Modeling for the Fabrication Process of a ϕ1185 mm C/C Composite Thermal Insulation Tube in an Isothermal Chemical Vapor Infiltration Reactor
Abstract
:1. Introduction
2. Simulation Model and Verification
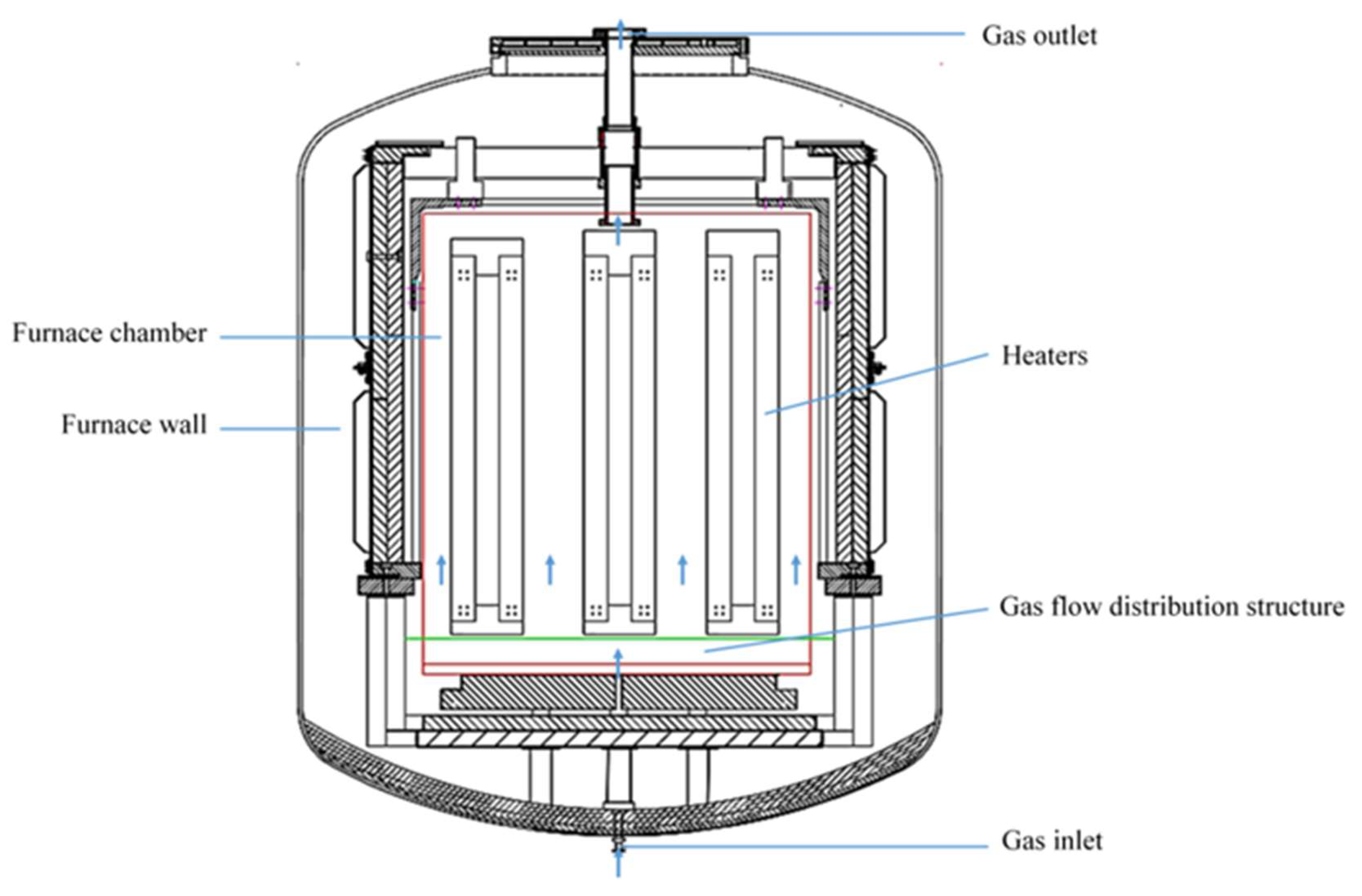
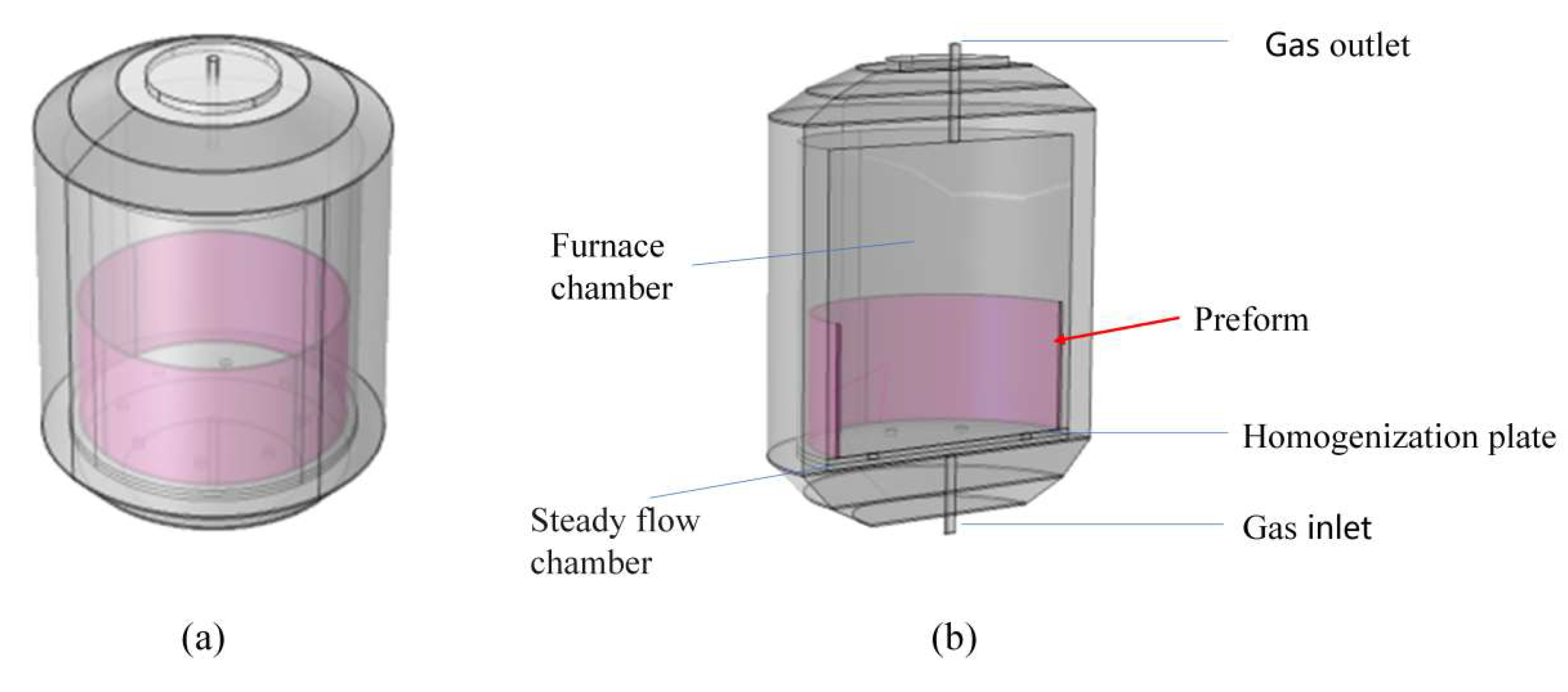
2.1. Geometric Modeling
2.2. Multi-Physics Field Model
- (1)
- Flow equation
- (2)
- Heat transfer equation
- (3)
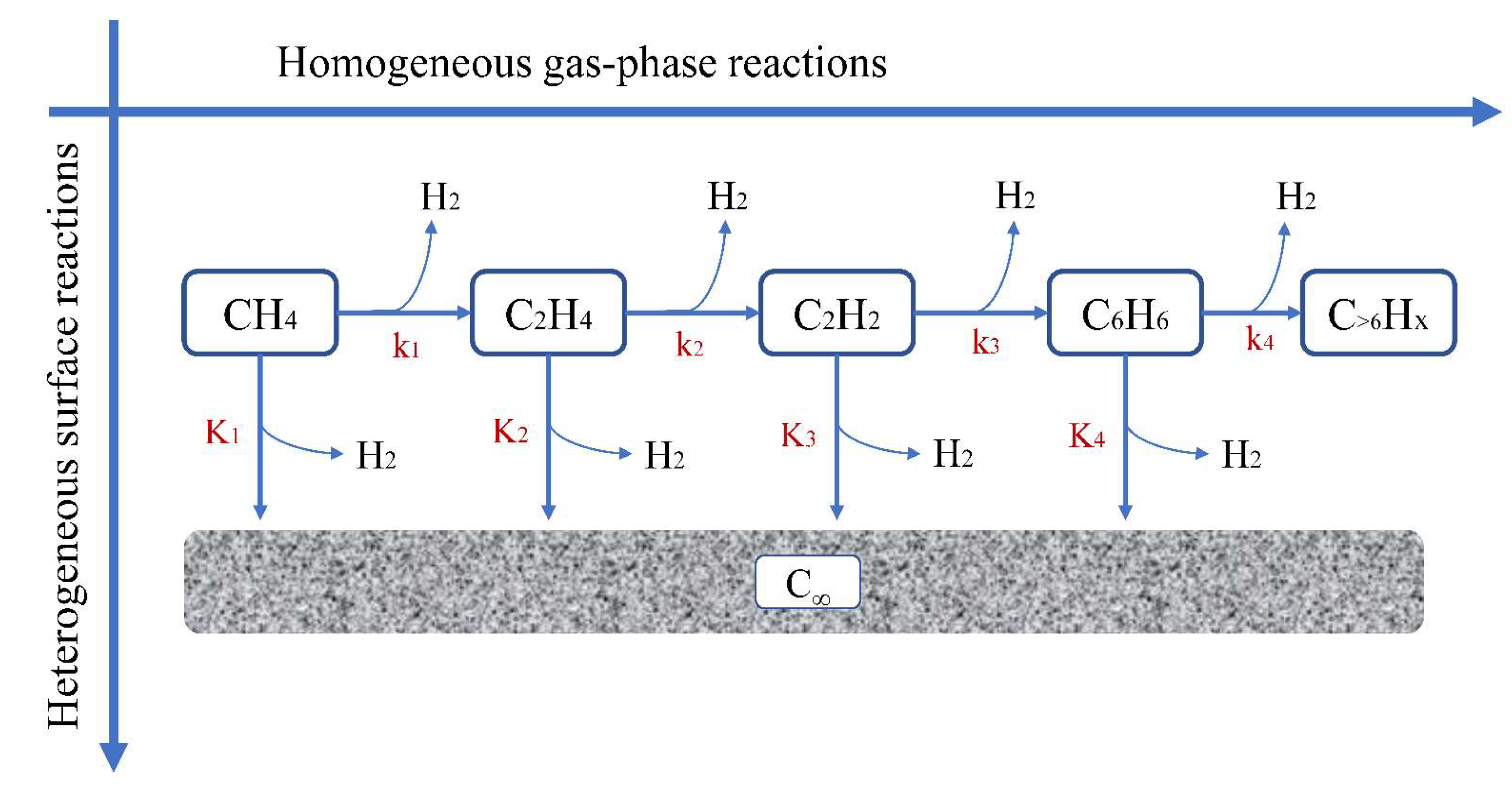
- Mass transfer function
2.3. Simulation Model Validation
3. Results and Discussion
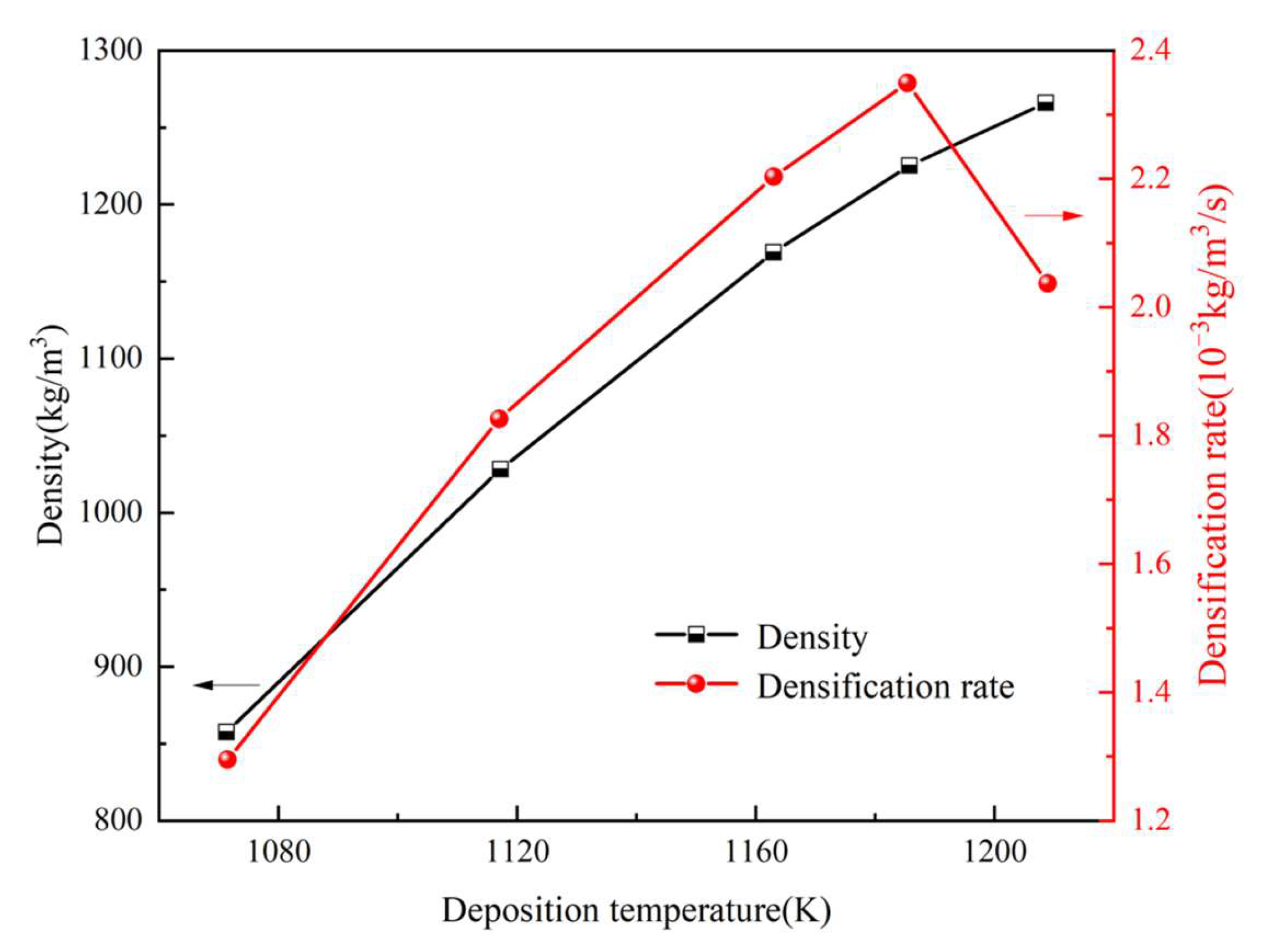
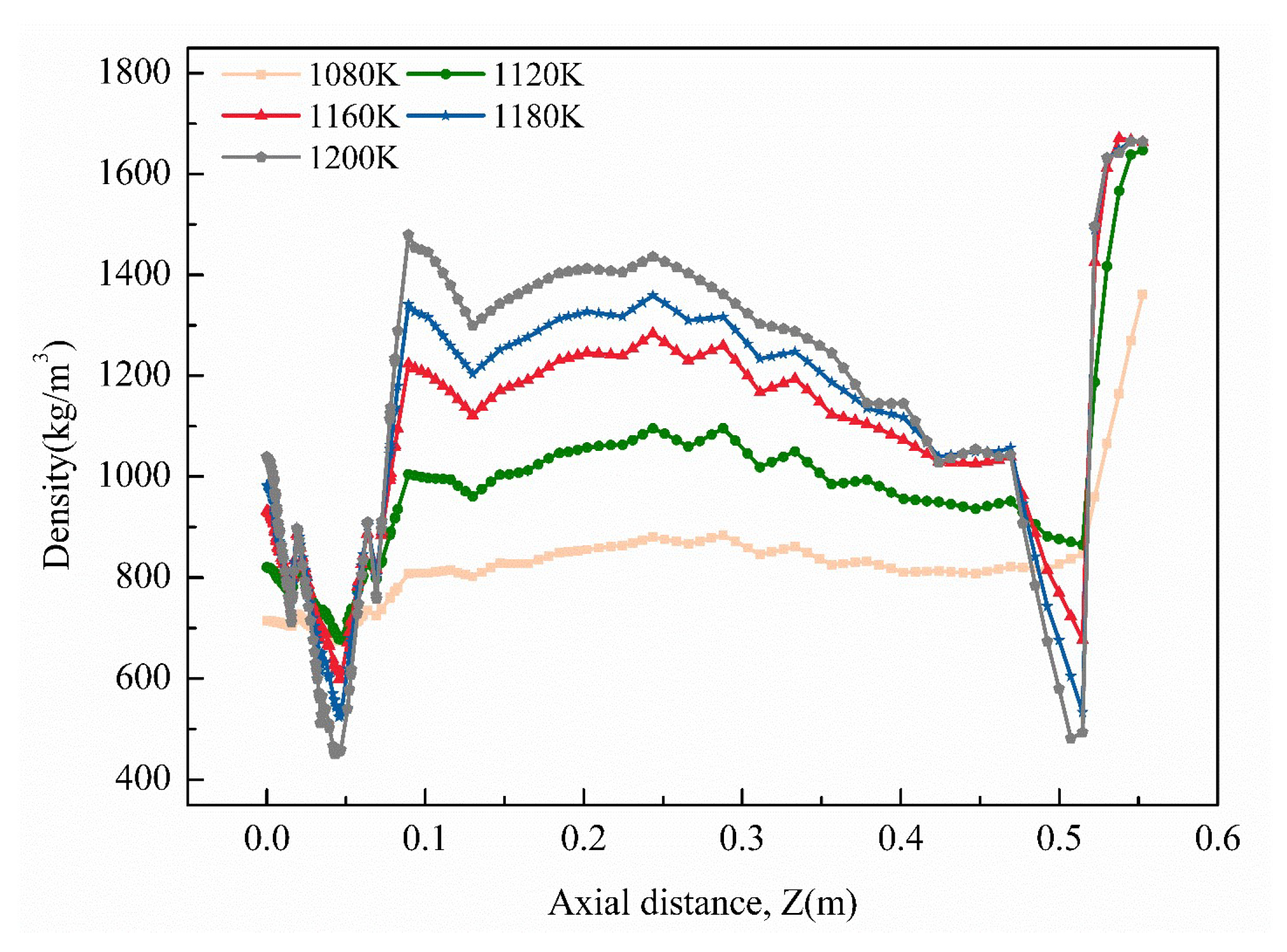
3.1. Determination of Deposition Temperature
3.2. Effect of Gas Pressure
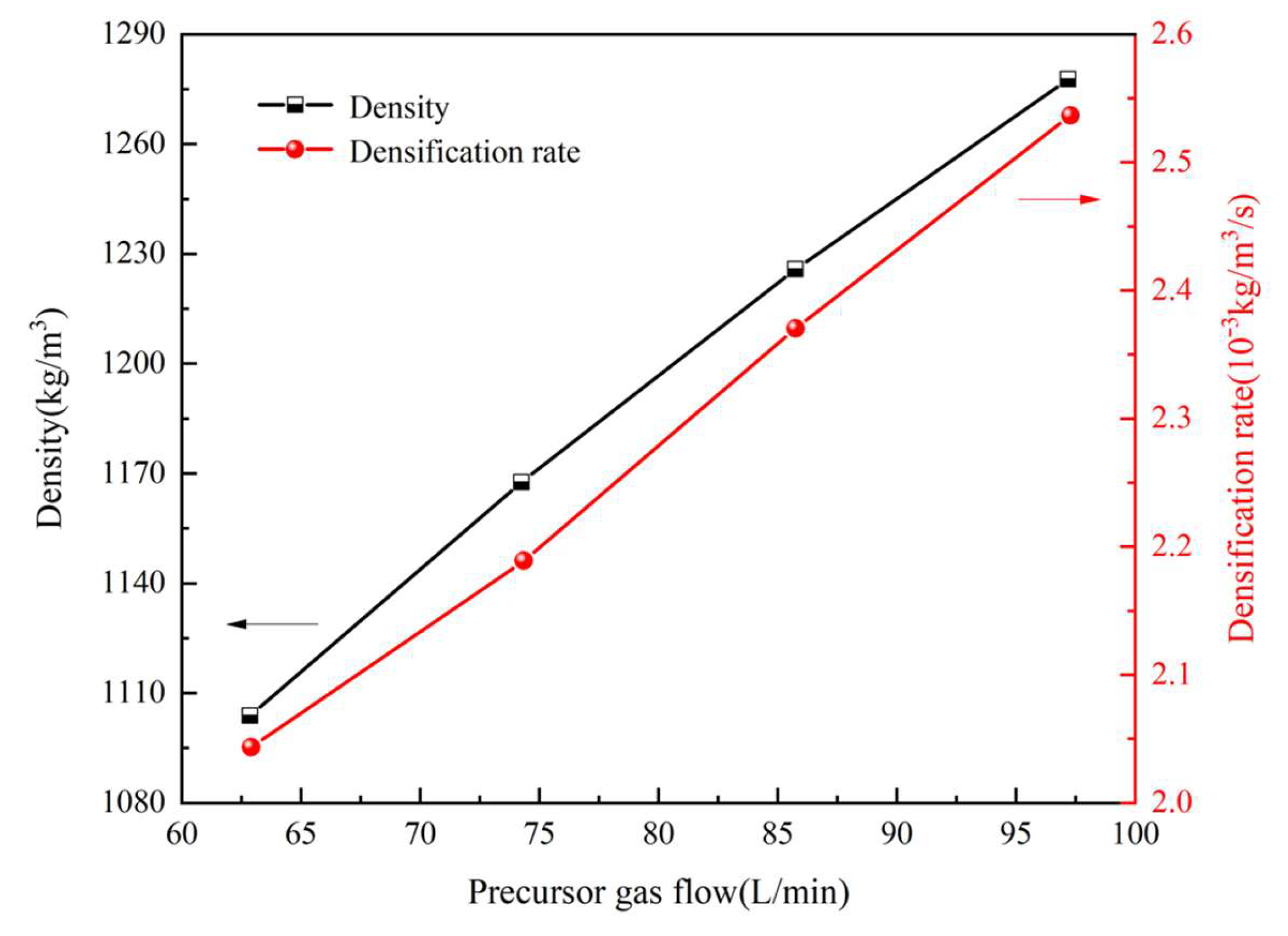
3.3. Influence of Precursor Gas Flow Rate
3.4. Synergistic Adjustment and Optimization of the Process
4. Conclusions
- (1)
- A multi-physics field coupling simulation model was established for the isothermal CVI process of a large-sized C/C composite thermal insulation tube, including parameters such as convection, heat conduction, diffusion, deposition reaction, and pore evolution. The densification behavior of the large-sized C/C composite during the CVI process was effectively predicted, and the process parameters were optimized.
- (2)
