Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parsley (PCE) Extraction
2.2. GC-MS Analysis of the Extract
2.3. FTIR Analysis of PCE
2.4. Steel Material Preparation and Solutions
2.5. Corrosion Inhibition Evaluation
2.5.1. Mass Loss Method
2.5.2. Electrochemical Measurements
2.5.3. UV–Vis Absorption Spectroscopy
2.5.4. SEM Analysis
2.5.5. XRD Analysis
3. Results and Discussion
3.1. Chemical Profile of the PCE Extract Using GC/MS
3.2. FTIR Analysis of PCE
3.3. Evaluation of the Corrosion Inhibition of PCE
3.3.1. Weight Loss Results
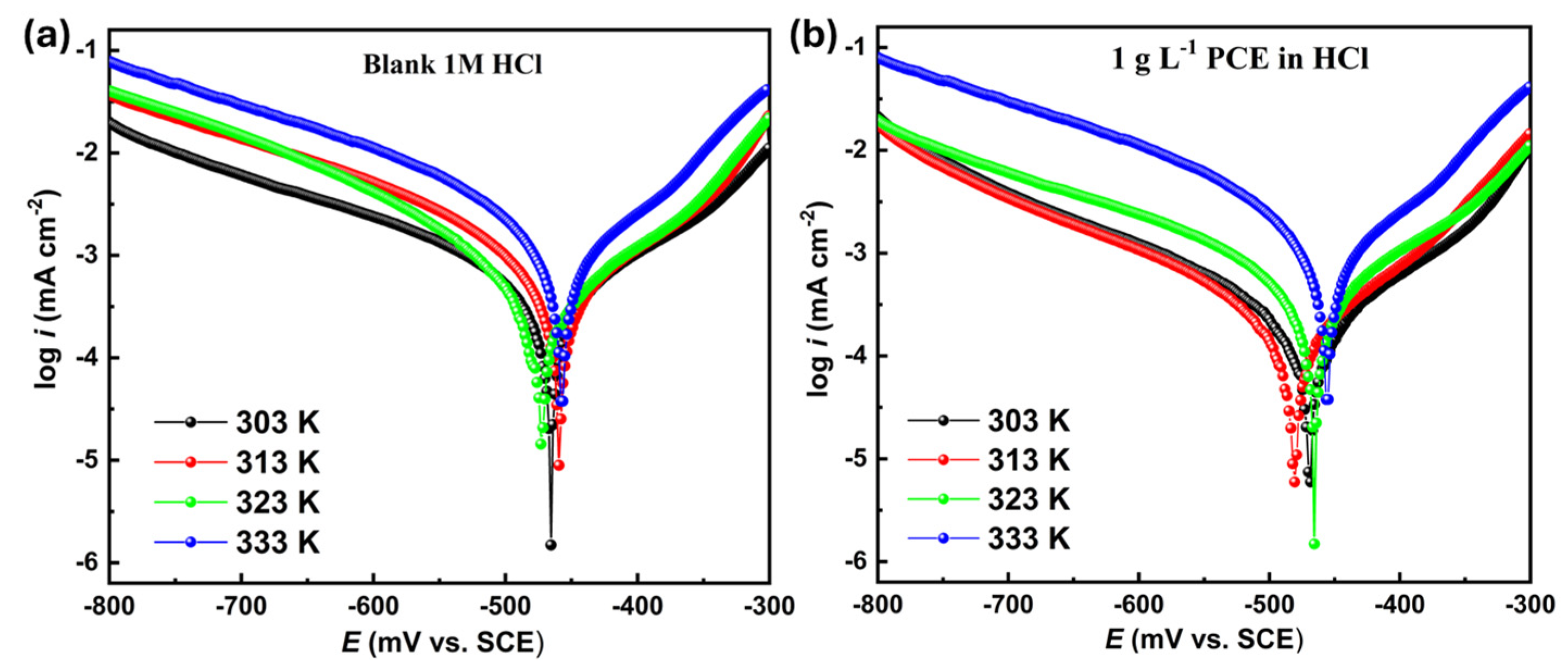
3.3.2. Potentiodynamic Polarization Measurements
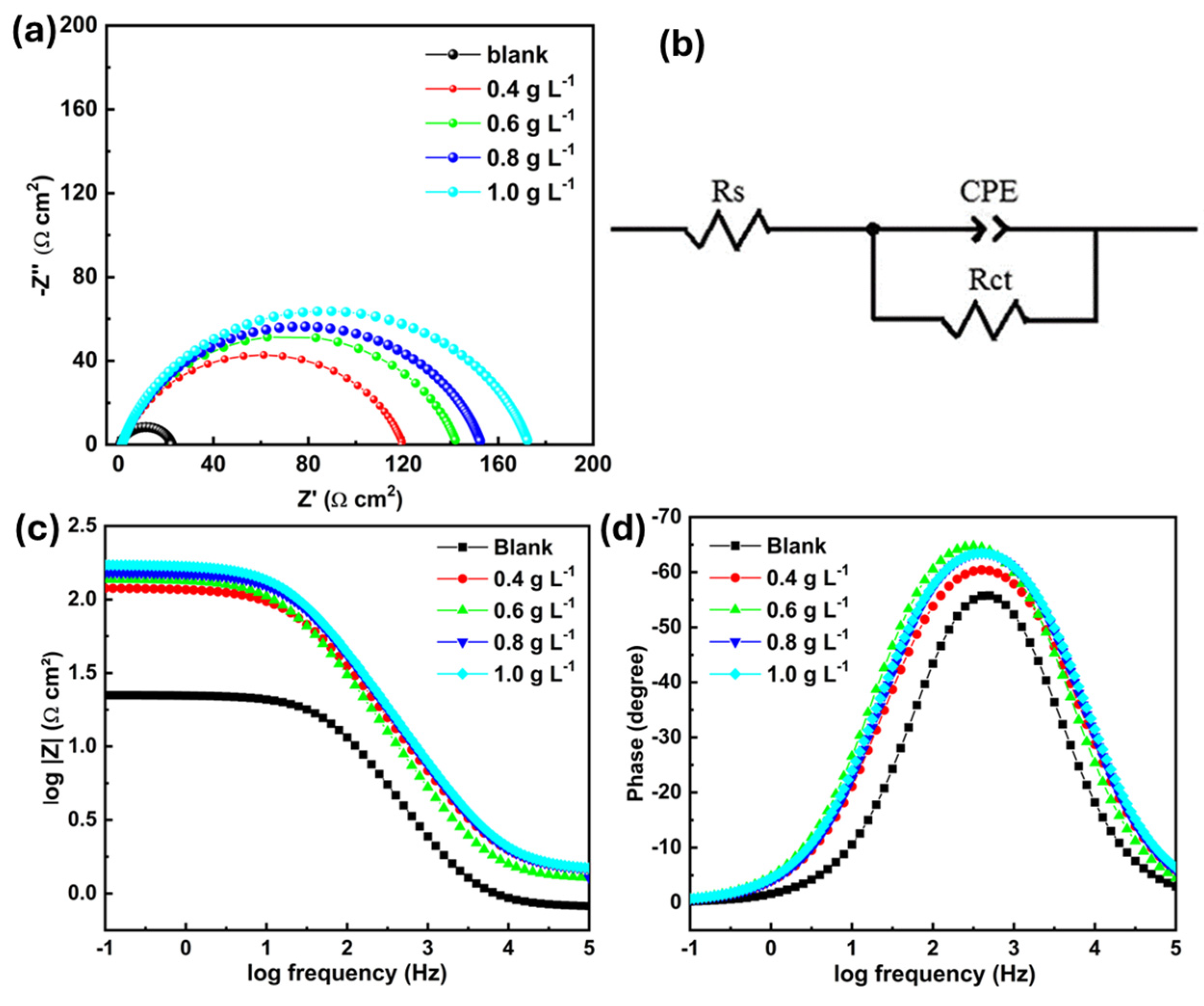
3.3.3. Electrochemical Impedance Spectroscopy (EIS)
3.4. Thermodynamics of the Process and Adsorption Isotherm
3.4.1. Temperature Effect
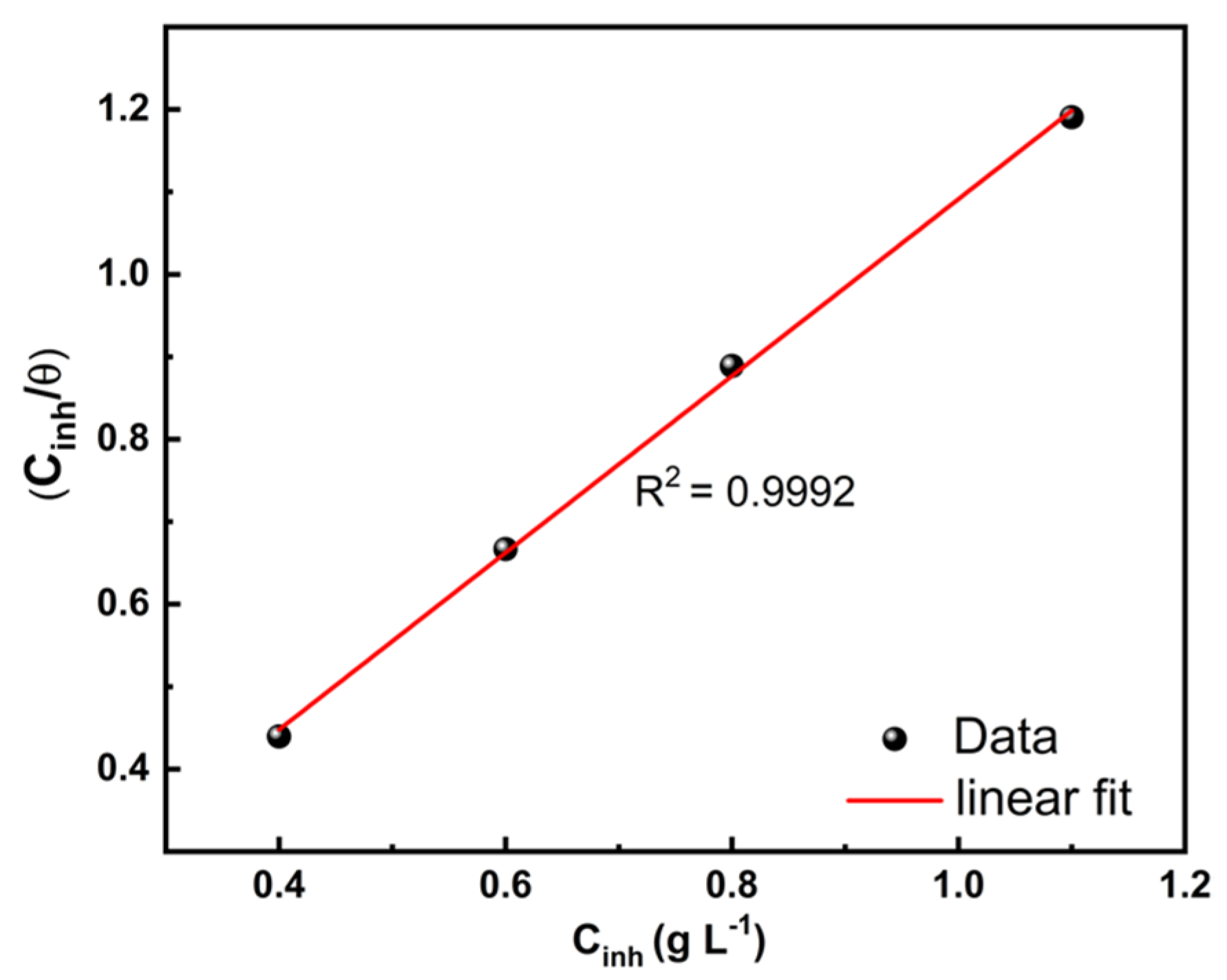
3.4.2. Adsorption Study
3.5. UV–Vis Absorption Spectroscopy
3.6. SEM Analysis
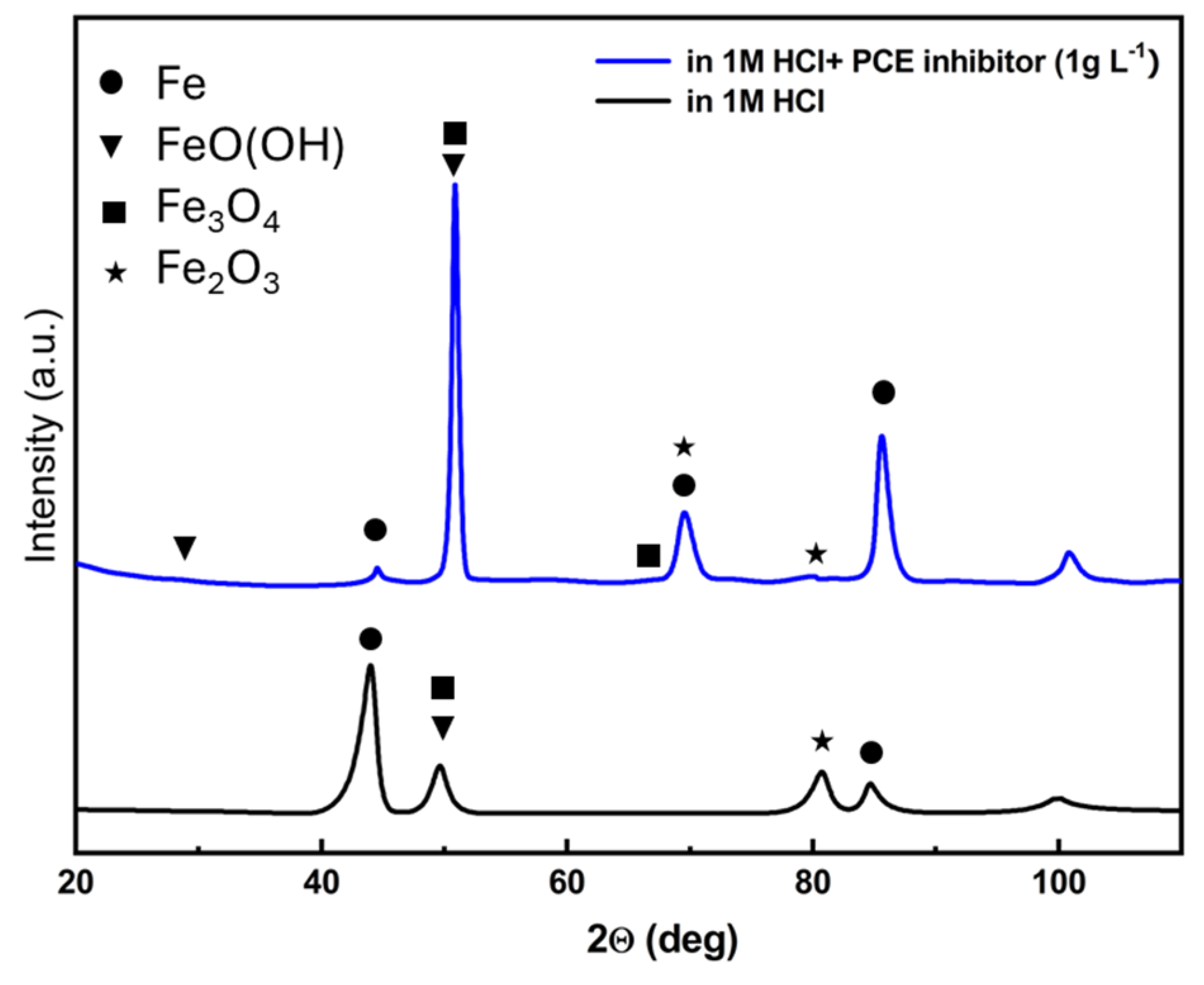
3.7. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Cao, L.; Li, M.; Wang, S.; Liu, F.; Lv, X.; Peng, X. Mechanical Properties and Durability of Alkali-Activated Steel Slag–Blastfurnace Slag Cement. J. Iron Steel Res. Int. 2023, 30, 1342–1355. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Galal, A. Corrosion Protection of Metals and Alloys Using Graphene and Biopolymer Based Nanocomposites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Güler, E.S.; Büyüklüoğlu, B. The Effects of Coating Conditions on Friction and Corrosion Resistance of Carbon Steel. Emerg. Mater. Res. 2020, 9, 383–387. [Google Scholar] [CrossRef]
- Timothy, U.J.; Umoren, P.S.; Solomon, M.M.; Igwe, I.O.; Umoren, S.A. An Appraisal of the Utilization of Natural Gums as Corrosion Inhibitors: Prospects, Challenges, and Future Perspectives. Int. J. Biol. Macromol. 2023, 253, 126904. [Google Scholar] [CrossRef] [PubMed]
