Preparation of Fe3O4/NiO Nanomaterials by Electrodeposition and Their Adsorption Performance for Fluoride Ions
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Instruments
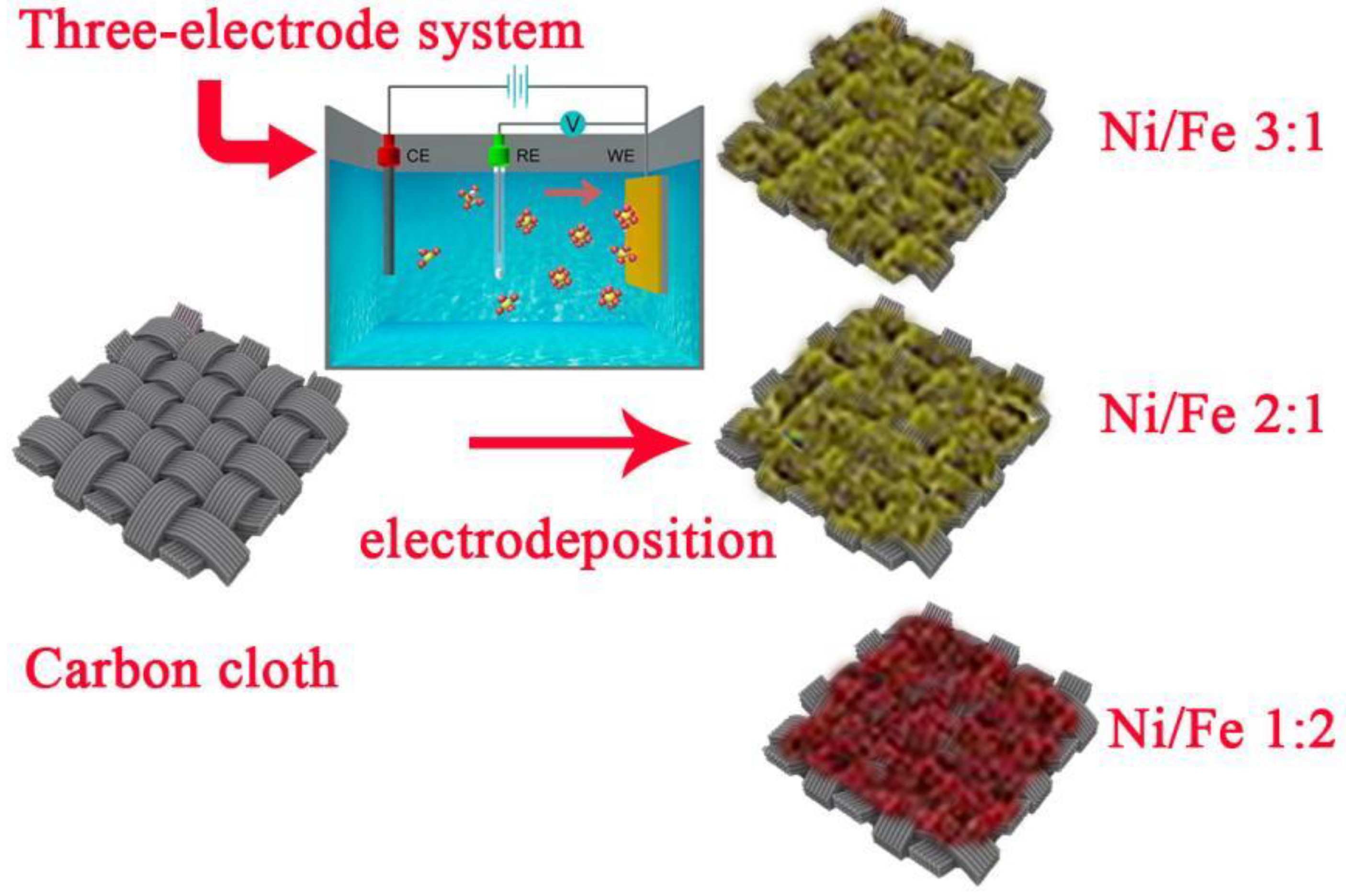