- It could be observed that the deposition temperature, gas pressure, and precursor gas flow rate were the key factors influencing the densification of the thermal insulation tube. An increase in the deposition temperature was found to notably enhance the degree of densification; however, it can lead to premature pore closure and a decrease in the densification rate. An increase in gas pressure reduced both the average density and densification rate. Further, the increase in the precursor gas flow rate enhanced both the average density and densification rate.
- (3)
- Synergistic process optimization was achieved. It was found that at a temperature of 1160 K, a gas pressure of 0.05 MPa, and a methane flow rate of 95 L·min−1, the average density of the thermal insulation tube increased by 5.7% and the densification rate increased by 26.5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, B.; Wang, Y.; Hu, L.; Lin, L.; Ma, C.; Zhang, C.; Lu, Y.; Sun, K.; Wu, X. Preparation and properties of lightweight carbon/carbon fiber composite thermal field insulation materials for high-temperature furnace. J. Eng. Fibers Fabr. 2019, 14, 1558925019884691. [Google Scholar] [CrossRef]
- Beaumont, P.W.R.; Zweben, C.H. Ceramic and Carbon Matrix Composites—5.16 Applications of Carbon/Carbon Composites. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 5, pp. 421–436. [Google Scholar]
- Albano, M.; Delfini, A.; Pastore, R.; Micheli, D.; Marchetti, M. A new technology for production of high thickness carbon/carbon composites for launchers application. Acta Astronaut. 2016, 128, 277–285. [Google Scholar] [CrossRef]
- Alghamdi, A.; Alharthi, H.; Alamoudi, A.; Alharthi, A.; Kensara, A.; Taylor, S. Effect of needling parameters and manufacturing porosities on the effective thermal conductivity of a 3D carbon–carbon composite. Materials 2019, 12, 3750. [Google Scholar] [CrossRef] [PubMed]
- Muruganantham, R.; Yang, C.W.; Wang, H.J.; Huang, C.H.; Liu, W.R. Industrial silicon-wafer-wastage-derived carbon-enfolded Si/Si-C/C nanocomposite anode material through plasma-assisted discharge process for rechargeable Li-ion storage. Nanomaterials 2022, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Tao, M. Inorganic photovoltaic solar cells: Silicon and beyond. Electrochem. Soc. Interface 2008, 17, 30–35. [Google Scholar] [CrossRef]
- Su, W.; Zuo, R.; Mazaev, K.; Kalaev, V. Optimization of crystal growth by changes of flow guide, radiation shield and sidewall insulation in Cz Si furnace. J. Cryst. Growth 2010, 312, 495–501. [Google Scholar] [CrossRef]
- Gruner, S.; Kranert, C.; Jauß, T.; Sorgenfrei, T.; Reimann, C.; Friedrich, J. Investigation of Facetted Growth in Heavily Doped Silicon Crystals Grown in Mirror Furnaces. Crystals 2022, 12, 1575. [Google Scholar] [CrossRef]