- Amegroud, H.; Boudalia, M.; Elhawary, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Electropolymerized Conducting Polyaniline Coating on Nickel-Aluminum Bronze Alloy for Improved Corrosion Resistance in Marine Environment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133909. [Google Scholar] [CrossRef]
- Boudalia, M.; Laourayed, M.; El Moudane, M.; Sekkat, Z.; Campos, O.S.; Bellaouchou, A.; Guenbour, A.; José Garcia, A.; Amin, H.M.A. Phosphate Glass Doped with Niobium and Bismuth Oxides as an Eco-Friendly Corrosion Protection Matrix of Iron Steel in HCl Medium: Experimental and Theoretical Insights. J. Alloys Compd. 2023, 938, 168570. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced Micro/Nanocapsules for Self-Healing Smart Anticorrosion Coatings. J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Chaibi, N.; Mernari, B.; Vezin, H.; Lagrenée, M. 2,5-Bis(n-Methoxyphenyl)-1,3,4-Oxadiazoles Used as Corrosion Inhibitors in Acidic Media: Correlation between Inhibition Efficiency and Chemical Structure. Corros. Sci. 2002, 44, 2271–2289. [Google Scholar] [CrossRef]
- Plotnikova, M.D.; Shein, A.B.; Scherban, M.G.; Vasyanin, A.N.; Rubtsov, A.E. Experimental and Theoretical Investigation of (E)-5-{[4-(Dimethylamino)Benzylidene]Amino}-1,3,4-Thiadiazole-2(3H)-Thione (DATT) as an Acid Corrosion Inhibitor of Mild Steel. Int. J. Corros. Scale Inhib. 2023, 12, 1365–1391. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J. Recent Progress and Challenges of Using Smart Corrosion Inhibitors in Reinforced Concrete Structures. Constr. Build. Mater. 2024, 411, 134595. [Google Scholar] [CrossRef]
- Najem, A.; Campos, O.S.; Girst, G.; Raji, M.; Hunyadi, A.; García-Antón, J.; Bellaouchou, A.; Amin, H.M.A.; Boudalia, M. Experimental and DFT Atomistic Insights into the Mechanism of Corrosion Protection of Low-Carbon Steel in an Acidic Medium by Polymethoxyflavones from Citrus Peel Waste. J. Electrochem. Soc. 2023, 170, 093512. [Google Scholar] [CrossRef]
