The Effect of Different Coating Agents on the Microhardness, Water Sorption, and Solubility of EQUIA Forte® HT
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Size
2.2. Specimen Preparation
2.3. Microhardness
2.4. Water Sorption and Solubility
2.5. Statistical Analysis
3. Results
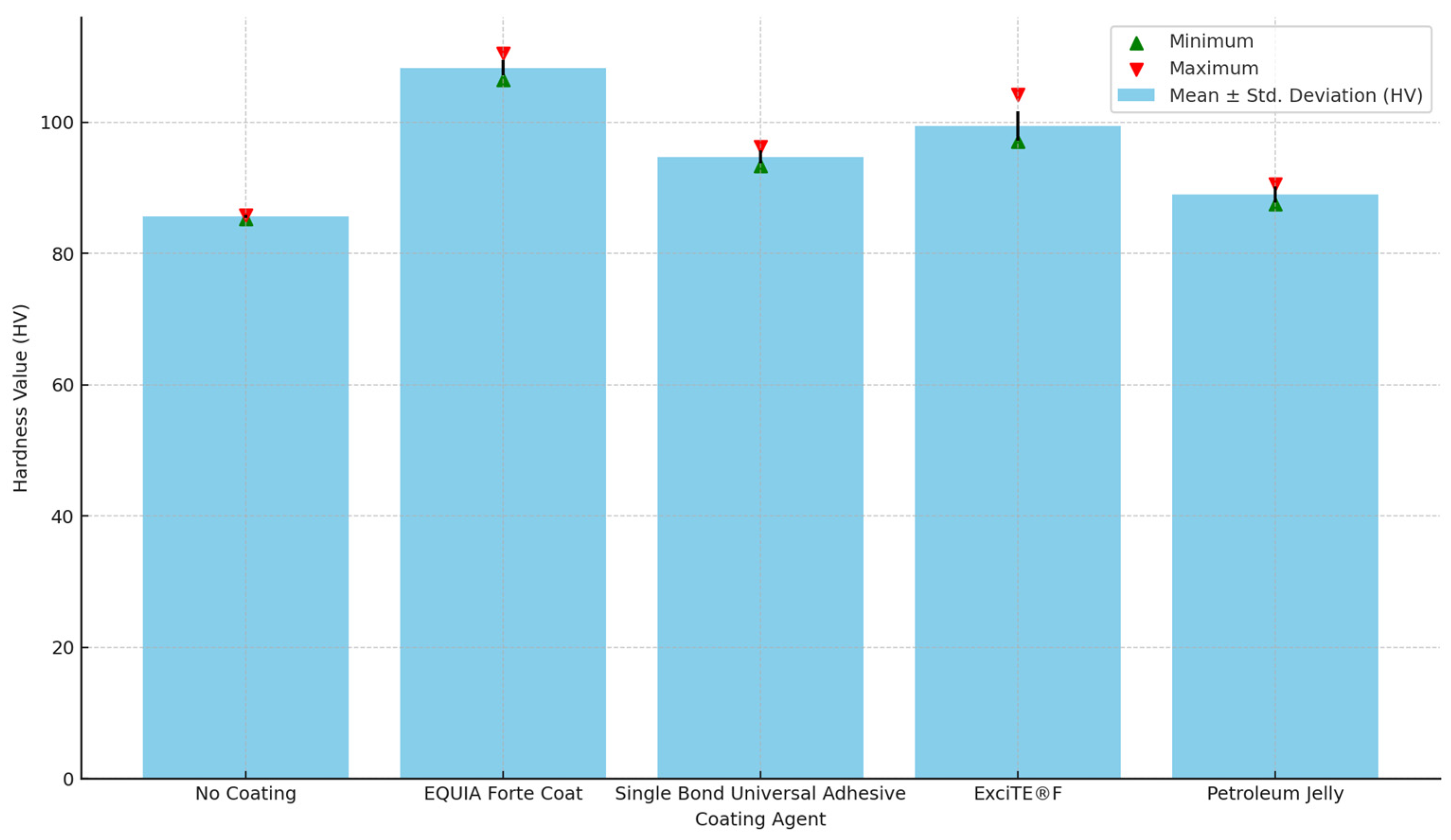
3.1. Microhardness
3.2. Water Sorption
3.3. Water Solubility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. A New Translucent Cement for Dentistry. The Glass Ionomer Cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, C.C.; Werner, A.; Kleverlaan, C.J. Coating Glass-Ionomer Cements with a Nanofilled Resin. Acta Odontol. Scand. 2012, 70, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.P.; Nicholson, J.W. Nicholson 423 Glass Ionomer Cement and Adjunct Professor. Pediatr. Dent. 2002, 24. [Google Scholar]
- Preston, A.; Agalamanyi, E.; Higham, S.; Mair, L. The Recharge of Esthetic Dental Restorative Materials with Fluoride In Vitro—Two Years’ Results. Dent. Mater. 2003, 19, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Brkanović, S.; Ivanišević, A.; Miletić, I.; Mezdić, D.; Krmek, S.J. Effect of Nano-Filled Protective Coating and Different Ph Enviroment on Wear Resistance of New Glass Hybrid Restorative Material. Materials 2021, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.E.; Arita, K.; Nishino, M. Toughness, Bonding and Fluoride-Release Properties of Hydroxyapatite-Added Glass Ionomer Cement. Biomaterials 2003, 24, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Yap, A.U.J.; Cheang, P.; Khor, K.A. Effects of Incorporation of HA/ZrO2 into Glass Ionomer Cement (GIC). Biomaterials 2005, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Dowling, A.H.; Stamboulis, A.; Fleming, G.J.P. The Influence of Montmorillonite Clay Reinforcement on the Performance of a Glass Ionomer Restorative. J. Dent. 2006, 34, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, I.M. Reinforcement of Conventional Glass-Ionomer Restorative Material with Short Glass Fibers. J. Mech. Behav. Biomed. Mater. 2009, 2, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Al Zraikat, H.; Palamara, J.E.A.; Messer, H.H.; Burrow, M.F.; Reynolds, E.C. The Incorporation of Casein Phosphopeptide–Amorphous Calcium Phosphate into a Glass Ionomer Cement. Dent. Mater. 2011, 27, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Leitune, V.C.B.; Balbinot, G.D.S.; Samuel, S.M.W.; Collares, F.M. Influence of Niobium Pentoxide Addition on the Properties of Glass Ionomer Cements. Acta Biomater. Odontol. Scand. 2016, 2, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gaintantzopoulou, M.D.; Gopinath, V.K.; Zinelis, S. Evaluation of Cavity Wall Adaptation of Bulk Esthetic Materials to Restore Class II Cavities in Primary Molars. Clin. Oral. Investig. 2017, 21, 1063–1070. [Google Scholar] [CrossRef]