- Li, H.; Li, A.; Bai, R.; Li, K. Numerical simulation of chemical vapor infiltration of propylene into C/C composite materials with reduced multi-step kinetic models. Carbon 2005, 43, 2937–2950. [Google Scholar] [CrossRef]
- Vignole, G.L. Modeling of chemical vapor infiltration processes. In Advances in Composite materials Manufacturing and Process Design; Woodhead Publishing: Sawston, UK, 2015; pp. 415–458. [Google Scholar]
- Hou, X.; Li, H.; Chen, Y.; Li, K. Modeling of chemical vapor infiltration process for fabrication of carbon–carbon composite materials by finite difference methods. Carbon 1999, 37, 669–677. [Google Scholar] [CrossRef]
- McAllister, P.; Wolf, E. Simulation of a multiple substrate reactor for chemical vapor infiltration of pyrolytic carbon within carbon-carbon composite materials. AIChE J. 1993, 39, 1196–1209. [Google Scholar] [CrossRef]
- Ibrahim, J.; Paolucci, S. Transient solution of chemical vapor infiltration/deposition in a reactor. Carbon 2011, 49, 915–930. [Google Scholar] [CrossRef]
- Tang, Z.; Li, A.; Hatakeyama, T.; Shuto, H.; Hayashi, J.I.; Norinaga, K. Transient three-dimensional simulation of densification process of carbon fibre preforms via chemical vapor infiltration of carbon matrix from methane. Chem. Eng. Sci. 2018, 176, 107–115. [Google Scholar] [CrossRef]