- Eddahhaoui, F.-Z.; Najem, A.; Elhawary, M.; Boudalia, M.; Campos, O.S.; Tabyaoui, M.; José Garcia, A.; Bellaouchou, A.; Amin, H.M.A. Experimental and Computational Aspects of Green Corrosion Inhibition for Low Carbon Steel in HCl Environment Using Extract of Chamaerops Humilis Fruit Waste. J. Alloys Compd. 2024, 977, 173307. [Google Scholar] [CrossRef]
- de Souza Morais, W.R.; da Silva, J.S.; Queiroz, N.M.P.; de Paiva e Silva Zanta, C.L.; Ribeiro, A.S.; Tonholo, J. Green Corrosion Inhibitors Based on Plant Extracts for Metals and Alloys in Corrosive Environment: A Technological and Scientific Prospection. Appl. Sci. 2023, 13, 7482. [Google Scholar] [CrossRef]
- Wong, P.; Kitts, D. Studies on the Dual Antioxidant and Antibacterial Properties of Parsley (Petroselinum crispum) and Cilantro (Coriandrum sativum) Extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and Analysis of Chemical Compositions of Natural Products and Plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- dos S. Franciscato, L.M.S.; Mendes, S.S.; Frederico, C.; Gonçalves, J.E.; Faria, M.G.I.; Gazim, Z.C.; Ruiz, S.P. Parsley (Petroselinum crispum): Chemical Composition and Antibacterial Activity of Essential Oil from Organic against Foodborne Pathogens. Aust. J. Crop. Sci. 2022, 16, 605–611. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.S.; Rahimi, R.; Farzaei, F. Parsley: A Review of Ethnopharmacology, Phytochemistry and Biological Activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Soliman, K.A.; Al Jahdaly, B.A.; Al-Fahemi, J.H.; Hawsawi, H.; Altass, H.M.; Motawea, M.S.; Al-Juaid, S.S. Natural Parsley Oil as a Green and Safe Inhibitor for Corrosion of X80 Carbon Steel in 0.5 M H2SO4solution: A Chemical, Electrochemical, DFT and MC Simulation Approach. RSC Adv. 2022, 12, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, P.; Drużyńska, B.; Obiedziński, M. Optimization of Extraction Conditions of Some Polyphenolic Compounds from Parsley Leaves (Petroselinum crispum). Acta Sci. Pol. Technol. Aliment. 2014, 13, 145–154. [Google Scholar] [CrossRef]