- Akman, H.; Tosun, G. Clinical Evaluation of Bulk-Fill Resins and Glass Ionomer Restorative Materials: A 1-Year Follow-up Randomized Clinical Trial in Children. Niger. J. Clin. Pract. 2020, 23, 489–497. [Google Scholar] [CrossRef]
- Klinke, T.; Daboul, A.; Turek, A.; Frankenberger, R.; Hickel, R.; Biffar, R. Clinical Performance during 48 Months of Two Current Glass Ionomer Restorative Systems with Coatings: A Randomized Clinical Trial in the Field. Trials 2016, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Türkün, L.S.; Kanik, O. A Prospective Six-Year Clinical Study Evaluating Reinforced Glass Ionomer Cements with Resin Coating on Posterior Teeth: Quo Vadis? Oper. Dent. 2016, 41, 587–598. [Google Scholar] [CrossRef]
- Gurgan, S.; Kutuk, Z.B.; Yalcin Cakir, F.; Ergin, E. A Randomized Controlled 10 Years Follow up of a Glass Ionomer Restorative Material in Class I and Class II Cavities. J. Dent. 2020, 94, 103175. [Google Scholar] [CrossRef]
- Gok Baba, M.; Kirzioglu, Z.; Ceyhan, D. One-year Clinical Evaluation of Two High-viscosity Glass-ionomer Cements in Class II Restorations of Primary Molars. Aust. Dent. J. 2021, 66, 32–40. [Google Scholar] [CrossRef]
- Miletić, I.; Baraba, A.; Basso, M.; Pulcini, M.G.; Marković, D.; Perić, T.; Ozkaya, C.A.; Turkun, L.S. Clinical Performance of a Glass-Hybrid System Compared with a Resin Composite in the Posterior Region: Results of a 2-Year Multicenter Study. J. Adhes. Dent. 2020, 22, 235–247. [Google Scholar] [CrossRef]
- Heck, K.; Frasheri, I.; Diegritz, C.; Manhart, J.; Hickel, R.; Fotiadou, C. Six-Year Results of a Randomized Controlled Clinical Trial of Two Glass Ionomer Cements in Class II Cavities. J. Dent. 2020, 97, 103333. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, M.; Navas, A.; Jahedmanesh, N.; Shah, K.C.; Moshaverinia, A.; Ansari, S. Comparative Evaluation of the Physical Properties of a Reinforced Glass Ionomer Dental Restorative Material. J. Prosthet. Dent. 2019, 122, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, S.N.; Turgut, H.; Küde, C. Comparison of Surface Roughness and Microhardness of Reinforced Glass Ionomer Cements and Microhybrid Composite. J. Dent. Indones. 2021, 28, 131–138. [Google Scholar] [CrossRef]
- Savas, S.; Colgecen, O.; Yasa, B.; Kucukyilmaz, E. Color Stability, Roughness, and Water Sorption/Solubility of Glass Ionomer–Based Restorative Materials. Niger. J. Clin. Pract. 2019, 22, 824. [Google Scholar] [CrossRef]
- Aydın, N.; Karaoğlanoğlu, S.; Aybala-Oktay, E.; Çetinkaya, S.; Erdem, O. Investigation of Water Sorption and Aluminum Releases from High Viscosity and Resin Modified Glass Ionomer. J. Clin. Exp. Dent. 2020, 12, e844–e851. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U. Dental Glass Ionomer Cements as Permanent Filling Materials?—Properties, Limitations and Future Trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef]