- Kim, H.G.; Ji, W.; Kwon, H.J.; Yoon, S.; Kim, J.I.; Bae, S.; Cho, N.C. Full-scale multi-physics numerical analysis of an isothermal chemical vapor infiltration process for manufacturing C/C composite materials. Carbon 2021, 172, 174–188. [Google Scholar] [CrossRef]
- Albano, M.; Pastore, R.; Delfini, A.; Micheli, D.; Volpini, F.; Marchetti, M. Densification of high thickness C/C composite materials by chemical vapor infiltration. Procedia Eng. 2015, 19, 381–389. [Google Scholar] [CrossRef]
- Hu, Z.J.; Zhang, W.G.; Hüttinger, K.J.; Reznik, B.; Gerthsen, D. Influence of pressure, temperature and surface area/volume ratio on the texture of pyrolytic carbon deposited from methane. Carbon 2003, 41, 749–758. [Google Scholar] [CrossRef]
- Tang, Z.H.; Qu, D.N.; Xiong, J.; Zou, Z.Q. Effects of infiltration conditions on the densification behavior of C/C c-omposite materials prepared by a directional-flow thermal gradient CVI process. Carbon 2003, 41, 2703–2710. [Google Scholar] [CrossRef]
- Reuge, N.; Vignoles, G.L. Modeling of isobaric–isothermal chemical vapor infiltration: Effects of reactor control parameters on a densification. J. Mater. Process. Technol. 2005, 166, 15–29. [Google Scholar] [CrossRef]