- Boutoumit, A.; Boudalia, M.; Hakiki, A.; Guenbour, A.; Kartah, B.; Bellaouchou, A.; Moussadaq, M. Electrochemical and Thermodynamic Investigation: Anti-Corrosive Properties of Parsley Extract for Carbon Steel in Hydrochloric Acid Media. Mater. Environ. Sci. 2018, 8, 4693–4704. [Google Scholar]
- Reedy, C.L.; Corbett, R.A.; Burke, M. Electrochemical Tests as Alternatives to Current Methods for Assessing Effects of Exhibition Materials on Metal Artifacts. Stud. Conserv. 1998, 43, 183–196. [Google Scholar] [CrossRef]
- Irfan, M.I.; Amjad, F.; Abbas, A.; Rehman, M.F.; Kanwal, F.; Saeed, M.; Ullah, S.; Lu, C. Novel Carboxylic Acid-Capped Silver Nanoparticles as Antimicrobial and Colorimetric Sensing Agents. Molecules 2022, 27, 3363. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Hussain, M.A.; Sher, M.; Irfan, M.I.; Tahir, M.N.; Tremel, W.; Hussain, S.Z.; Hussain, I. Design, characterization and evaluation of hydroxyethylcellulose based novel regenerable supersorbent for heavy metal ions uptake and competitive adsorption. Int. J. Biol. Macromol. 2017, 102, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.A.; Singh, A.; Singh, V.K.; Yadav, D.K.; Singh, A.K. Green Approach to Corrosion Inhibition of Mild Steel in Hydrochloric Acid and Sulphuric Acid Solutions by the Extract of Murraya Koenigii Leaves. Mater. Chem. Phys. 2010, 122, 114–122. [Google Scholar] [CrossRef]
- Ogunleye, O.O.; Arinkoola, A.O.; Eletta, O.A.; Agbede, O.O.; Osho, Y.A.; Morakinyo, A.F.; Hamed, J.O. Green Corrosion Inhibition and Adsorption Characteristics of Luffa Cylindrica Leaf Extract on Mild Steel in Hydrochloric Acid Environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef] [PubMed]
- Rguiti, M.M.; Chadili, M.; Ibrahimi, B.E.; Baddouh, A.; Bazzi, L.; Hilali, M.; Bazzi, L. Iron corrosion inhibition by olive mill wastewaters in acid medium. Moroc. J. Chem. 2018, 6, 307–317. [Google Scholar] [CrossRef]
