Efficient Recycling and Utilization Strategy for Steel Spent Pickling Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing Methods
2.3. Measurement of the Content of Fe3+ and Fe2+
2.4. Measurement of Raw Material Utilization Rate
2.5. Measurement of Acidity
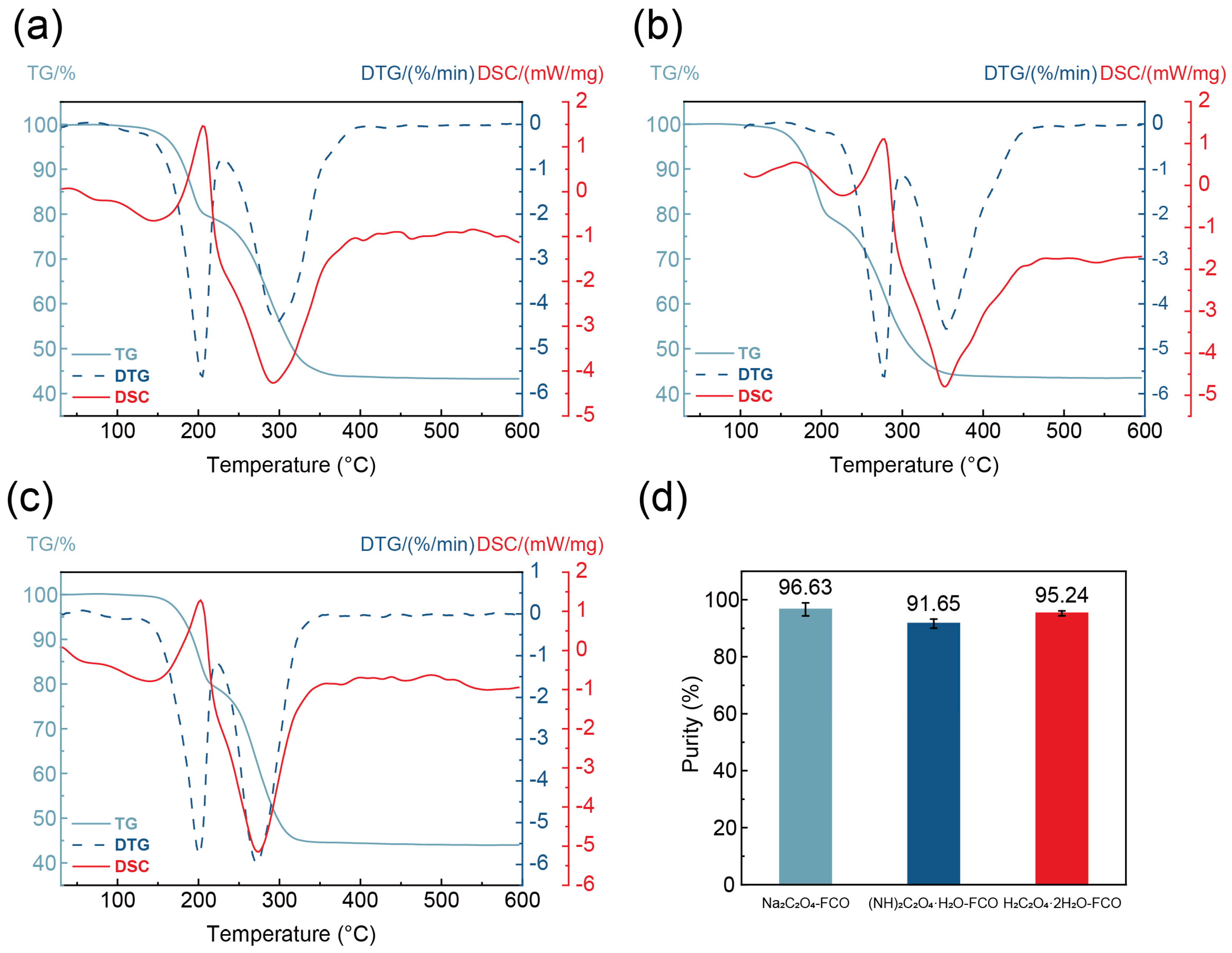
2.6. Purity Determination of FeC2O4·2H2O
2.7. Characterization
3. Results and Discussion
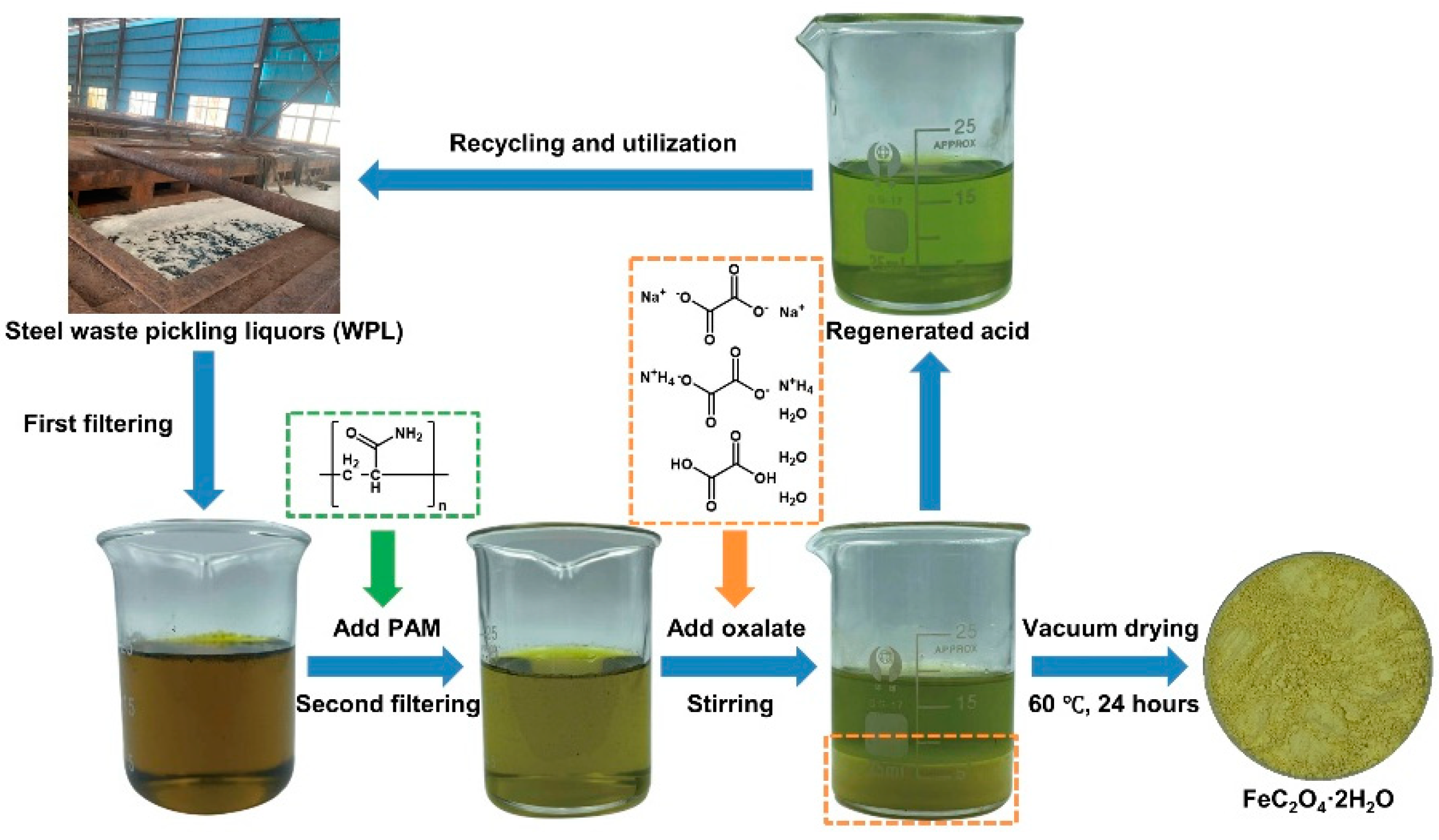
3.1. Strategy for Recycling and Utilization of the Steel SPS
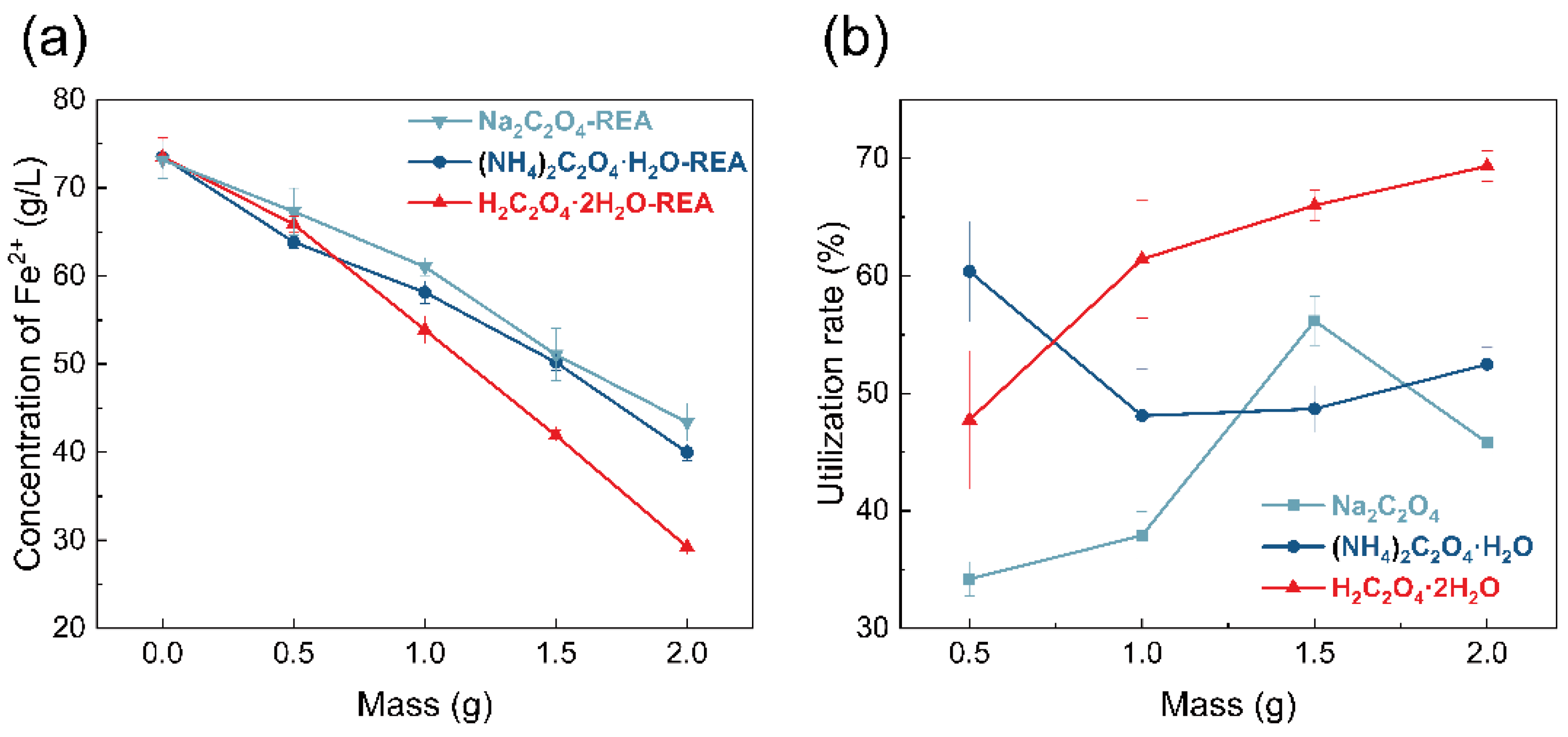