2.2. Preparation of MNN Materials
2.3. Adsorption Experiment
2.4. Characterization
3. Results and Discussion
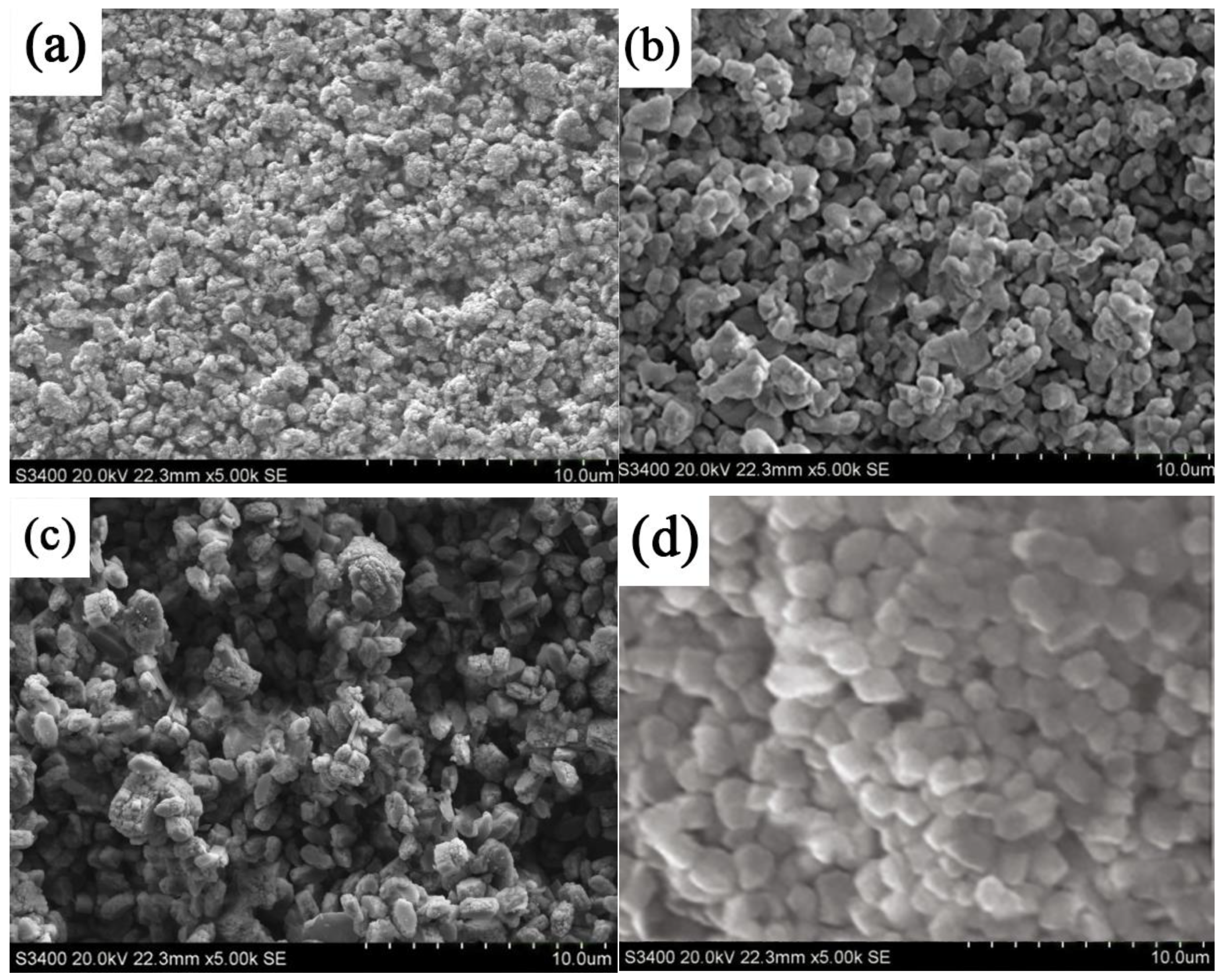
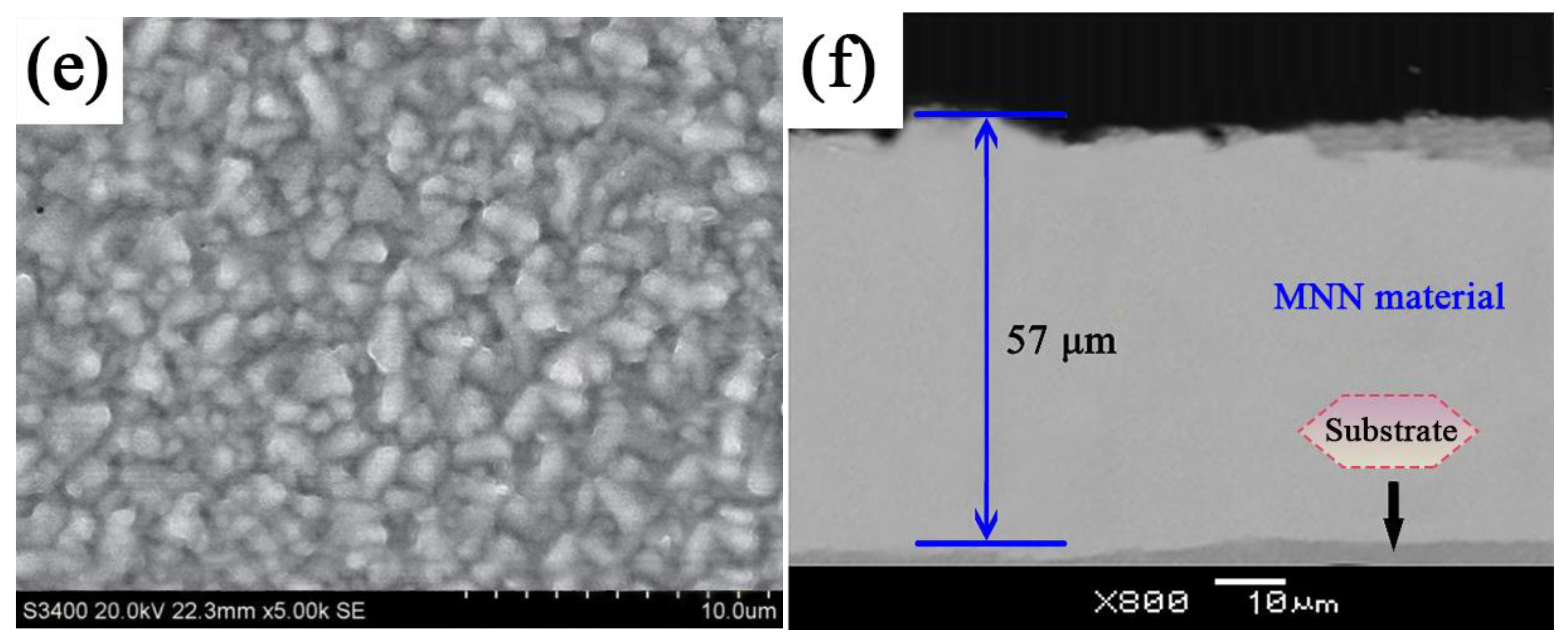
3.1. Surface Morphology of MNN Material