- Mahoney, P.; Povitsky, A. Modeling of Chemical Vapor Infiltration for Fiber-Reinforced Silicon Carbide Composites Using Meshless Method of Fundamental Solutions. Math. Comput. Appl. 2024, 29, 27. [Google Scholar] [CrossRef]
- Steinbrueck, M.; Grosse, M.; Stegmaier, U.; Braun, J.; Lorrette, C. Oxidation of silicon carbide composites for nuclear applications at very high temperatures in steam. Coatings 2022, 12, 875. [Google Scholar] [CrossRef]
- Ye, C.; Huang, D.; Li, B.; Yang, P.; Liu, J.; Wu, H.; Yang, J.; Li, X. Ablation behavior of the SiC-coated three-dimensional highly thermal conductive mesophase-pitch-based carbon-fiber-reinforced carbon matrix composite under plasma flame. Materials 2019, 12, 2723. [Google Scholar] [CrossRef]
- Li, A.; Deutschmann, O. Transient modeling of chemical vapor infiltration of methane using multi-step reaction and deposition models. Chem. Eng. Sci. 2007, 62, 4976–4982. [Google Scholar] [CrossRef]
- Li, A.; Norinaga, K.; Zhang, W.; Deutschmann, O. Modeling and simulation of materials synthesis: Chemical vapor deposition and infiltration of pyrolytic carbon. Compos. Mater. Sci. Technol. 2008, 68, 1097–1104. [Google Scholar] [CrossRef]
- Masoodi, R.; Pillai, K.M. Darcy’s law-based model for wicking in paper-like swelling porous media. AIChE J. 2010, 56, 2257–2267. [Google Scholar] [CrossRef]
- COMSOL. CFD Module User’s Guide, COMSOL Multiphysics® v. 5.6; COMSOL: Burlington, MA, USA, 2020; pp. 591–605. [Google Scholar]