3.2. Effect of Addition Quality on the Concentration of Fe2+ and Utilization Rate
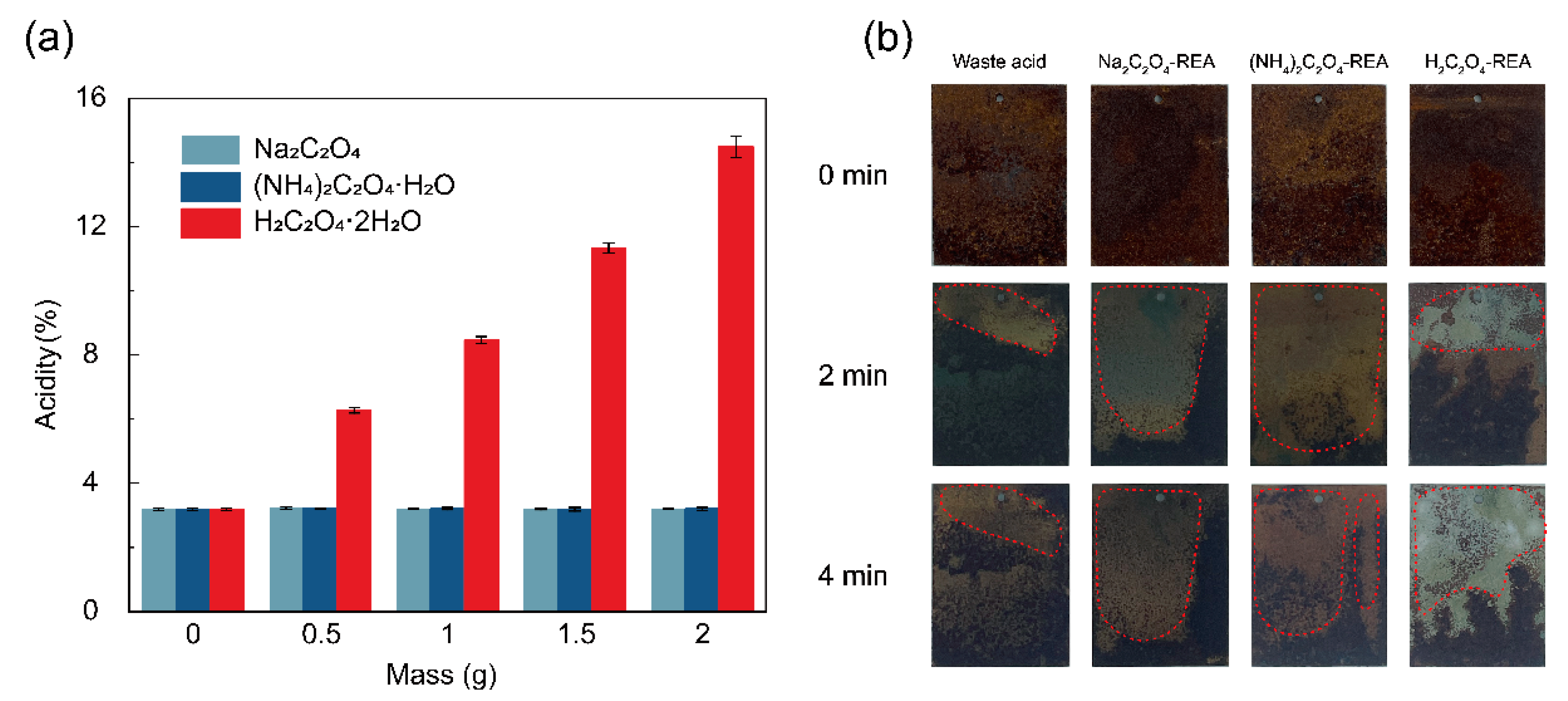
3.3. Effect of Regenerated Acid on Acidity and Actual Pickling
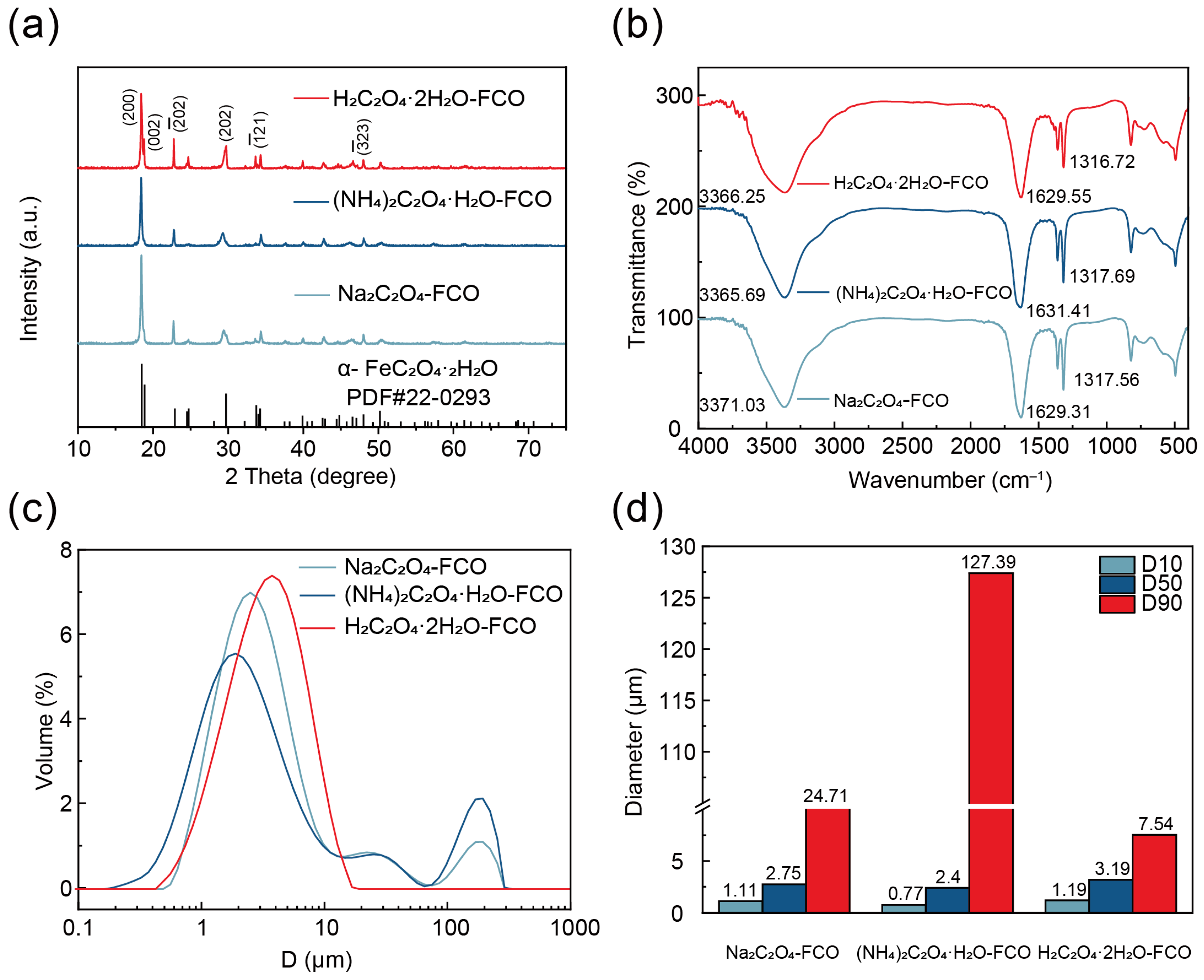
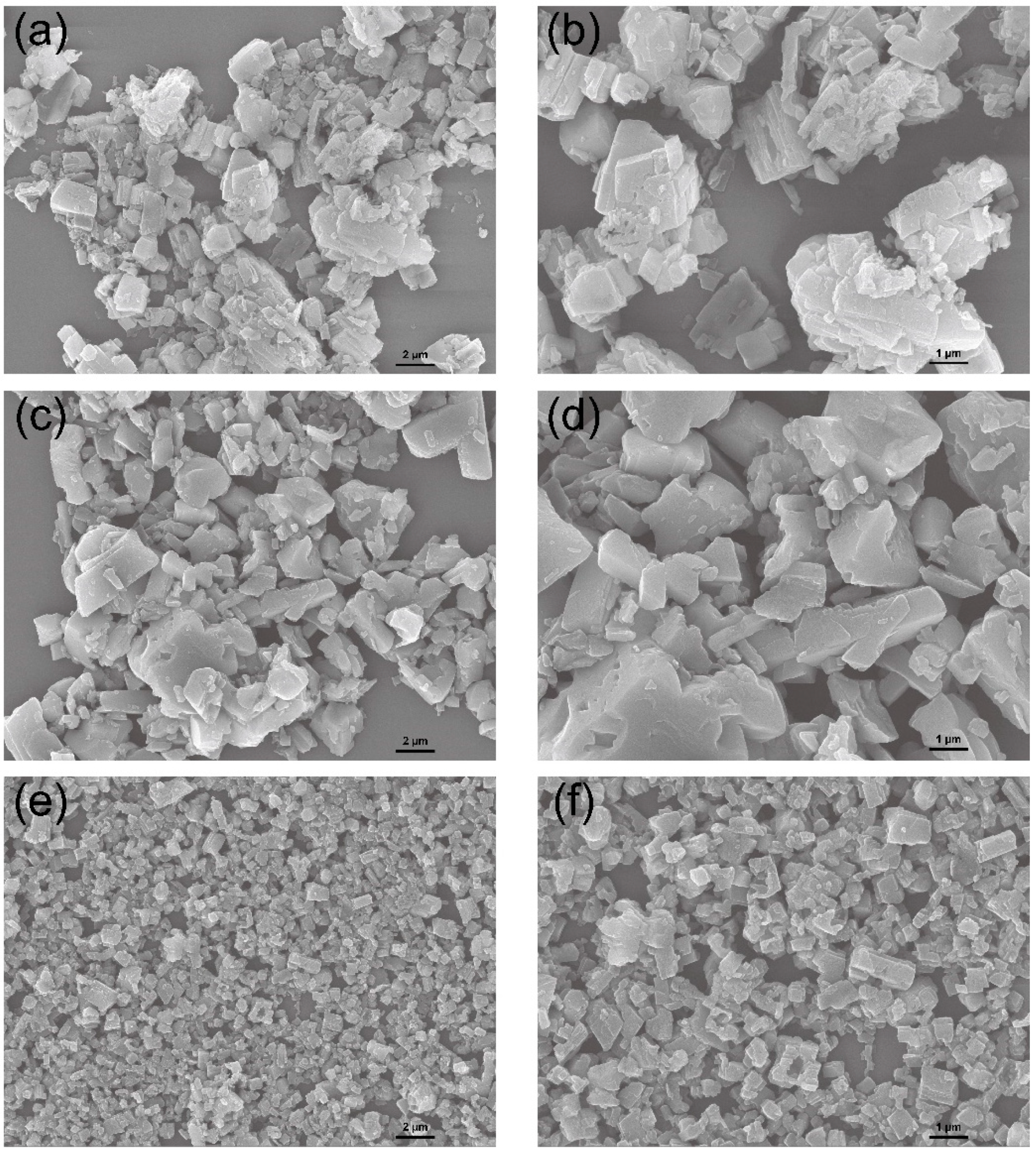
3.4. Characterization of Na2C2O4-FCO, (NH4)2C2O4·H2O-FCO, and H2C2O4·2H2O-FCO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santa, A.C.; Montoya, D.A.; Tamayo, J.A.; Gómez, M.A.; Castaño, J.G.; Baena, L.M. Atmospheric corrosion of carbon steel: Results of