- Ong, J.; Yap, A.; Hong, J.; Eweis, A.; Yahya, N. Viscoelastic Properties of Contemporary Bulk-Fill Restoratives: A Dynamic-Mechanical Analysis. Oper. Dent. 2018, 43, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Moore, B.K.; Onose, H. Effect of Surface Coatings on Flexural Properties of Glass Ionomers. Eur. J. Oral. Sci. 1996, 104, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.R.; Velasco, L.G.; Bonini, G.A.V.C.; Imparato, J.C.P.; Raggio, D.P. Glass Ionomer Cement Hardness after Different Materials for Surface Protection. J. Biomed. Mater. Res. A 2009, 93A, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.C.; Navarro, M.F.; Freitas, S.F.; Carvalho, R.M.; Cury, J.A.; Retief, D.H. Glass Ionomer Cement Surface Protection. Am. J. Dent. 1994, 7, 203–206. [Google Scholar] [PubMed]
- Sidhu, S. Glass-Ionomer Cement Restorative Materials: A Sticky Subject? Aust. Dent. J. 2011, 56, 23–30. [Google Scholar] [CrossRef]
- Marquezan, M.; Osorio, R.; Clamponi, A.L.; Toledano, M. Resistance to Degradation of Bonded Restorations to Simulated Caries-Affected Primary Dentin. Am. J. Dent. 2010, 23, 47–52. [Google Scholar] [PubMed]
- Krajangta, N.; Dulsamphan, C.; Chotitanmapong, T. Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement. Dent. J. 2022, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Handoko, M.W.; Tjandrawinata, R. Octarina the Effect of Nanofilled Resin Coating on the Hardness of Glass Ionomer Cement. Sci. Dent. J. 2020, 4, 97. [Google Scholar] [CrossRef]
- Faraji, F.; Heshmat, H.; Banava, S. Effect of Protective Coating on Microhardness of a New Glass Ionomer Cement: Nanofilled Coating versus Unfilled Resin. J. Conserv. Dent. 2017, 20, 260. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.; Park, H.; Lee, J.; Seo, H. Effect of Nano-Filled Protective Coating on Microhardness and Wear Resistance of Glass-Ionomer Cements. J. Korean Acad. Pedtatric Dent. 2019, 46, 226–232. [Google Scholar] [CrossRef]
- Yilmaz, M.N.; Gul, P.; Kiziltunc, A. Water Sorption and Solubility of a High-Viscous Glass-Ionomer Cement after the Application of Different Surface-Coating Agents. Eur. J. Gen. Dent. 2020, 9, 118–121. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. Fundamentals of Materials Science. In Craig’s Restorative Dental Materials; Mosby: Maryland Height, MI, USA, 2012; pp. 33–81. [Google Scholar]
- Yanıkoğlu, N.D.; Sakarya, R.E. Test Methods Used in the Evaluation of the Structure Features of the Restorative Materials: A Literature Review. J. Mater. Res. Technol. 2020, 9, 9720–9734. [Google Scholar] [CrossRef]
- Mazumdar, P.; Chowdhury, D. Manual of Laboratory Testing Methods for Dental Restorative Materials, 1st ed.; Wiley: Hoboken, NJ, USA, 2021; ISBN 9781119687993. [Google Scholar]
- Dulsamphan, C.; Chotitanmapong, T.; Klaisiri, A.; Krajangta, N. Effect of Protective Surface Coating on Hardness of Recent Uncoated High Viscosity Glass Ionomer Cement. J. Int. Dent. Med. Res. 2022, 15, 1036–1042. [Google Scholar]