- Atta, N.F.; Amin, H.M.A.; Khalil, M.W.; Galal, A. Nanotube Arrays as Photoanodes for Dye Sensitized Solar Cells Using Metal Phthalocyanine Dyes. Int. J. Electrochem. Sci. 2011, 6, 3316–3332. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Awad, M.K.; Rezk, M. Three Novel Di-Quaternary Ammonium Salts as Corrosion Inhibitors for API X65 Steel Pipeline in Acidic Solution. Part I: Experimental Results. Corros. Sci. 2014, 81, 54–64. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; Bouhlal, F.; Kouri, H.; Chellouli, M.; Benmessaoud, M.; Dahrouch, A.; Labjar, N.; El Hajjaji, S. Use of Omeprazole as Inhibitor for C38 Steel Corrosion in 1.0 M H3PO4 Medium. J. Fail. Anal. Prev. 2020, 20, 563–571. [Google Scholar] [CrossRef]
- Lukács, Z.; Kristóf, T. A Generalized Model of the Equivalent Circuits in the Electrochemical Impedance Spectroscopy. Electrochim. Acta 2020, 363, 137199. [Google Scholar] [CrossRef]
- Gateman, S.M.; Gharbi, O.; Gomes de Melo, H.; Ngo, K.; Turmine, M.; Vivier, V. On the Use of a Constant Phase Element (CPE) in Electrochemistry. Curr. Opin. Electrochem. 2022, 36, 101133. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Al-Moubaraki, A.H.; Bakhouch, M.; El Yazidi, M.; Ko, Y.G. Electrochemical Behavior and Interfacial Bonding Mechanism of New Synthesized Carbocyclic Inhibitor for Exceptional Corrosion Resistance of Steel Alloy: DFTB, MD and Experimental Approaches. Arab. J. Chem. 2022, 15, 104323. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abdul-Hadi, A.A. Kinetic and Mathematical Approaches to the Corrosion of Mild Steel in Nitric Acid. React. Kinet. Mech. Catal. 2014, 112, 15–26. [Google Scholar] [CrossRef]
- Manimegalai, S.; Manjula, P. Thermodynamic and Adsorption Studies for Corrosion Inhibition of Mild Steel in Aqueous Media by Sargasam swartzii (Brown algae). J. Mater. Environ. Sci. 2015, 6, 1629–1637. [Google Scholar]