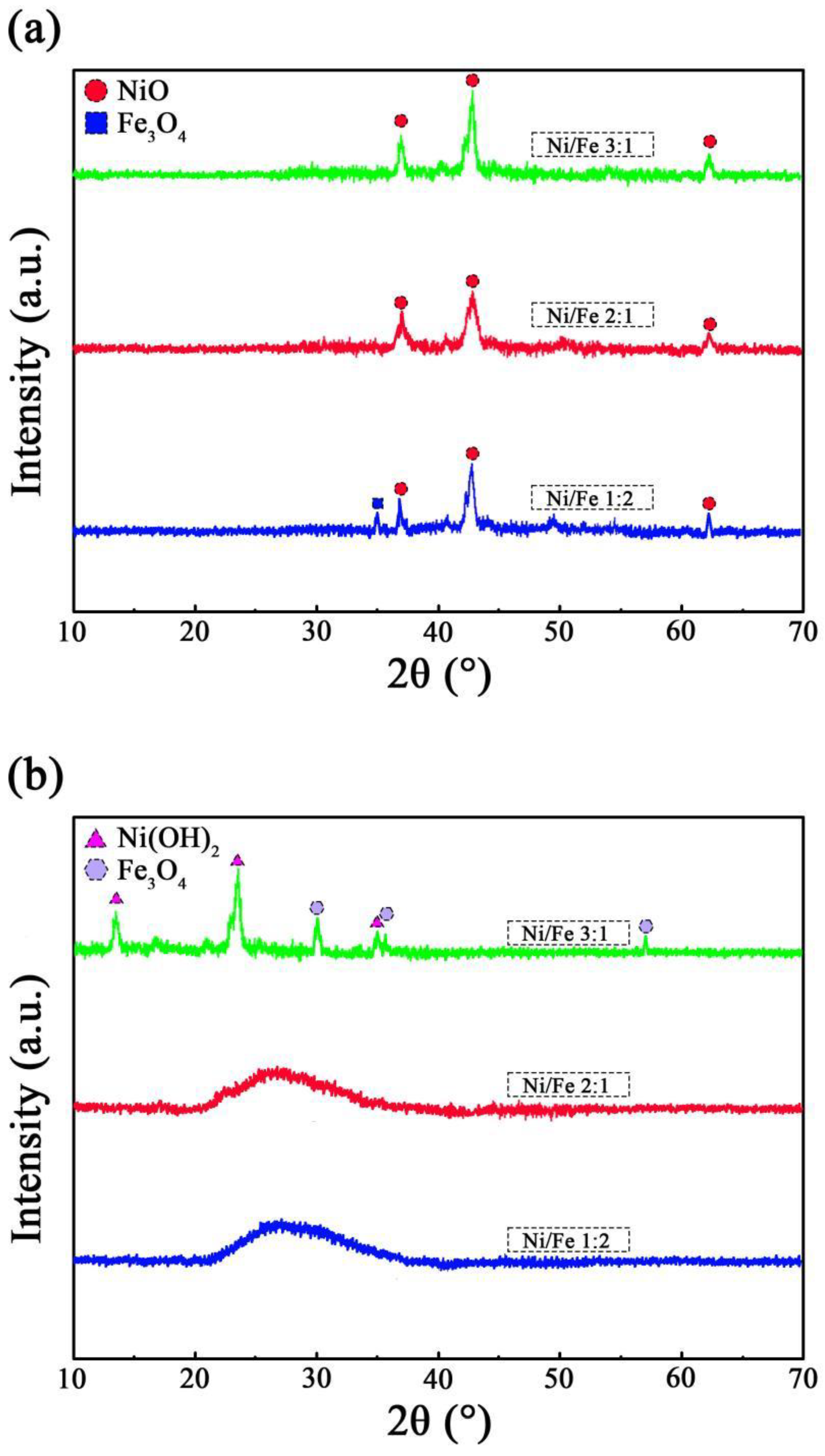
3.2. Phase Composition of MNN Material
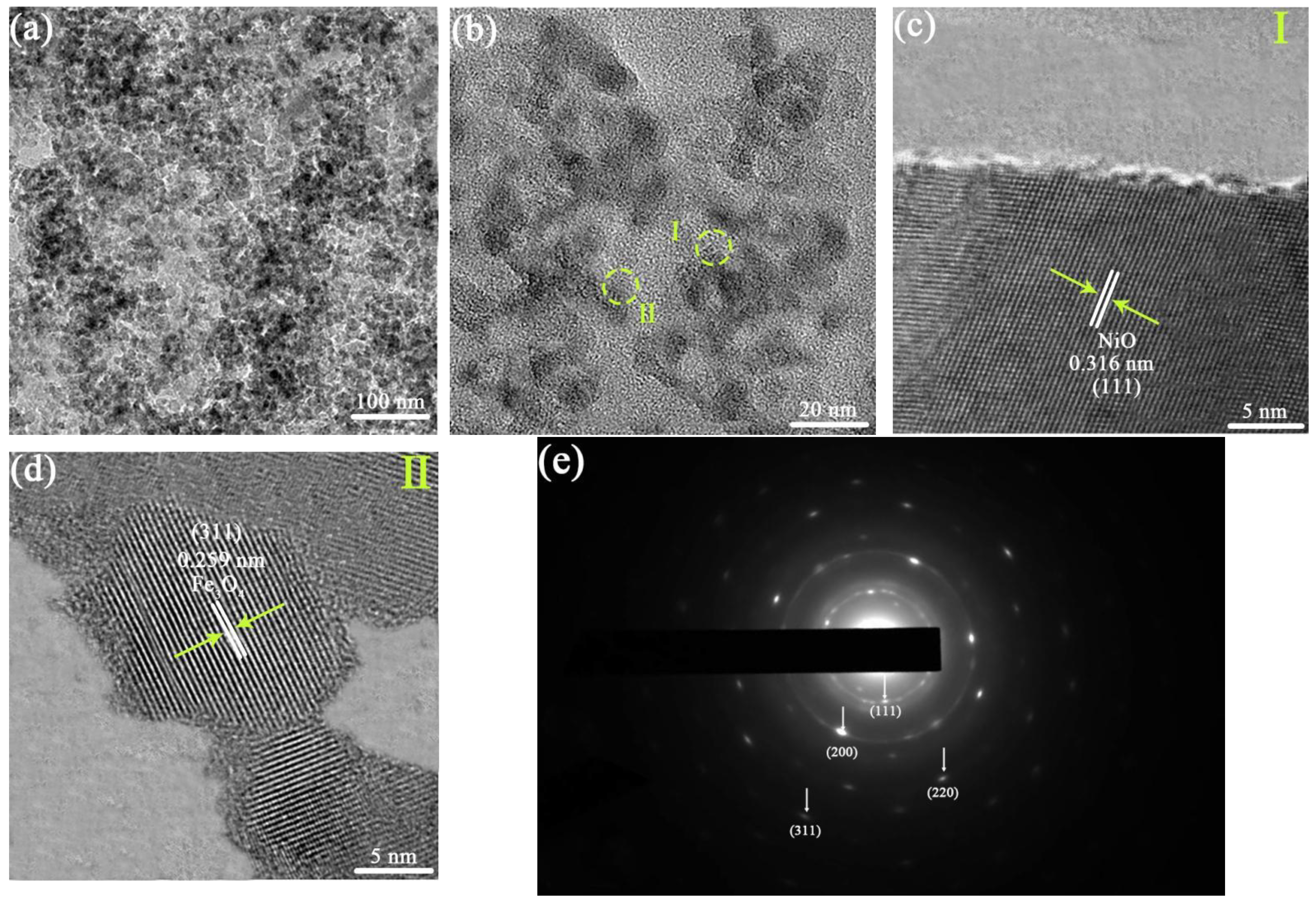
3.3. Microstructure Observation of MNN Material
3.4. BET Analysis of MNN Material