- Kim, K.-H.; Ong, J.L.; Okuno, O. The Effect of Filler Loading and Morphology on the Mechanical Properties of Contemporary Composites. J. Prosthet. Dent. 2002, 87, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Swartz, M.L.; Phillips, R.W.; Moore, B.K.; Roberts, T.A. Materials Science Effect of Filler Content and Size on Properties of Composites. J. Dent. Res. 1985, 64, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Habib, S. Fluoride Releasing/Recharging Ability of Bulk-Fill and Resin Modified Glass Ionomer Cements After the Application of Different Surface Coating Agents: An In-Vitro Study. Adv. Dent. J. 2020, 2, 80–92. [Google Scholar] [CrossRef]
- Kelić, K.; Par, M.; Peroš, K.; Šutej, I.; Tarle, Z. Fluoride-Releasing Restorative Materials: The Effect of a Resinous Coat on Ion Release. Acta Stomatol. Croat. 2020, 54, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Tarifard, A. The Effect of Adhesive Systems on Fluoride Release of a Conventional Glass Ionomer: An In Vitro Study. Master’s Thesis, Tufts University, Boston, MA, USA, 2014. [Google Scholar]
- Cole, B.O.I.; Welbury, R.R. The Atraumatic Restorative Treatment (ART) Technique: Does It Have a Place in Everyday Practice? Dent. Update 2000, 27, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Soanca, A.; Bondor, C.I.; Moldovan, M.; Roman, A.; Rominu, M. Water Sorption and Solubility of an Experimental Dental Material: Comparative Study. Appl. Med. Inform. 2011, 29, 27–33. [Google Scholar]
- da Silva, E.M.; Almeida, G.S.; Poskus, L.T.; Guimarães, J.G.A. Relationship between the Degree of Conversion, Solubility and Salivary Sorption of a Hybrid and a Nanofilled Resin Composite. J. Appl. Oral. Sci. 2008, 16, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Frankenberger, R.; Krejci, I.; Bouillaguet, S.; Pashley, D.H.; Carvalho, R.M.; Lai, C.N.S. Single-Bottle Adhesives Behave as Permeable Membranes after Polymerization. I. In Vivo Evidence. J. Dent. 2004, 32, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Fabre, H.S.C.; Fabre, S.; Cefaly, D.F.G.; de Oliveira Carrilho, M.R.; Garcia, F.C.P.; Wang, L. Water Sorption and Solubility of Dentin Bonding Agents Light-Cured with Different Light Sources. J. Dent. 2007, 35, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.D.O. Water Sorption/Solubility of Dental Adhesive Resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Small, I.C.B.; Watson, T.F.; Chadwick, A.V.; Sidhu, S.K. Water Sorption in Resin-Modified Glass-Ionomer Cements: An In Vitro Comparison with Other Materials. Biomaterials 1998, 19, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Satterthwaite, J.D.; Silikas, N. Long-Term Sorption and Solubility of Bulk-Fill and Conventional Resin-Composites in Water and Artificial Saliva. J. Dent. 2015, 43, 1511–1518. [Google Scholar] [CrossRef]
- Cattani-Lorente, M.A.; Dupuis, V.; Payan, J.; Moya, F.; Meyer, J.M. Effect of Water on the Physical Properties of Resin-Modified Glass Ionomer Cements. Dent. Mater. 1999, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]