- Talati, J.D.; Gandhi, D.K. N-Heterocyclic Compounds as Corrosion Inhibitors for Aluminium-Copper Alloy in Hydrochloric Acid. Corros. Sci. 1983, 23, 1315–1332. [Google Scholar] [CrossRef]
- Desai, P.D.; Pawar, C.B.; Avhad, M.S.; More, A.P. Corrosion Inhibitors for Carbon Steel: A Review. Vietnam. J. Chem. 2023, 61, 15–42. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion Inhibition of C38 Steel by Alkaloids Extract of Geissospermum Laeve in 1M Hydrochloric Acid: Electrochemical and Phytochemical Studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- El Hamdani, N.; Fdil, R.; Tourabi, M.; Jama, C.; Bentiss, F. Alkaloids Extract of Retama monosperma (L.) Boiss. Seeds Used as Novel Eco-Friendly Inhibitor for Carbon Steel Corrosion in 1 M HCl Solution: Electrochemical and Surface Studies. Appl. Surf. Sci. 2015, 357, 1294–1305. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus Tikoua Leaves Extract as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in HCl Media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-QTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef]
- Majd, M.T.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Probing molecular adsorption/interactions and anti-corrosion performance of poppy extract in acidic environments. J. Mol. Liq. 2020, 304, 112750. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Ghaderi, M.; Ramzani, A.; Mahdavian, M. Falcaria vulgaris leaves extract as an eco-friendly corrosion inhibitor for mild steel in hydrochloric acid media. Sci. Rep. 2023, 13, 3737. [Google Scholar] [CrossRef]
- Abd El-Maksoud, S.A.; Fouda, A.S.; El-Habab, A.T.; Ibrahim, A.R. Synthesis of Some Ethoxylated and Sulfonated Fatty Alcohol Surfactants and Their Inhibition Actions for C-Steel Corrosion in 1 M HCl. J. Bio-Tribo-Corros. 2021, 7, 44. [Google Scholar] [CrossRef]