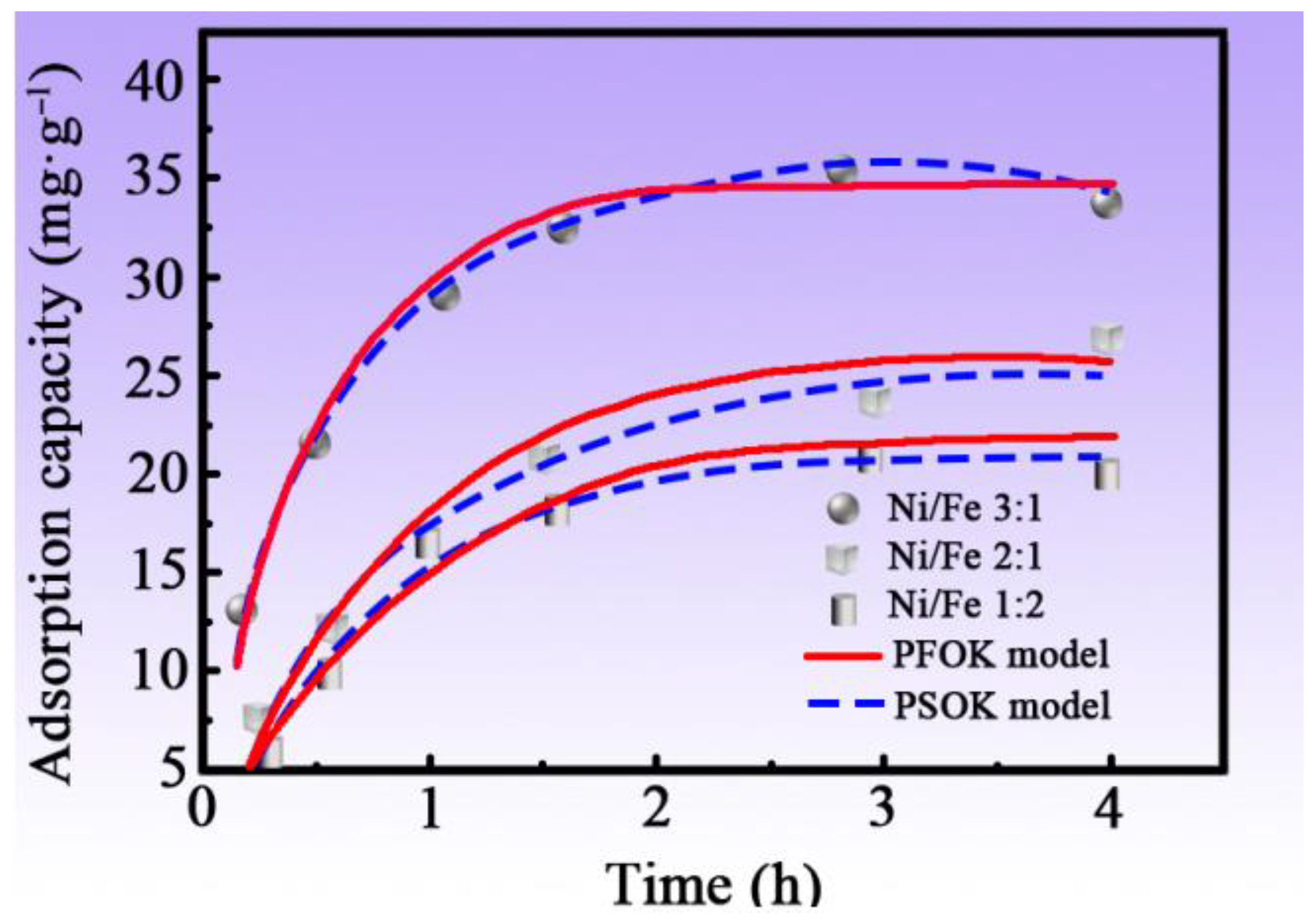
3.5. Adsorption Kinetics of MNN Material
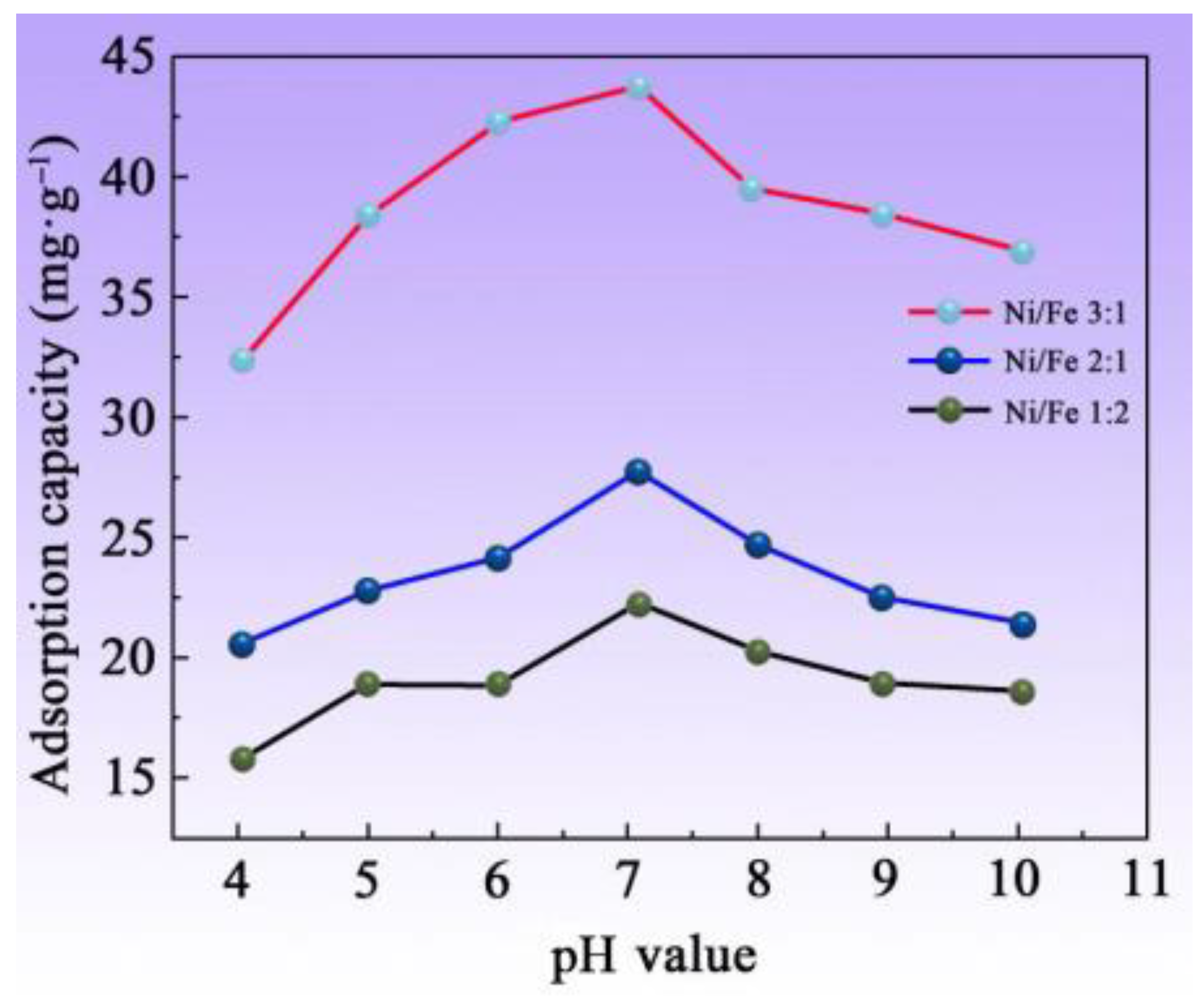
3.6. Impact of Various pH Values on Adsorption Characteristics
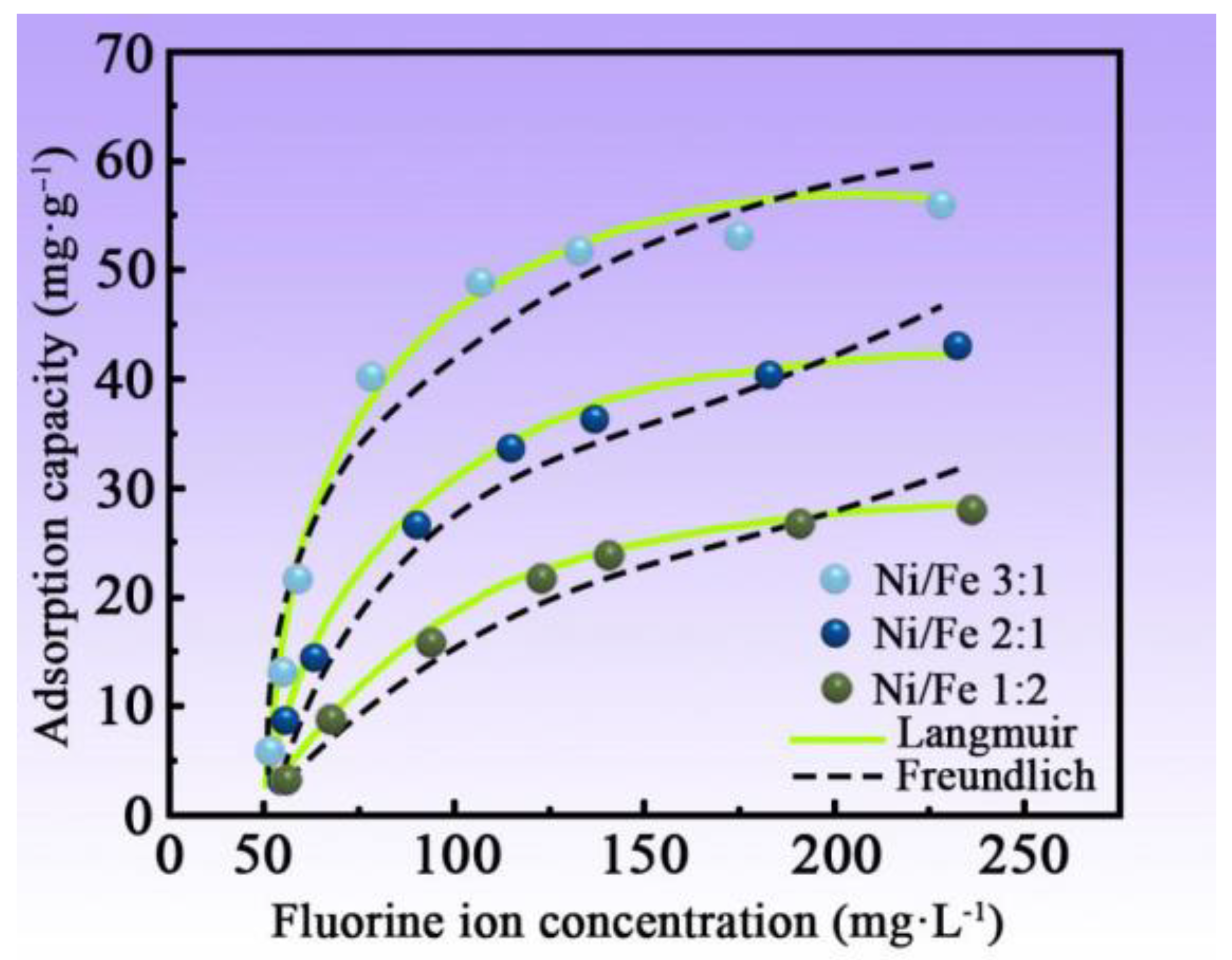
