrossRef]
- de Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Evaluation of corrosion behavior of surface modified Ti–6Al–4V ELI alloy in hanks solution. J. Appl. Electrochem. 2010, 40, 285–293. [Google Scholar] [CrossRef]
- Hamza, H.M.; Deen, K.M.; Haider, W. Microstructural examination and corrosion behavior of selective laser melted and conventionally manufactured Ti6Al4V for dental applications. Mater. Sci. Eng. C 2020, 113, 110980. [Google Scholar] [CrossRef] [PubMed]
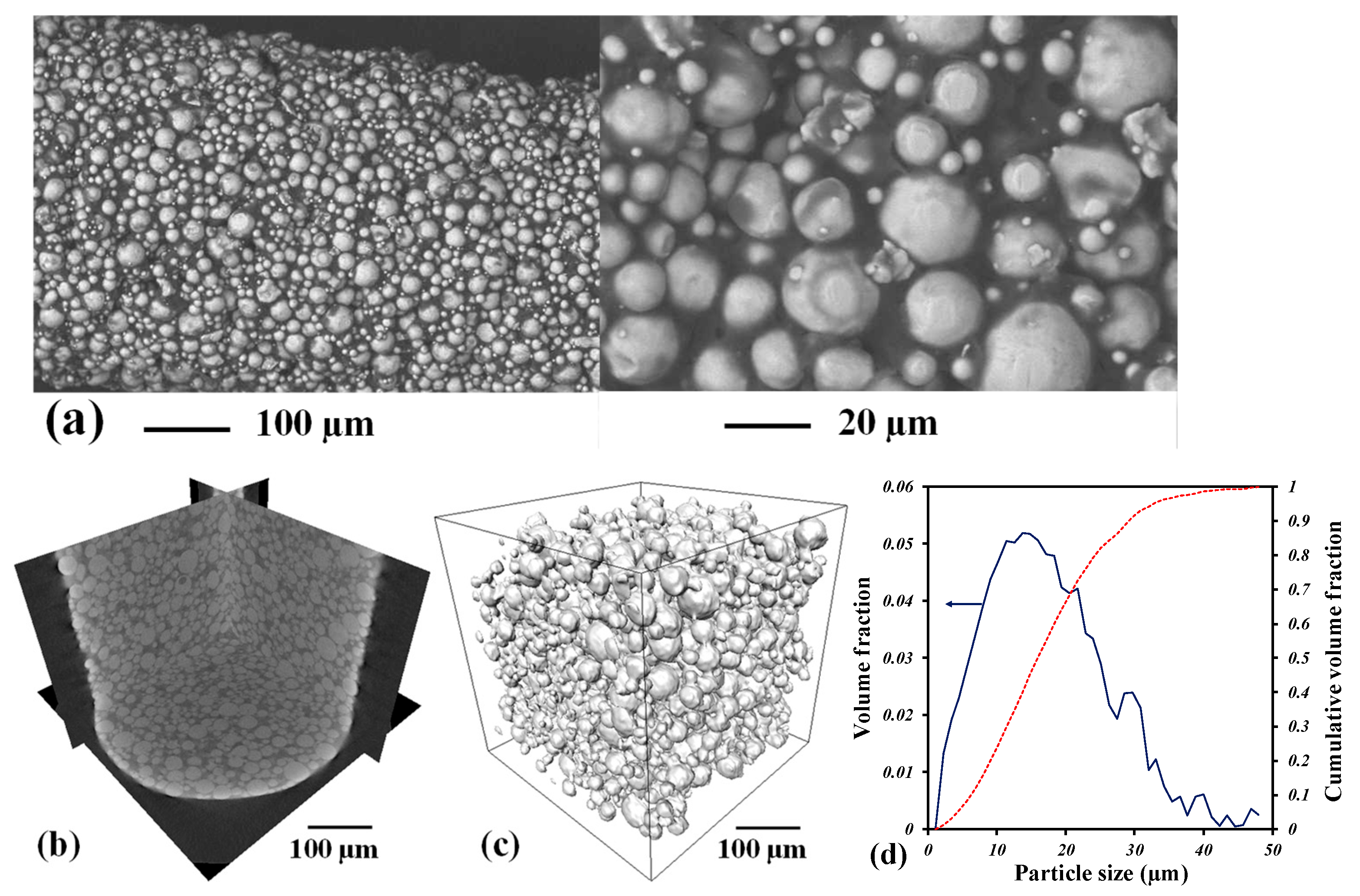

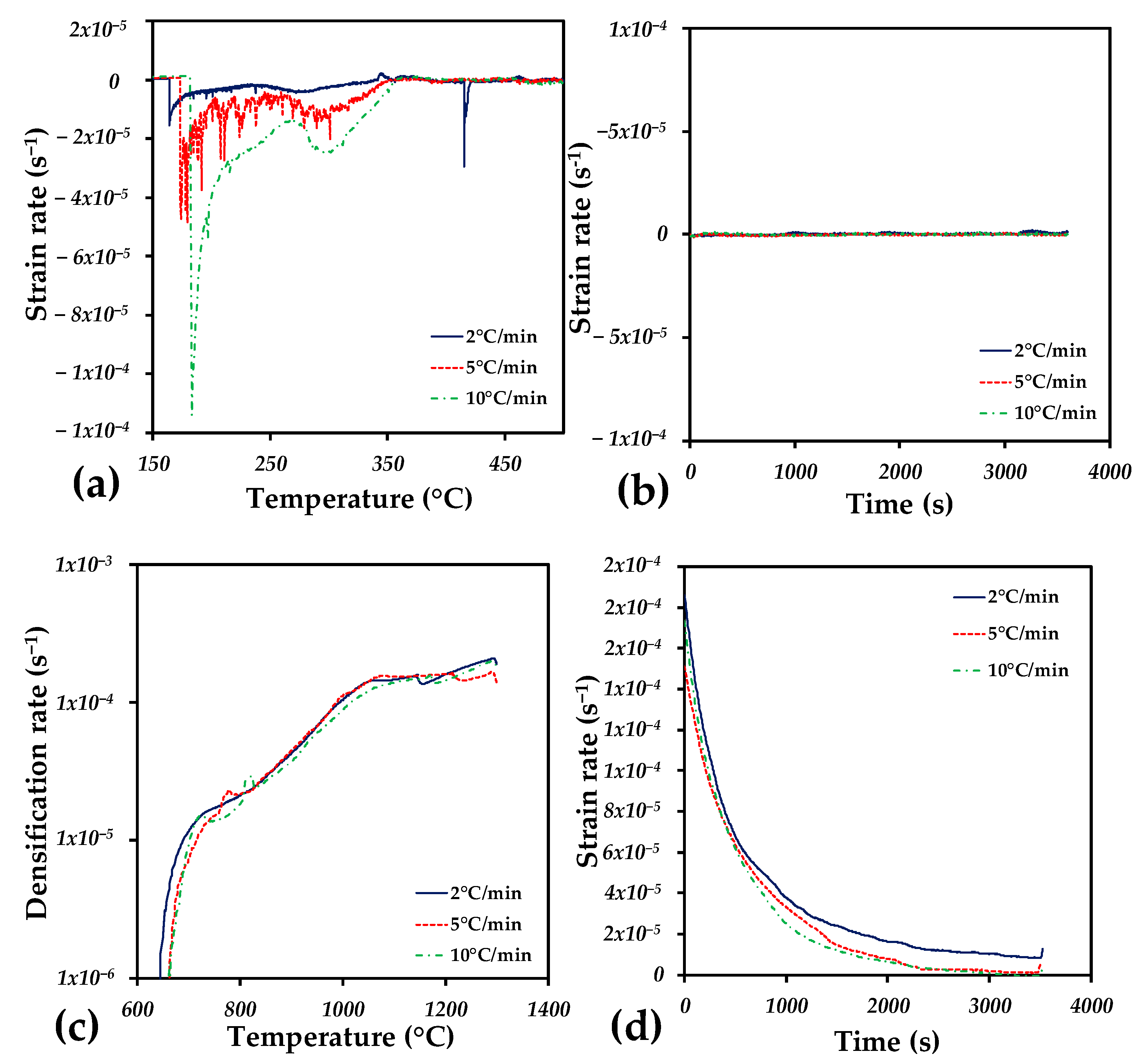
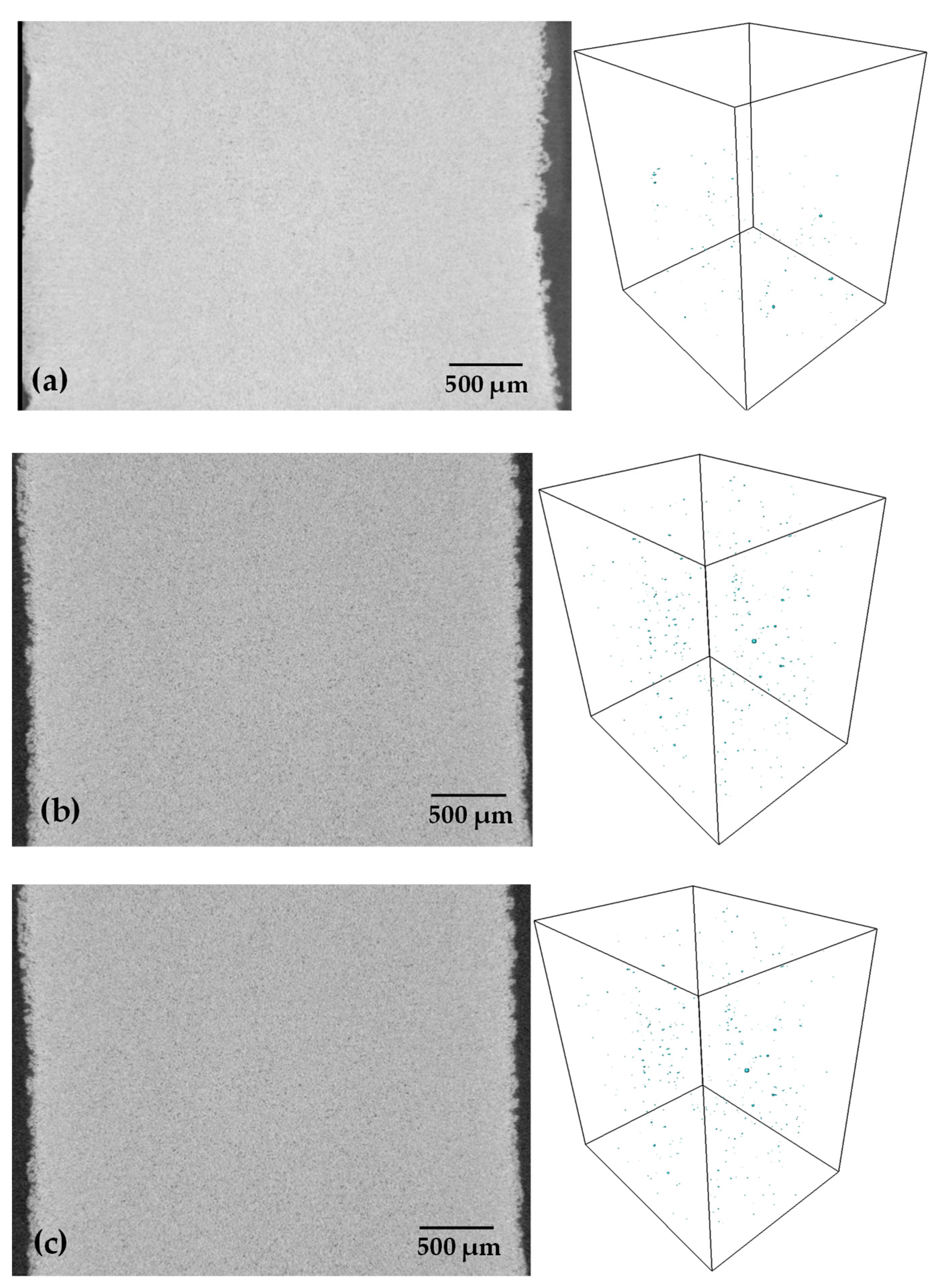

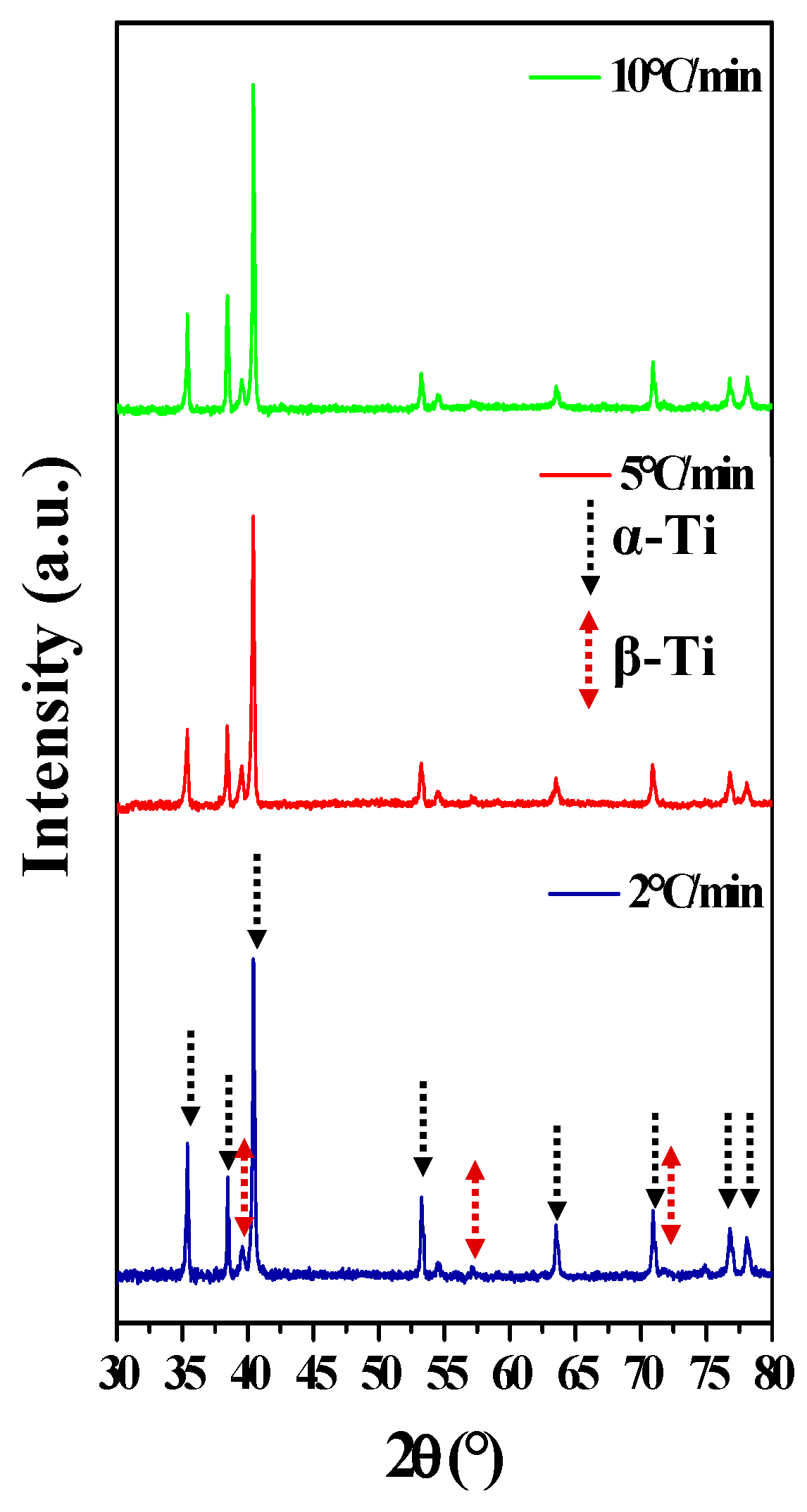

