The Effects of Preharvest Silicon Treatment and Passive MAP on Quality and Shelf Life of White Button Mushrooms in Thermoformed Recycled PET Packaging System
Abstract
1. Introduction
2. Materials and Methods
2.1. Mushroom Cultivation
2.2. Silicon Treatment Applications
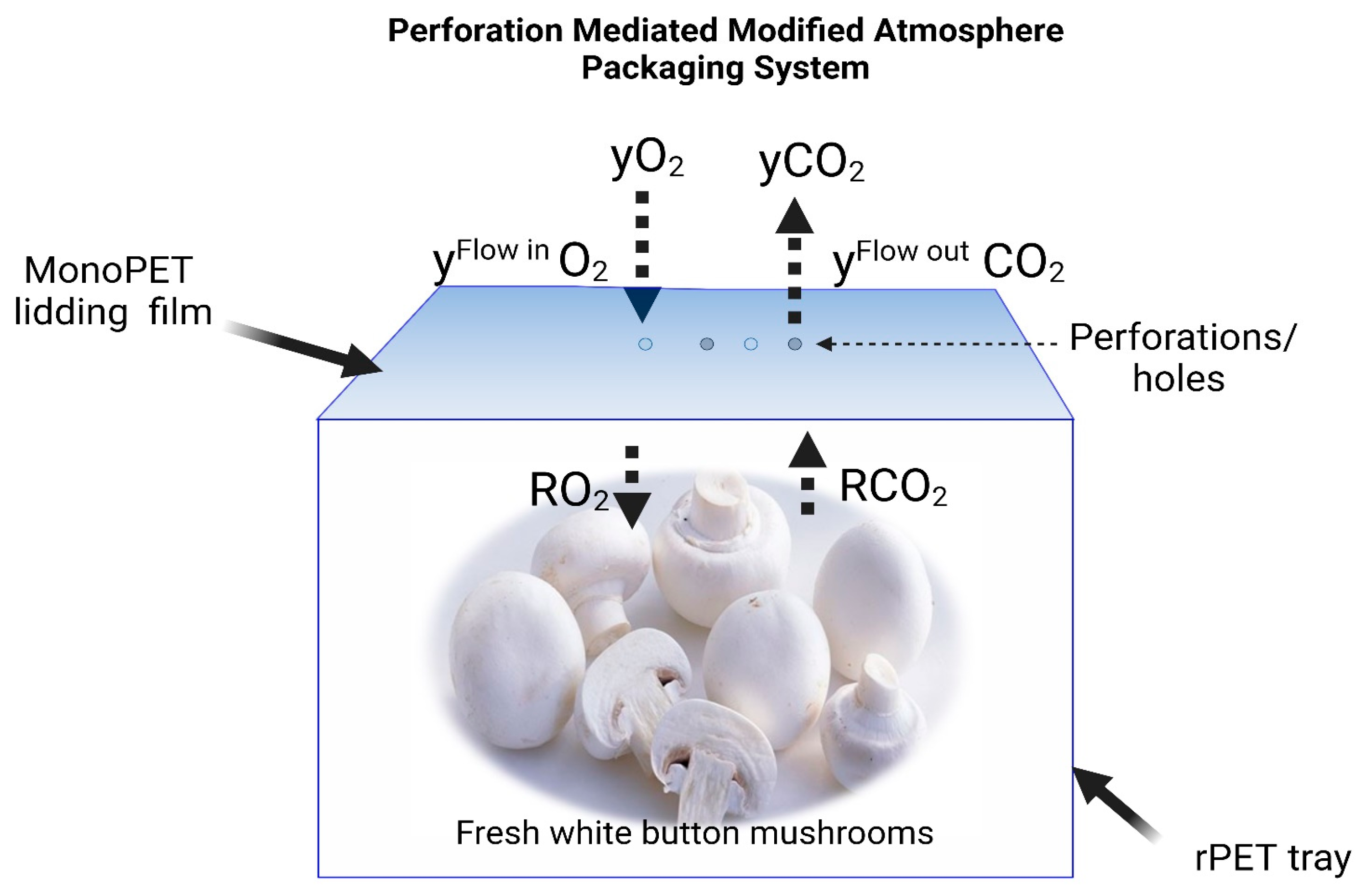
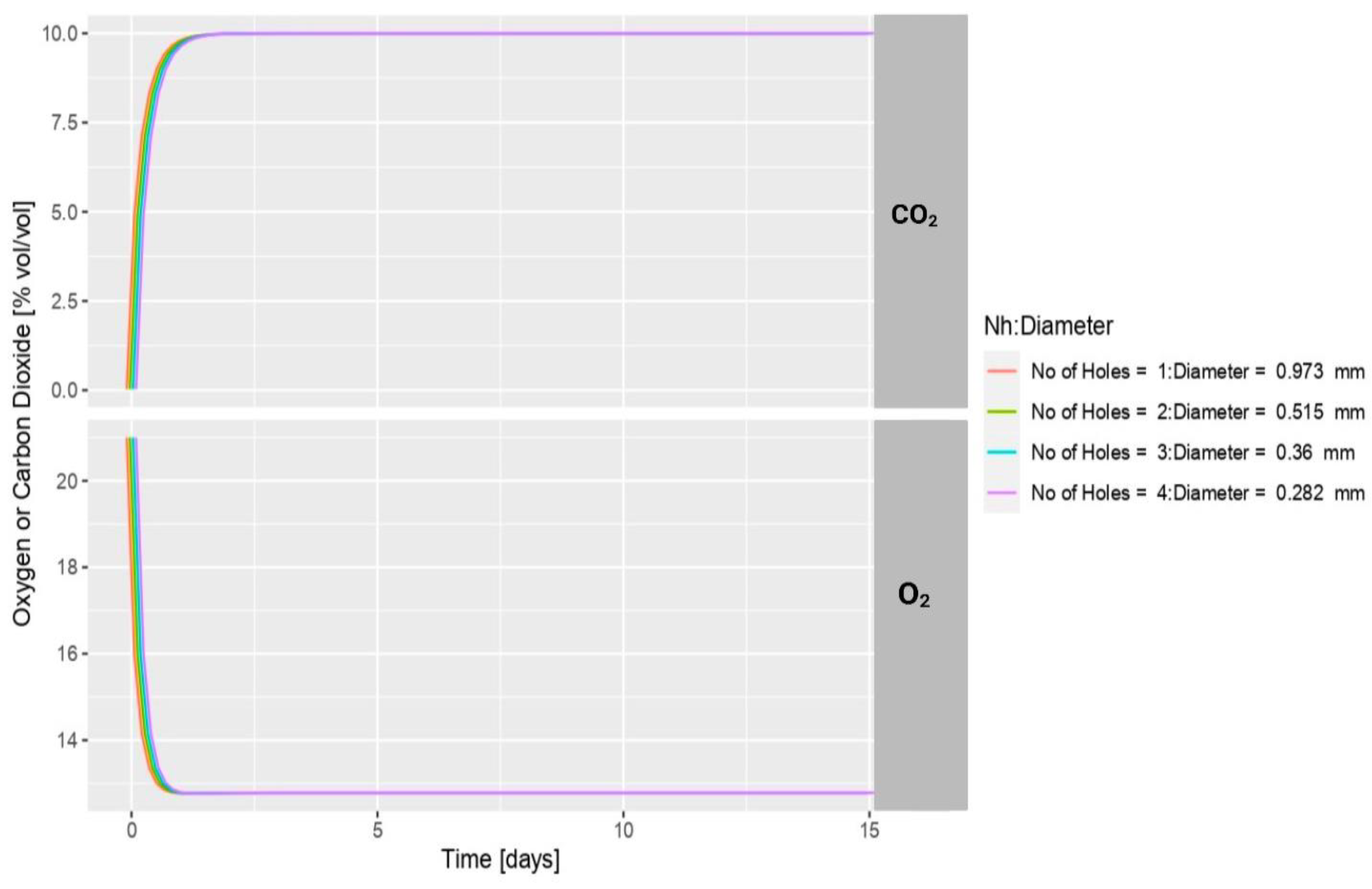
2.3. Optimization of the Passive Modified Atmosphere Packaging (MAP) Conditions