- Melhi, S.; Bedair, M.A.; Alosaimi, E.H.; Younes, A.A.O.; El-Shwiniy, W.H.; Abuelela, A.M. Effective Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Newly Synthesized Schiff Base Nano Co(ii) and Cr(iii) Complexes: Spectral, Thermal, Electrochemical and DFT (FMO, NBO) Studies. RSC Adv. 2022, 12, 32488–32507. [Google Scholar] [CrossRef]
- Wang, X.-F.; Liu, X.-Y.; Su, F.; Li, J.-S.; Zhu, Z.-M.; Sang, X.-J.; Zhang, L.-C. Enhanced Corrosion Resistance of Carbon Steel in Hydrochloric Acid Solution by Polyoxometalate-Estertin Derivatives. ACS Omega 2022, 7, 4429–4443. [Google Scholar] [CrossRef]





| Element | C | Fe | Mn | P | S | Cr | Cu | Ni | Mo | V |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 0.34 | 0.60 | 0.44 | 0.21 | 0.03 | 4.78 | 0.08 | 0.15 | 1.61 | 0.51 |
| Component | Formula | Chemical Name | Molar Mass (g mol−1) | Retention Time (min) | Area% |
|---|---|---|---|---|---|
| Myristicin | C11H12O3 | 6-Allyl-4-methoxy-1,3-benzodioxole | 192.21 | 18.2 | 28.4 |
| Apiol | C12H14O4 | 4,7-dimethoxy-5-prop-2-enyl-1,3-benzodioxole | 222.2 | 20.4 | 35.6 |
| Phellandrene | C10H16 | 2-methyl-5-propan-2-ylcyclohexa-1,3-diene | 136.23 | 10.6 | 12.3 |
| Limonene | C10H16 | 1-methyl-4-prop-1-en-2-ylcyclohexene | 136.23 | 8.5 | 6.7 |
| Eugenol | C10H12O2 | 4-Allyl-2-methoxyphenol | 164.2 | 15.9 | 5.4 |
| Alpha-Pinene | C10H16 | 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene; pin-2(3)-ene; Pinene isomer | 136.23 | 7.2 | 3.9 |
| Beta-Pinene | C10H16 | 6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane | 136.23 | 7.8 | 2.6 |
| Terpinolene | C10H16 | 1-methyl-4-propan-2-ylidenecyclohexene | 136.23 | 11.3 | 1.8 |
| Caryophyllene | C15H24 | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | 204.35 | 16.5 | 1.5 |
| Cinh (g L−1) | CR (mg cm−2 h−1) | ηwl% |
|---|---|---|
| Blank | 0.936 | -- |
| 0.4 | 0.150 | 79.9 |
| 0.6 | 0.127 | 85.4 |
| 0.8 | 0.090 | 89.5 |
| 1 | 0.088 | 90.0 |
| Cinh (g L−1) | Ecorr (mV vs. SCE) | icorr (µA cm−2) | βa (mV dec−1) | βc (mV dec−1) | ηPDP (%) |
|---|---|---|---|---|---|