| Heating Rate (°C/min) | Relative Density (%) | E (GPa) | σy (MPa) |
|---|---|---|---|
| 2 | 97.1 | 90.3 ± 2 | 827 ± 13 |
| 5 | 95.8 | 86.1 ± 0.5 | 800 ± 4 |
| 10 | 92.1 | 81.6 ± 1.3 | 775 ± 12 |
| Heating Rate | E0 (V) | ba (mV/DEC) | bc (mV/DEC) | Icorr (A/cm2) | Vcorr (mm/year) |
|---|---|---|---|---|---|
| 2 C/min | −0.322 | 432 | 187 | 1.079 × 10−7 | 0.0022 ± 1 × 10−4 |
| 5 C/min | −0.279 | 294 | 239 | 3.55 × 10−7 | 0.0073 ± 3 × 10−4 |
| 10 C/min | −0.312 | 567 | 267 | 6.88 × 10−7 | 0.0143 ± 7 × 10−4 |
| Heating Rate (°C/min) | Rs Ω·cm2 | CPE-T F·cm2·Sn−1 × 10−5 | CPE-P | Rp Ω·cm2 |
|---|---|---|---|---|
| 2 °C | 20.64 | 4.6267 | 0.86573 | 519,180 |
| 5 °C | 22.54 | 5.9358 | 0.81447 | 70,926 |
| 10 °C | 29.6 | 9.964 | 0.76706 | 70,637 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Pedraza, A.S.; Bouvard, D.; Missiaen, J.-M.; Olmos, L.; Vergara-Hernández, H.J.; Lemus-Ruiz, J.; Villalobos, J.C. Analyzing the Debinding Step of Ti64 Parts Fabricated by 3D Printing Extrusion. Coatings 2024, 14, 715. https://doi.org/10.3390/coatings14060715
González-Pedraza AS, Bouvard D, Missiaen J-M, Olmos L, Vergara-Hernández HJ, Lemus-Ruiz J, Villalobos JC. Analyzing the Debinding Step of Ti64 Parts Fabricated by 3D Printing Extrusion. Coatings. 2024; 14(6):715. https://doi.org/10.3390/coatings14060715
Chicago/Turabian StyleGonzález-Pedraza, Ana Silvia, Didier Bouvard, Jean-Michel Missiaen, Luis Olmos, Héctor Javier Vergara-Hernández, Jose Lemus-Ruiz, and Julio César Villalobos. 2024. "Analyzing the Debinding Step of Ti64 Parts Fabricated by 3D Printing Extrusion" Coatings 14, no. 6: 715. https://doi.org/10.3390/coatings14060715
APA StyleGonzález-Pedraza, A. S., Bouvard, D., Missiaen, J.-M., Olmos, L., Vergara-Hernández, H. J., Lemus-Ruiz, J., & Villalobos, J. C. (2024). Analyzing the Debinding Step of Ti64 Parts Fabricated by 3D Printing Extrusion. Coatings, 14(6), 715. https://doi.org/10.3390/coatings14060715