- Vf is the headspace (free volume) in the package
- y is the gas concentration (in molar fraction)
- e is the thickness of the polymeric film
- The package’s permeability, denoted as P, is the amount of gas exchanged per unit time and area.
- The respiration rate, denoted as R, is the volume of gas produced or consumed per unit time.
- M represents the weight of the product.
- The subscripts O2 and CO2 indicate oxygen and carbon dioxide, respectively.
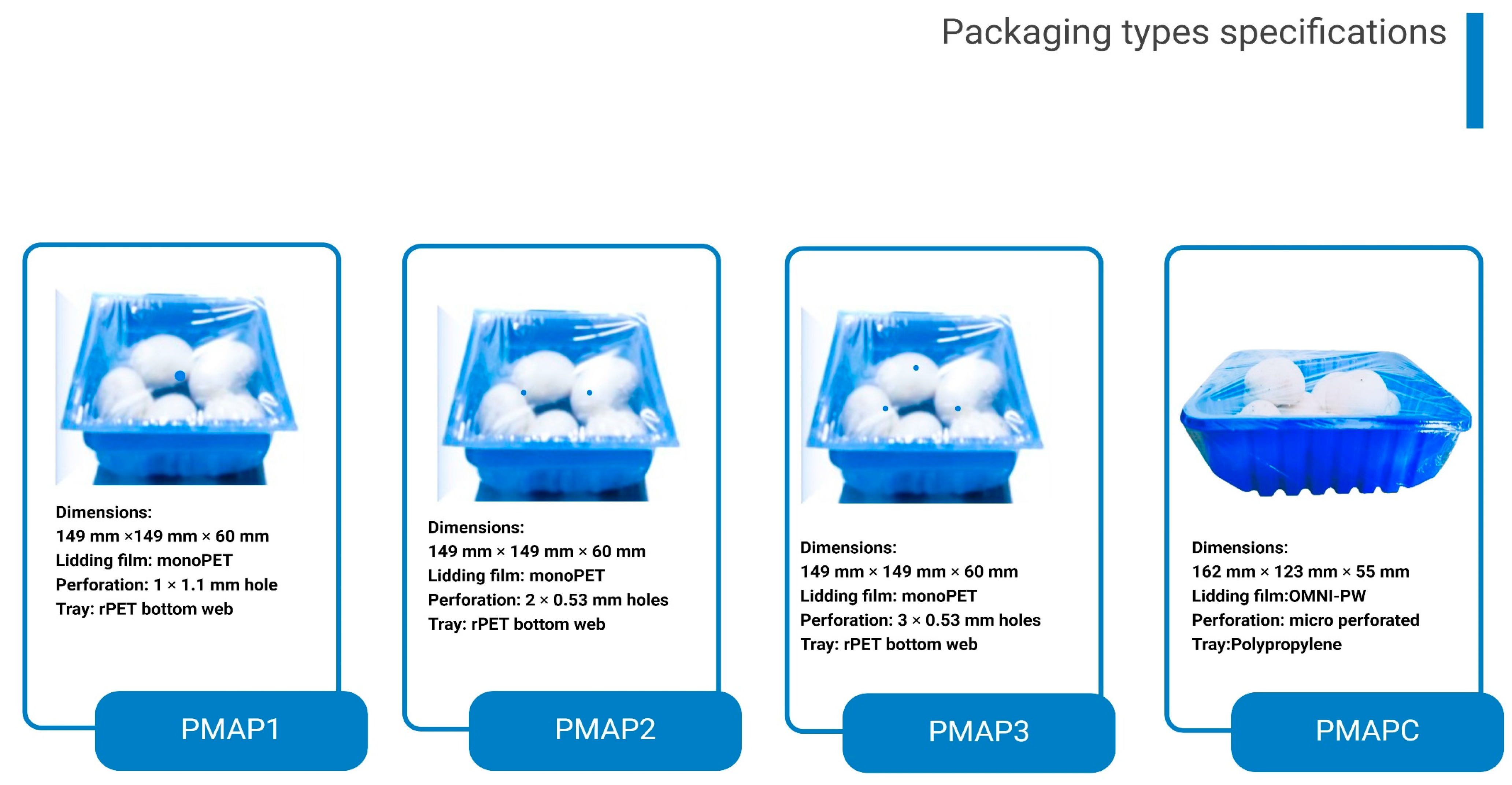
2.4. Packaging Treatments
2.5. Experimental Design
2.6. Analytical Methods
2.6.1. Sampling of Gas from the Packaging Atmosphere
2.6.2. Colour
2.6.3. Density
2.6.4. Electrolyte Leakage
2.6.5. Total Soluble Solids (TSS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Headspace