| Blank | −446 | 427.1 | 48.9 | −132.1 | -- |
| 0.2 | −460 | 202.5 | 112.6 | −192.1 | 52.5 |
| 0.4 | −461 | 88.5 | 96.4 | −211.0 | 79.2 |
| 0.6 | −443 | 69.0 | 102.4 | −249.7 | 83.8 |
| 0.8 | −478 | 64.5 | 107.5 | −207.6 | 84.8 |
| 1 | −468 | 34.3 | 89.6 | −197.5 | 91.9 |
| Cinh (g L−1) | Rs (Ω cm2) | Rct (Ω cm2) | Qdl (Ω−1 Sn cm2) | n | Cdl (µF cm−2) | ηEIS (%) |
|---|---|---|---|---|---|---|
| Blank | 2.1 | 24.8 | 3.07 × 10−4 | 0.837 | 537 | -- |
| 0.4 | 1.4 | 118.9 | 1.48 × 10−4 | 0.797 | 498 | 79.1 |
| 0.6 | 1.3 | 141.5 | 1.78 × 10−4 | 0.798 | 419 | 82.5 |
| 0.8 | 1.4 | 151.5 | 1.14 × 10−4 | 0.814 | 358 | 83.6 |
| 1 | 2.3 | 283.9 | 1.10 × 10−4 | 0.813 | 402 | 91.3 |
| Medium | T (K) | iorr (µA cm−2) | ηPDP (%) |
|---|---|---|---|
| Blank | 303 | 427 | 91.9 |
| PCE | 34 | ||
| Blank | 313 | 760 | 90.0 |
| PCE | 75 | ||
| Blank | 323 | 990 | 89.0 |
| PCE | 91 | ||
| Blank | 333 | 1300 | 84.1 |
| PCE | 206 |
| Medium | Ea (kJ mol−1) | ∆Ha (kJ mol−1) | ∆Sa (J mol−1 K−1) |
|---|---|---|---|
| Blank | 31.6 | 24.3 | −113.1 |
| 1 g L−1 PCE | 43.3 | 47.5 | −58.7 |
| Slope | Intercept (g L−1) | Kads (L g−1) | R2 | |
|---|---|---|---|---|
| PCE | 1.07 | 0.0188 | 55.6 | 0.9992 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutoumit, A.; Elhawary, M.; Bellaouchou, A.; Boudalia, M.; Hammani, O.; José Garcia, A.; Amin, H.M.A. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings 2024, 14, 783. https://doi.org/10.3390/coatings14070783
Boutoumit A, Elhawary M, Bellaouchou A, Boudalia M, Hammani O, José Garcia A, Amin HMA. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings. 2024; 14(7):783. https://doi.org/10.3390/coatings14070783
Chicago/Turabian StyleBoutoumit, Aomar, Maha Elhawary, Abdelkbir Bellaouchou, Maria Boudalia, Othmane Hammani, Anton José Garcia, and Hatem M. A. Amin. 2024. "Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl" Coatings 14, no. 7: 783. https://doi.org/10.3390/coatings14070783