3.7. Isothermal Adsorption Test of MNN Material
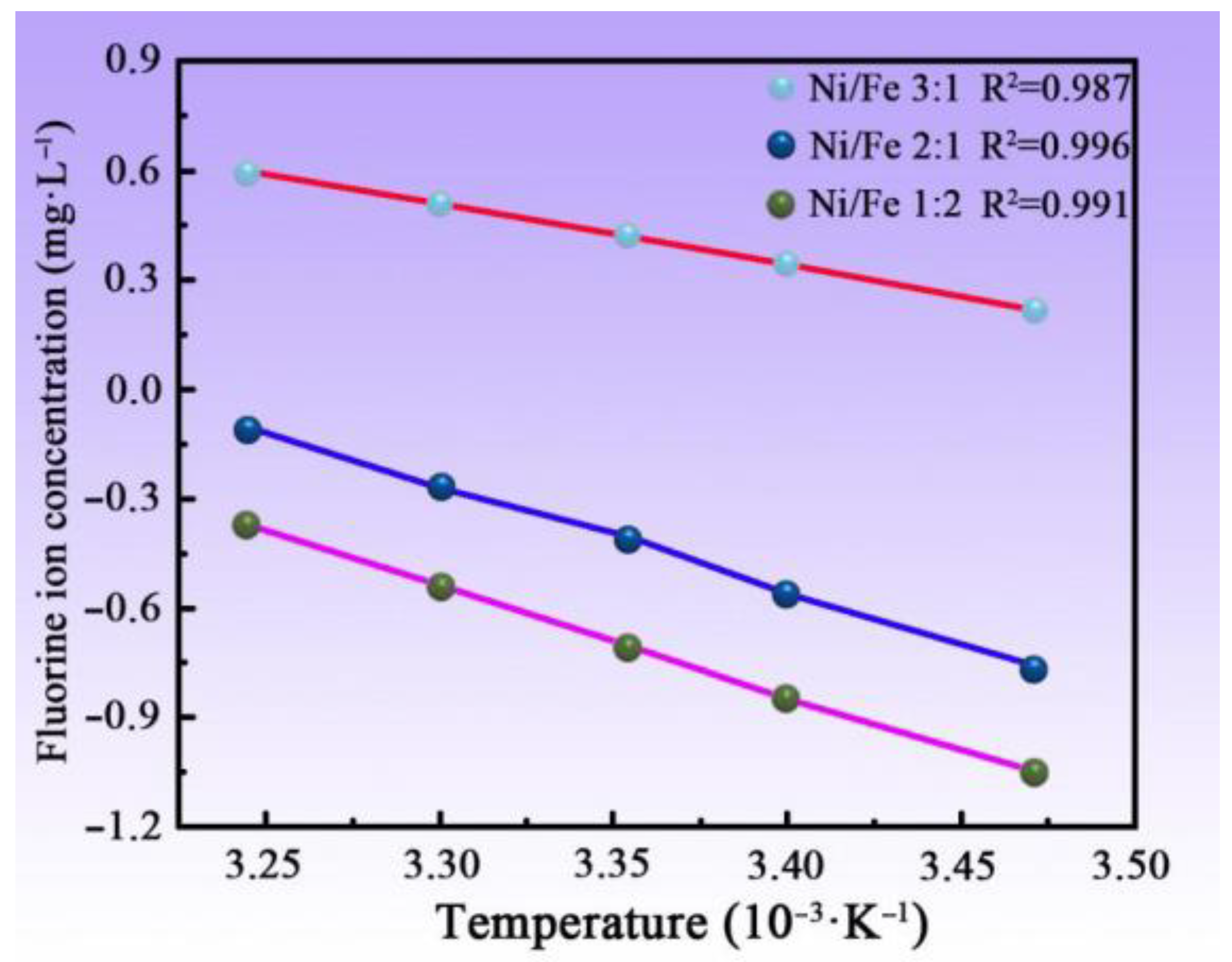
3.8. Adsorption Thermodynamic Test of MNN Material
4. Conclusions
- (1)
- MNN materials were successfully synthesized with varying nickel–iron ratios using the electrodeposition method. SEM and TEM analysis revealed that MNN material, particularly with a nickel–iron ratio of 3:1, exhibits a dense and uniformly dispersed surface.