Gas Composition during the Storage Days
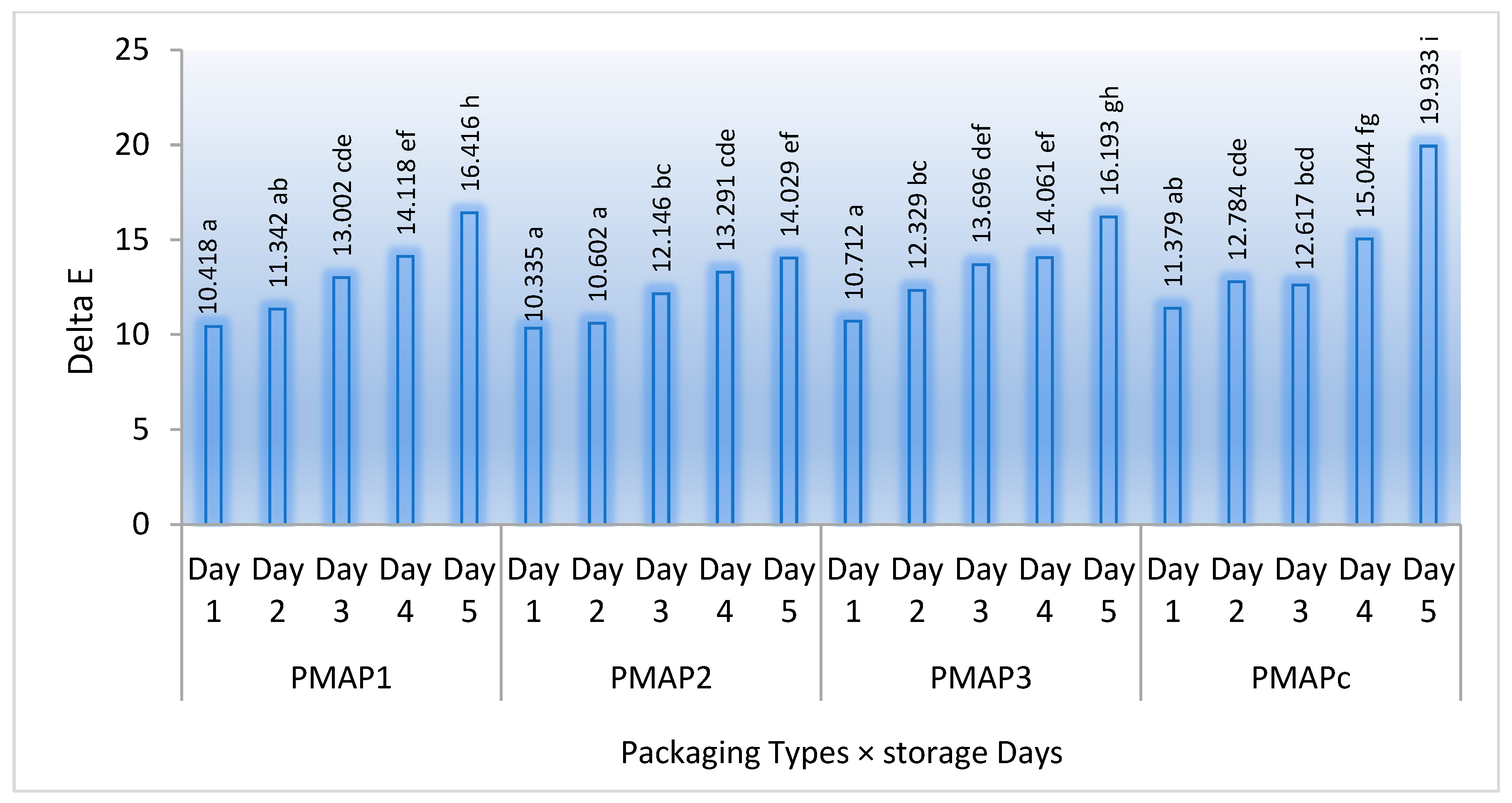
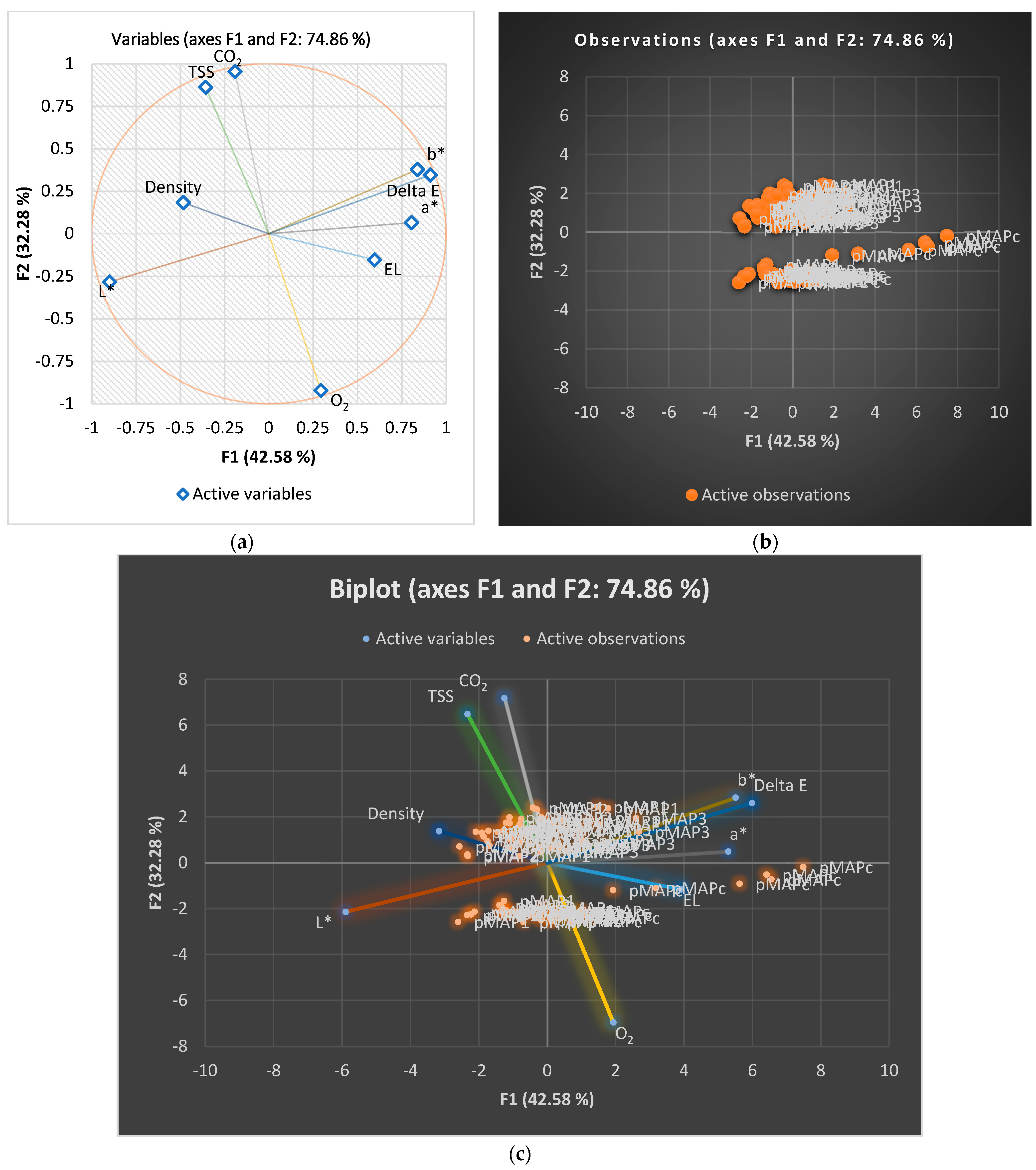
3.2. Colour Changes during the Storage Period
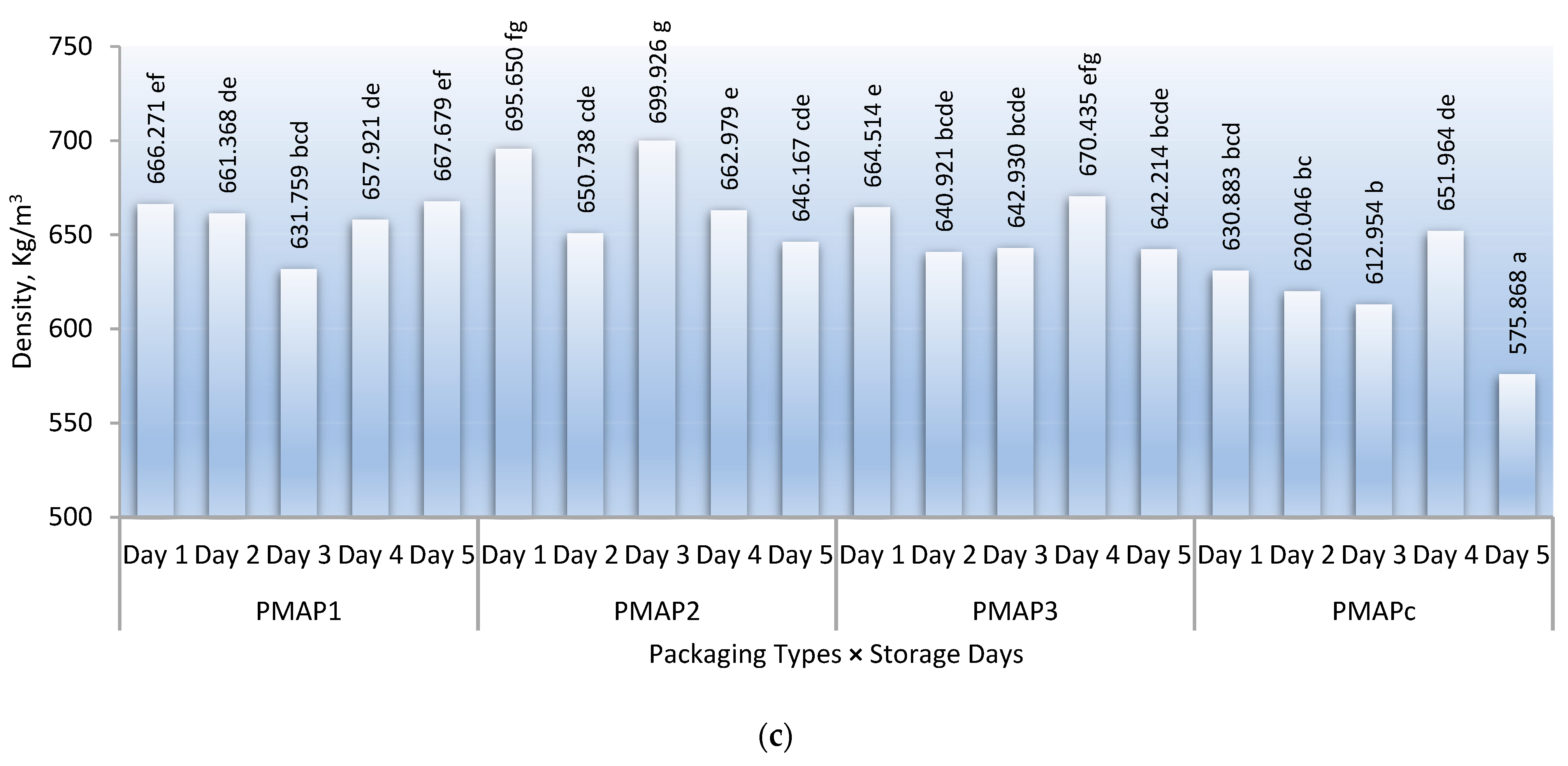
3.3. Electrolyte Leakage, TSS, and Density during the Storage Period
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Kamal, S.; Sharma, V. Status and trends in world mushroom production-III-World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2020, 29, 75. [Google Scholar] [CrossRef]
- Teagasc, ‘The Irish Mushroom Industry’, Teagasc Fact Sheet Hortic. 7—Mushroom Prod. No. V1 2020. 2020. Available online: https://www.teagasc.ie/media/website/rural-economy/rural-development/diversification/7-Mushroom-Production.pdf (accessed on 22 November 2023).