| Product | Manufacturer | Composition |
|---|---|---|
| EQUIA Forte® HT | GC, Tokyo, Japan | Powder: fluoroaluminosilicate glass, polyacrylic acid, iron oxide Liquid: polybasic carboxylic acid, water |
| EQUIA Forte Coat | GC, Tokyo, Japan | MMA * 25%–50%, photoinitiator 1%–5%, synergist 15%–5%, phosphoric acid ester monomer 15%–5%, BHT * <1% |
| Single Bond Universal Adhesive | 3M ESPE, St. Paul, MN, USA | MDP *, dimethacrylate resins, HEMA *, Vitrebond™ copolymer, filler, ethanol, water, initiators, silane |
| ExciTE®F Adhesive | Ivoclar vivadent, Schaan, Liechtenstein | Phosphoric acid acrylate, HEMA *, Bis-GMA *, alcohol, di-methacrylates, silicon dioxide, initiators, stabilizers |
| Petroleum jelly | Vaseline, Unilever Ltd., Dubai, United Arab Emirates | Mineral oils, paraffin, microcrystalline waxes |
| Mean ± Std. Deviation (HV) | Minimum | Maximum | p Value | |
|---|---|---|---|---|
| No Coating | 85.6 a ± 0.2 | 85.3 | 85.9 | <0.001 * |
| EQUIA Forte Coat | 108.3 a ± 1.2 | 106.5 | 110.5 | |
| Single Bond Universal Adhesive | 94.7 a ± 1.0 | 93.3 | 96.3 | |
| ExciTE®F | 99.4 a ± 2.2 | 97.1 | 104.2 | |
| Petroleum Jelly | 89.0 a ± 1.2 | 87.5 | 90.6 |
| 24 h | 7 days | p Value * | |
|---|---|---|---|
| Mean ± Std. Deviation (μg/mm3) | Mean ± Std. Deviation (μg/mm3) | ||
| No Coating | 108.5 ± 6.8 a | 95.9 ± 6.8 | <0.001 * |
| EQUIA Forte Coat | 74.4 ± 11.8 a | 91.5 ± 8.1 b | <0.001 * |
| Single Bond Universal Adhesive | 98.8 ± 16.8 | 109.2 ± 15.6 b | <0.001 * |
| ExciTE®F | 95.3 ± 7.8 | 103.6 ± 9.3 | <0.001 * |
| Petroleum Jelly | 95.5 ± 41.1 | 93.3 ± 21.0 | 0.9 * |
| p value ** | 0.015 ** | 0.024 ** |
| Mean (μg/mm3) | Std. Deviation | Minimum | Maximum | |
|---|---|---|---|---|
| No Coating | −43.0 a,b,c | 21.5 | −100.6 | −19.7 |
| Single Bond Universal Adhesive | −12.2 a,d | 6.3 | −28.0 | −5.7 |
| Petroleum Jelly | 18.4 b,d,e | 12.1 | 7.0 | 43.9 |
| EQUIA Forte Coat | −20.6 e,f | 40.0 | −133.1 | 2.5 |
| ExciTE®F | 9.0 c,f | 5.3 | 0.0 | 17.2 |
| p value | <0.001 * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqasabi, S.Y.; Sulimany, A.M.; Almohareb, T.; Alayad, A.S.; Bawazir, O.A. The Effect of Different Coating Agents on the Microhardness, Water Sorption, and Solubility of EQUIA Forte® HT. Coatings 2024, 14, 751. https://doi.org/10.3390/coatings14060751
Alqasabi SY, Sulimany AM, Almohareb T, Alayad AS, Bawazir OA. The Effect of Different Coating Agents on the Microhardness, Water Sorption, and Solubility of EQUIA Forte® HT. Coatings. 2024; 14(6):751. https://doi.org/10.3390/coatings14060751
Chicago/Turabian StyleAlqasabi, Saleh Y., Ayman M. Sulimany, Thamer Almohareb, Abdullah S. Alayad, and Omar A. Bawazir. 2024. "The Effect of Different Coating Agents on the Microhardness, Water Sorption, and Solubility of EQUIA Forte® HT" Coatings 14, no. 6: 751. https://doi.org/10.3390/coatings14060751