- (2)
- X-ray diffraction data along with the analysis of specific surface area and pore size distribution highlighted that, during F− adsorption, the MNN material featuring a nickel–iron ratio of 2:1 undergoes hydration and electrostatic adsorption processes, achieving an adsorption capacity of 58.3 mg·g−1 for environmental F−.
- (3)
- The MNN material exhibited maximum defluoridation efficiency at a neutral pH. The use of a pseudo-second-order kinetics model is followed by the adsorption process, which aligns with the Langmuir model, indicating a chemisorption mechanism involving monolayer coverage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.L.; Shi, Y.B.; Huang, Y.; Xing, A.L.; Xue, H. The Effect of Governance on Industrial Wastewater Pollution in China. Int. J. Environ. Res. Public Health 2022, 19, 9316. [Google Scholar] [CrossRef] [PubMed]
- Savun-Hekimoglu, B.; Isler, Z.; Hekimoglu, M.; Burak, S.; Karli, D.; Yucekaya, A.; Akpinar, E.; Ediger, V.S. Optimization of wastewater treatment systems for growing industrial parks. Sci. Total Environ. 2023, 905, 167223. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, H.Q. Spatiotemporal coupling measurement of industrial wastewater discharge and industrial economy in China. Environ. Sci. Pollut. Res. 2021, 28, 46319–46333. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.L.; Huang, L.; Yan, J.; Ma, B.Y.; Huang, X.J.; Luo, Z.X.; Zhang, H.G.; Xiao, T.F. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Siddiqi, H.; Bai, M.; Meikap, B.C. Calcium and zirconium modified acid activated alumina for adsorptive removal of fluoride: Performance evaluation, kinetics, isotherm, characterization and industrial wastewater treatment. Adv. Power Technol. 2020, 31, 2045–2060. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Seczkowska, M.; Derylo-Marczewska, A.; Blachnio, M. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: Effect of temperature. Adsorpt. J. Int. Adsorpt. Soc. 2016, 22, 777–790. [Google Scholar] [CrossRef]
- Rezaei, N.; Ehsani, M.H.; Aghazadeh, M.; Karimzadeh, L. An Investigation on Magnetic-Interacting Fe3O4 Nanoparticles Prepared by Electrochemical Synthesis Method. J. Supercond. Nov. Magn. 2018, 31, 2139–2147. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shen, D.K.; Shen, F.; Li, T.Y. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.Z.; Huang, T.; Zhang, N.; Wang, Y. Fabricating the ternary CeO2@CNTs/CdSe composite with synchronously enhanced adsorption and photocatalytic activity toward water-soluble pollutants removal. Chem. Eng. J. 2023, 476, 146574. [Google Scholar] [CrossRef]
- Ju, F.; Wang, M.; Wu, T.; Ling, H. The Role of NiO in Reactive Adsorption Desulfurization Over NiO/ZnO-Al2O3-SiO2 Adsorbent. Catalysts 2019, 9, 79. [Google Scholar] [CrossRef]
- Baumagartner, M.; Spichtinger, P. Homogeneous nucleation from an asymptotic point of view. Theor. Comput. Fluid Dyn. 2019, 33, 83–106. [Google Scholar] [CrossRef]
- Karar, Y.; Boundinar, S.S.; Kadri, A.; Lepretre, J.C.; Benbrahim, N.; Chainet, E. Ammonium chloride effects on bismuth electrodeposition in a choline chloride-urea deep eutectic solvent. Electrochim. Acta 2021, 367, 137481. [Google Scholar] [CrossRef]
- Jebraeil, S.M.; Eslami, A. Hydrothermal synthesis of graphene oxide/NiO and its influence on the therma decomposition of ammonium perchlorate. Indian J. Chem. 2022, 61, 278–284. [Google Scholar]
- Baral, A.; Satish, L.; Padhy, S.K.; Das, D.P.; Tripathy, B.C.; Ghosh, M.K. Electrodeposition of nanoMnO2 from mineral leach liquor and the investigation on conformational changes of hemoglobin induced by the nanomaterial. Colloids Surf. A Physicochem. Eng. Asp. 2019, 585, 124102. [Google Scholar] [CrossRef]
- Lim, J.H.; Rotaru, A.; Min, S.G.; Malkinski, L.; Wiley, J.B. Synthesis of mild-hard AAO templates for studying magnetic interactions between metal nanowires. J. Mater. Chem. 2010, 20, 9246–9252. [Google Scholar] [CrossRef]
- Ursueguia, D.; Diaz, E.; Ordonez, S. Adsorption of methane and nitrogen on Basolite MOFs: Equilibrium and kinetic studies. Microporous Mesoporous Mater. 2020, 298, 110048. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Yao, L.M.; Li, H.X.; Jia, X. Investigation of the mechanical properties and corrosion behaviors of Ni-BN-TiC layers constructed via laser cladding technique. Ceram. Int. 2022, 49, 6671–6677. [Google Scholar] [CrossRef]