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh mushroom preservation techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.M.B.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. NPJ Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent developments on umami ingredients of edible mushrooms—A review. Trends Food Sci. Technol. 2013, 33, 78–92. [Google Scholar] [CrossRef]
- Xue, Z.; Hao, J.; Yu, W.; Kou, X. Effects of Processing and Storage Preservation Technologies on Nutritional Quality and Biological Activities of Edible Fungi: A Review. J. Food Process Eng. 2017, 40, e12437. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Das, S.; Singh, B.K.; Kishore Dubey, N. Green facile synthesis of cajuput (Melaleuca cajuputi Powell.) essential oil loaded chitosan film and evaluation of its effectiveness on shelf-life extension of white button mushroom. Food Chem. 2023, 401, 134114. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, H.; Sun, Y.; Xia, R.; Hou, Z.; Li, Y.; Wang, Y.; Pan, S.; Fan, Y.; Zhu, J.; et al. Review of packaging for improving storage quality of fresh edible mushrooms. Packag. Technol. Sci. 2023, 36, 629–646. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Qi, Z.; Deng, Z.; Han, A.; Long, H.; Wang, J.; Yao, W.; et al. Advances in Postharvest Storage and Preservation Strategies for Pleurotus eryngii. Foods 2023, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zhang, M.; Wang, D.; Mujumdar, A.S.; Bhandari, B.; Zhang, L. New preservation and detection technologies for edible mushrooms: A review. J. Sci. Food Agric. 2023, 103, 3230–3248. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Y.Y.; Pan, L.H.; Li, Q.M.; Luo, J.P.; Zha, X.Q. Co-encapsulation systems for delivery of bioactive ingredients. Food Res. Int. 2022, 155, 111073. [Google Scholar] [CrossRef] [PubMed]
- Villaescusa, R.; Gil, M.I. Quality improvement of Pleurotus mushrooms by modified atmosphere packaging and moisture absorbers. Postharvest Biol. Technol. 2003, 28, 169–179. [Google Scholar] [CrossRef]
- Oliveira, F.; Sousa-Gallagher, M.J.; Mahajan, P.V.; Teixeira, J.A. Evaluation of MAP engineering design parameters on quality of fresh-sliced mushrooms. J. Food Eng. 2012, 108, 507–514. [Google Scholar] [CrossRef]
- Gholami, R.; Ahmadi, E.; Ahmadi, S. Investigating the effect of chitosan, nanopackaging, and modified atmosphere packaging on physical, chemical, and mechanical properties of button mushroom during storage. Food Sci. Nutr. 2020, 8, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.V.; Oliveira, F.A.R.; Montanez, J.C.; Frias, J. Development of user-friendly software for design of modified atmosphere packaging for fresh and fresh-cut produce. Innov. Food Sci. Emerg. Technol. 2007, 8, 84–92. [Google Scholar] [CrossRef]
- Jafri, M.; Jha, A.; Bunkar, D.S.; Ram, R.C. Quality retention of oyster mushrooms (Pleurotus florida) by a combination of chemical treatments and modified atmosphere packaging. Postharvest Biol. Technol. 2013, 76, 112–118. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Modelling approaches for designing and evaluating the performance of modified atmosphere packaging (MAP) systems for fresh produce: A review. Food Packag. Shelf Life 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Teagasc. No more PET peeves? TResearch Cream of the crop. In TResearch Magazine; Catriona, B., Ed.; Teagasc: Carlow, Ireland, 2023; Volume 18. [Google Scholar]
- Hernández-Muñoz, P.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Catalá, R.; Gavara, R. Nanotechnology in food packaging. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–232. [Google Scholar] [CrossRef]
- Wang, T.; Yun, J.; Zhang, Y.; Bi, Y.; Zhao, F.; Niu, Y. Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2021, 176, 111501. [Google Scholar] [CrossRef]
- Zheng, E.; Zheng, Z.; Ren, S.; Zhou, H.; Yang, H. Postharvest quality and reactive oxygen species metabolism improvement of Coprinus comatus mushroom using allyl isothiocyanate fumigation. Food Qual. Saf. 2022, 6, fyac031. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 175–187. [Google Scholar] [CrossRef]
- Artyszak, A. Effect of silicon fertilization on crop yield quantity and quality—A literature review in Europe. Plants 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Dodgson, J.; Weston, A.K.; Marks, D.J. Tomato Firmness and Shelf-Life Increased by Application of Stimulated Calcium. Crops 2023, 3, 251–265. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Ziogas, V.; Bravos, N.; Hussain, S.B. Preharvest Foliar Application of Si–Ca-Based Biostimulant Affects Postharvest Quality and Shelf-Life of Clementine Mandarin (Citrus clementina Hort. Ex Tan). Horticulturae 2022, 8, 996. [Google Scholar] [CrossRef]
- Rojas-Rodríguez, M.L.; Ramírez-Gil, J.G.; González-Concha, L.F.; Balaguera-López, H.E. Biostimulants Improve Yield and Quality in Preharvest without Impinging on the Postharvest Quality of Hass Avocado and Mango Fruit: Evaluation under Organic and Traditional Systems. Agronomy 2023, 13, 1917. [Google Scholar] [CrossRef]
- Rombolà, A.D.; Quartieri, M.; Rodríguez-Declet, A.; Minnocci, A.; Sebastiani, L.; Sorrenti, G. Canopy-applied silicon is an effective strategy for reducing sweet cherry cracking. Hortic. Environ. Biotechnol. 2023, 64, 371–378. [Google Scholar] [CrossRef]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Zied, D.C.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barros, L. Influence of calcium silicate on the chemical properties of Pleurotus ostreatus var. Florida (Jacq.) P. Kumm. J. Fungi 2020, 6, 299. [Google Scholar] [CrossRef]
- EN ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space (ISO/CIE 11664-4:2019). National Standards Authority of Ireland: Dublin, Ireland, 2019.