- Leutard, D.; Vignoles, G.L.; Lamouroux, F.; Bernard, B. Monitoring density and temperature in C/C composite materials processing by CVI with induction heating. J. Mater. Synth. Process. 2001, 9, 259–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Tan, Z. Compression shear properties of adhesively bonded single-lap joints of C/C composite materials at high temperatures. Symmetry 2019, 11, 1437. [Google Scholar] [CrossRef]
- Delfini, A.; Albano, M.; Vricella, A.; Santoni, F.; Rubini, G.; Pastore, R.; Marchetti, M. Advanced radar absorbing ceramic-based materials for multifunctional applications in space environment. Materials 2018, 11, 1730. [Google Scholar] [CrossRef]
- Yin, T.; Jiang, B.Y.; Su, Z.A.; Fan, Z.Q.; Huang, Q.Z. Numerical simulation of carrier gas effects on flow field, species concentration and deposition rate in the chemical vapor deposition of carbon. New Carbon Mater. 2018, 33, 357–363. [Google Scholar] [CrossRef]
- Vignoles, G.L.; Goyhénèche, J.M.; Sébastian, P.; Puiggali, J.R.; Lines, J.F.; Lachaud, J.; Trinquecoste, M. The film-boiling densification process for C/C composite fabrication: From local scale to overall optimization. Chem. Eng. Sci. 2006, 61, 5636–5653. [Google Scholar] [CrossRef]




| Chemical Species | Ri1 (m3/mol∙s) | Ri2 (m3/mol∙s) |
|---|---|---|