- Li, C.; Xia, F.; Ma, C.; Li, Q. Research on the Corrosion Behavior of Ni-SiC Nanocoating Prepared Using a Jet Electrodeposition Technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Xia, F.F.; Yan, P.; Ma, C.Y.; Wang, B.J.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J. Mater. Res. Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Rohrer, G.S.; Chesser, I.; Krause, A.R.; Naghoibzadeh, S.K.; Xu, Z.P.; Dayal, K.; Holm, E.A. Grain Boundary Migration in Polycrystals. Annu. Rev. Mater. Res. 2023, 53, 347–369. [Google Scholar] [CrossRef]
- Kutluay, S.; Sahin, O.; Ece, M.S. Fabrication and characterization of Fe3O4/perlite, Fe3O4/perlite@SiO2, and Fe3O4/perlite@SiO2@sulfanilamide magnetic nanomaterials. Appl. Phys. A Mater. Sci. Process. 2022, 128, 222. [Google Scholar] [CrossRef]
- Wu, Y.H.; Guo, Q.B.; Liu, H.; Wei, S.S.; Wang, L. Effect of Fe doping on the surface properties of ?-MnO2 nanomaterials and its decomposition of formaldehyde at room temperature. J. Environ. Chem. Eng. 2022, 10, 108277. [Google Scholar] [CrossRef]
- Eris, S.; Bashiri, H. Kinetic study of the adsorption of dyes onto activated carbon. Prog. React. Kinet. Mech. 2016, 41, 109–119. [Google Scholar] [CrossRef]
- Cavusoglu, H. Exploring the Role of pH on the Physical and Optoelectronic Attributes of Nanostructured NiO Thin Films. J. Nanoelectron. Optoelectron. 2019, 14, 645–652. [Google Scholar] [CrossRef]
- Dong, X.X.; Song, M.; Jin, B.S.; Zhou, Z.; Yang, X. The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions. Catalysts 2017, 7, 51. [Google Scholar] [CrossRef]
- Balarak, D.; Mahvi, A.H.; Shim, M.J.; Lee, S.M. Adsorption of ciprofloxacin from aqueous solution onto synthesized NiO: Isotherm, kinetic and thermodynamic studies. Desalination Water Treat. 2021, 212, 390–400. [Google Scholar] [CrossRef]
- Li, H.X.; Xia, F.F.; Ma, C.Y.; Li, C.Y. Magnetic field-assisted electrodeposition of ZnCo2O4-rGo nanoelectrodes for enhanced supercapacitor performance. Chem. Eng. J. 2024; in press. [Google Scholar] [CrossRef]
- Pore, O.C.; Fulari, A.V.; Parale, V.G.; Park, H.H.; Shejwal, R.V.; Fulari, V.J.; Lohar, G.M. Facile hydrothermal synthesis of NiO/rGO nanocomposite electrodes for supercapacitor and nonenzymatic glucose biosensing application. J. Porous Mater. 2022, 29, 1991–2001. [Google Scholar] [CrossRef]
- Chen, P.; Richter, J.; Wang, G.; Li, D.Q.; Pietsch, T.; Ruck, M. Ionometallurgical Step-Electrodeposition of Zinc and Lead and its Application in a Cycling-Stable High-Voltage Zinc-Graphite Battery. Small 2021, 17, 2102058. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Al-Shihri, A.S.; Kalam, A.; Du, G.H.; Ahmad, T. Microwave synthesis, optical properties and surface area studies of NiO nanoparticles. J. Mol. Struct. 2014, 1058, 56–61. [Google Scholar] [CrossRef]




| Chemical Reagents | Value |
|---|---|
| Ni(NO3)2·6H2O (g·L−1) | 23.76–96.03 |
| Fe(NO3)3·9(H2O) (g·L−1) | 66.6 |
| HFM (g·L−1) | 15 |
| H3BO3 (g·L−1) | 30 |
| C12H25SO4Na (g·L−1) | 0.1 |
| Item | Value |
|---|---|
| Plating bath temperature (°C) | 55 |
| Plating current density (A·dm−2) | 3 |
| Stirring speed (r·min−1) | 800 |
| Duty cycle (%) | 20 |
| Activation time (min) | 30 |
| pH | 4.0 |
| Pulse frequency (Hz) | 500 |
| Ni/Fe | PFOD Model | PSOK Model | ||||
|---|---|---|---|---|---|---|
| qe/(mg·g−1) | K1 | R2 | qe/(mg·g−1) | K1 | R2 | |
| 3:1 | 36.28 | 2.01 | 0.8394 | 40.13 | 0.073 | 0.9396 |
| 2:1 | 27.36 | 1.19 | 0.9187 | 31.98 | 0.070 | 0.9641 |
| 1:2 | 23.15 | 1.57 | 0.9719 | 26.64 | 0.053 | 0.9862 |
| Ni/Fe | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Q/(mg·g−1) | KL | R2 | Kf/(mg·g−1) | n | R2 | |
| 3:1 | 63.86 | 0.062 | 0.9749 | 8.90 | 2.64 | 0.9521 |
| 2:1 | 51.14 | 0.034 | 0.9956 | 5.12 | 2.13 | 0.9660 |
| 1:2 | 35.29 | 0.020 | 0.9875 | 2.73 | 1.98 | 0.9807 |
| Ni/Fe | ∆H0/(KJ·mol−1) | ∆S0/(J·mol−1·K−1) | ∆G0/(KJ·mol−1) | ||
|---|---|---|---|---|---|
| 287.36 K | 296.36 K | 312.36 K | |||
| 3:1 | 13.67 | 51.06 | −0.46 | −1.15 | −1.68 |
| 2:1 | 26.31 | 80.29 | 1.72 | 1.01 | 0.36 |
| 1:2 | 27.93 | 81.86 | 2.61 | 1.84 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Z.; Ma, C.; He, H.; Wei, L.; Xia, F. Preparation of Fe3O4/NiO Nanomaterials by Electrodeposition and Their Adsorption Performance for Fluoride Ions. Coatings 2024, 14, 739. https://doi.org/10.3390/coatings14060739
Zhang H, Li Z, Ma C, He H, Wei L, Xia F. Preparation of Fe3O4/NiO Nanomaterials by Electrodeposition and Their Adsorption Performance for Fluoride Ions. Coatings. 2024; 14(6):739. https://doi.org/10.3390/coatings14060739
Chicago/Turabian StyleZhang, Hongbin, Zhiping Li, Chunyang Ma, Hongxin He, Lixin Wei, and Fafeng Xia. 2024. "Preparation of Fe3O4/NiO Nanomaterials by Electrodeposition and Their Adsorption Performance for Fluoride Ions" Coatings 14, no. 6: 739. https://doi.org/10.3390/coatings14060739