- Ajlouni, S.O. Quality Characteristics of Two Hybrids of the Cultivated Mushrooms (Agaricus bisporus) and the Improvem Ent of Their Shelf Life Using Stipe Trimming and Gamma Irradiation; The Pennsylvania State University: University Park, PA, USA, 1991. [Google Scholar]
- Hatsugai, N.; Katagiri, F. Quantification of Plant Cell Death by Electrolyte Leakage Assay. Bio-Protocol 2018, 8, e2758. [Google Scholar] [CrossRef]
- Sandhya. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT-Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yang, H.; Wang, A. Effect of exogenous glycine betaine on qualities of button mushrooms (Agaricus bisporus) during postharvest storage. Eur. Food Res. Technol. 2015, 240, 41–48. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Kitazawa, H.; Wang, Y. Storage temperature effect on quality related with cell wall metabolism of shiitake mushrooms (Lentinula edodes) and its modeling. Food Packag. Shelf Life 2022, 32, 100865. [Google Scholar] [CrossRef]
- Shonte, T.T.; Mulla, M.F.; Foley, L.; Pathania, S. Mechanisms of Action and Preservation Effects of Packaging Systems for Mushrooms: Novel Approaches to Preserve Irish Edible Mushrooms. Coatings 2024, 14, 172. [Google Scholar] [CrossRef]
- Chang, T.-S.; Tseng, M.; Ding, H.; Tai, S.S.-K. Isolation and characterization of Streptomyces hiroshimensis strain TI-C3 with anti-tyrosinase activity. J. Soc. Cosmet. Chem. 2009, 31, 72. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Haudecoeur, R.; Carotti, M.; Gouron, A.; Maresca, M.; Buitrago, E.; Hardre, R.; Bergantino, E.; Jamet, H.; Belle, C.; Reglier, M.; et al. 2-Hydroxypyridine- N-oxide-Embedded Aurones as Potent Human Tyrosinase Inhibitors. ACS Med. Chem. Lett. 2016, 8, 55–60. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Wei, W.; Lv, P.; Xia, Q.; Tan, F.; Sun, F.; Yu, W.; Jia, L.; Cheng, J. Fresh-keeping effects of three types of modified atmosphere packaging of pine-mushrooms. Postharvest Biol. Technol. 2017, 132, 62–70. [Google Scholar] [CrossRef]
- Jiang, T. Effect of alginate coating on physicochemical and sensory qualities of button mushrooms (Agaricus bisporus) under a high oxygen modified atmosphere. Postharvest Biol. Technol. 2013, 76, 91–97. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Klaus, A.; Cheng, A.; Salis, S.A.; Abdul Halim-Lim, S. Total quality index of commercial oyster mushroom Pleurotus sapidus in modified atmosphere packaging. Br. Food J. 2019, 121, 1871–1883. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Wang, Y.Y.; Bu, H.; Dong, T. Changes in cell wall metabolism and flavor qualities of mushrooms (Agaricus bernardii) under EMAP treatments during storage. Food Packag. Shelf Life 2021, 29, 100732. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, D.; Wu, Y.; Yuan, M.; Li, L.; Yang, J. Effect of PLA/PCL/cinnamaldehyde antimicrobial packaging on physicochemical and microbial quality of button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 99, 73–79. [Google Scholar] [CrossRef]