| CH4, c1 | ||
| C2H4, c2 | ||
| C2H2, c3 | ||
| C6H6, c4 | ||
| H2, c5 |
| Parameters | Parameter Value Range | Average Density (kg·m−3) | Densification Rate (kg·m−3·s−1) | Uniformity (%) |
|---|---|---|---|---|
| Deposition Temperature (K) | [1120–1180] | [1–1.29] × 103 | [1.73–2.59] × 10−3 | [85.1–92.5] |
| Pressure (MPa) | [0.05–0.1] | |||
| Flow rate (L·min−1) | [85.1–92.5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhan, J.; Liang, H.; Guo, Y.; Zhao, B.; Hao, L.; Zhang, T.; Jiang, B. Modeling for the Fabrication Process of a ϕ1185 mm C/C Composite Thermal Insulation Tube in an Isothermal Chemical Vapor Infiltration Reactor. Coatings 2024, 14, 756. https://doi.org/10.3390/coatings14060756
Zhou J, Zhan J, Liang H, Guo Y, Zhao B, Hao L, Zhang T, Jiang B. Modeling for the Fabrication Process of a ϕ1185 mm C/C Composite Thermal Insulation Tube in an Isothermal Chemical Vapor Infiltration Reactor. Coatings. 2024; 14(6):756. https://doi.org/10.3390/coatings14060756
Chicago/Turabian StyleZhou, Jicheng, Jianyong Zhan, Huiling Liang, Yan Guo, Baoxing Zhao, Linzhao Hao, Tong Zhang, and Bingchun Jiang. 2024. "Modeling for the Fabrication Process of a ϕ1185 mm C/C Composite Thermal Insulation Tube in an Isothermal Chemical Vapor Infiltration Reactor" Coatings 14, no. 6: 756. https://doi.org/10.3390/coatings14060756