- Li, Y.; Ishikawa, Y.; Satake, T.; Kitazawa, H.; Qiu, X.; Rungchang, S. Effect of active modified atmosphere packaging with different initial gas compositions on nutritional compounds of shiitake mushrooms (Lentinus edodes). Postharvest Biol. Technol. 2014, 92, 107–113. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Changes in color, antioxidant, and free radical scavenging enzyme activity of mushrooms under high oxygen modified atmospheres. Postharvest Biol. Technol. 2012, 69, 1–6. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hu, H.; Sun, Y.; Wang, Y.; Zhao, Y. High carbon dioxide and low oxygen storage effects on reactive oxygen species metabolism in Pleurotus eryngii. Postharvest Biol. Technol. 2013, 85, 141–146. [Google Scholar] [CrossRef]
- Parentelli, C.; Ares, G.; Corona, M.; Lareo, C.; Gambaro, A.; Soubes, M.; Lema, P. Sensory and microbiological quality of shiitake mushrooms in modified-atmosphere packages. J. Sci. Food Agric. 2007, 87, 1645–1652. [Google Scholar] [CrossRef]

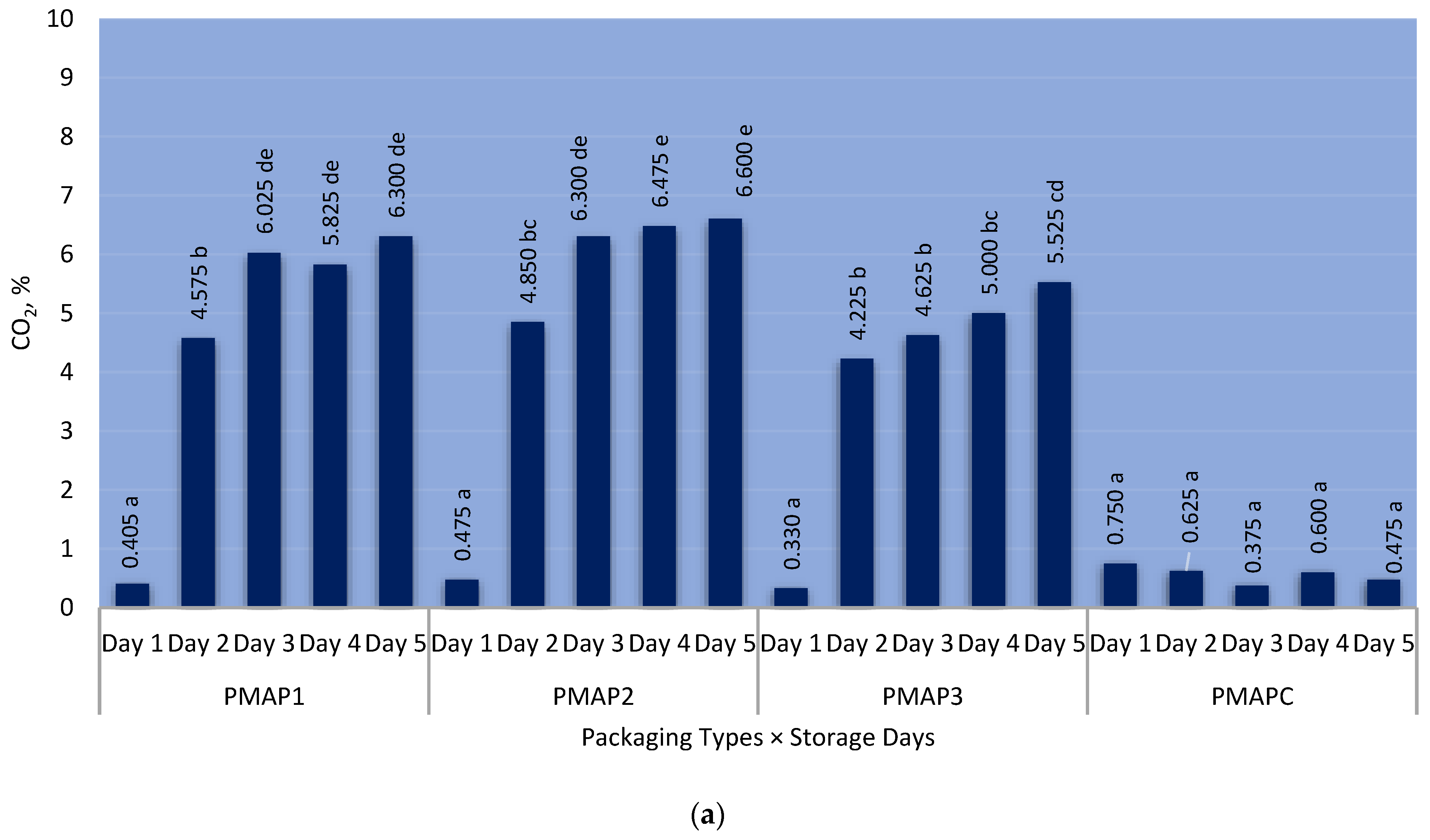


| Category | Model | SD | PHT | PT | SD×PHT | SD×PT | PHT×PT | SD×PHT×PT |
|---|---|---|---|---|---|---|---|---|
| CO2 | <0.0001 | <0.0001 | 0.731 | <0.0001 | 0.996 | <0.0001 | 0.896 | 1.000 |
| O2 | <0.0001 | <0.0001 | 0.650 | <0.0001 | 0.992 | <0.0001 | 0.838 | 1.000 |
| L* | <0.0001 | <0.0001 | 0.471 | <0.0001 | 0.855 | <0.0001 | 0.958 | 0.179 |
| a* | <0.0001 | <0.0001 | 0.126 | <0.0001 | 0.094 | <0.0001 | 0.688 | 0.637 |
| b* | <0.0001 | <0.0001 | 0.023 | <0.0001 | 0.928 | 0.258 | 0.553 | 0.999 |
| ΔE | <0.0001 | <0.0001 | 0.104 | <0.0001 | 0.954 | <0.0001 | 0.622 | 1.000 |
| EL | <0.0001 | 0.005 | 0.135 | <0.0001 | 0.147 | 0.240 | 0.016 | 0.130 |
| Density | <0.0001 | 0.001 | 0.072 | <0.0001 | 0.008 | 0.002 | 0.004 | 0.001 |
| TSS | <0.0001 | <0.0001 | 0.531 | <0.0001 | <0.0001 | <0.0001 | 0.023 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shonte, T.T.; Grogan, H.; Frias Celayeta, J.M.; Giordano, F.S.; Reynolds, A.; O’Halloran, O.; Foley, L.; Pathania, S. The Effects of Preharvest Silicon Treatment and Passive MAP on Quality and Shelf Life of White Button Mushrooms in Thermoformed Recycled PET Packaging System. Coatings 2024, 14, 754. https://doi.org/10.3390/coatings14060754
Shonte TT, Grogan H, Frias Celayeta JM, Giordano FS, Reynolds A, O’Halloran O, Foley L, Pathania S. The Effects of Preharvest Silicon Treatment and Passive MAP on Quality and Shelf Life of White Button Mushrooms in Thermoformed Recycled PET Packaging System. Coatings. 2024; 14(6):754. https://doi.org/10.3390/coatings14060754
Chicago/Turabian StyleShonte, Tigist T., Helen Grogan, Jesus Maria Frias Celayeta, Francesco S. Giordano, Andrew Reynolds, Orla O’Halloran, Lorraine Foley, and Shivani Pathania. 2024. "The Effects of Preharvest Silicon Treatment and Passive MAP on Quality and Shelf Life of White Button Mushrooms in Thermoformed Recycled PET Packaging System" Coatings 14, no. 6: 754. https://doi.org/10.3390/coatings14060754
APA StyleShonte, T. T., Grogan, H., Frias Celayeta, J. M., Giordano, F. S., Reynolds, A., O’Halloran, O., Foley, L., & Pathania, S. (2024). The Effects of Preharvest Silicon Treatment and Passive MAP on Quality and Shelf Life of White Button Mushrooms in Thermoformed Recycled PET Packaging System. Coatings, 14(6), 754. https://doi.org/10.3390/coatings14060754