one-year exposure in an andean tropical atmosphere in Colombia. Heliyon 2024, 10, e29391. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.J.; Hu, Z.H.; Liu, X.J.; Su, C.W.; Hao, L. Understanding in compositional phases of carbon steel rust layer with a long-term atmospheric exposure. Mater. Lett. 2022, 315, 131968. [Google Scholar] [CrossRef]
- SArreola-Villa, S.A.; Vergara-Hernández, H.J.; Solorio-Diáz, G.; Pérez-Alvarado, A.; Vázquez-Gómez, O.; Chávez-Campos, G.M. Kinetic study of oxide growth at high temperature in low carbon steel. Metals 2022, 12, 147. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Y.; Chen, S.; Xu, X.; Yao, M.; Fang, J.; Liu, G. Hot-dip galvanizing process and the influence of metallic elements on composite coatings. J. Compos. Sci. 2024, 8, 160. [Google Scholar] [CrossRef]
- Wu, M.T.; Li, Y.L.; Guo, Q.; Shao, D.W.; He, M.M.; Qi, T. Harmless treatment and resource utilization of stainless steel pickling sludge via direct reduction and magnetic separation. J. Clean. Prod. 2019, 240, 118187. [Google Scholar] [CrossRef]
- El Kacimi, Y.; Touir, R.; Galai, M.; Alaoui, K.; Dkhireche, N.; Touhami, M.E. Relationship between silicon, phosphorus content and grain number in mild steels and its corrosion resistance in pickling hydrochloric acid. Int. J. Ind. Chem. 2020, 11, 111–122. [Google Scholar] [CrossRef]
- Manna, M.; Dutta, M. Effect of prior electro or electroless Ni plating layer in galvanizing and galvannealing behavior of high strength steel sheet. Surf. Coat. Technol. 2017, 316, 48–58. [Google Scholar] [CrossRef]
- Miranda-Alcantará, B.; Castañeda-Záldivar, F.; Ortíz-Frade, L.; Antaño, R.; Rivera, F.F. Electrochemical study of iron deposit in acid media for its recovery from spent pickling baths regeneration. J. Electroanal. Chem. 2021, 901, 115805. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef]
- Covaliu-Mierlă, C.I.; Păunescu, O.; Iovu, H. Recent advances in membranes used for nanofiltration to remove heavy metals from wastewater: A review. Membranes 2023, 13, 643. [Google Scholar] [CrossRef]
- Tang, B.; Yuan, L.; Shi, T.; Yu, L.; Zhu, Y. Preparation of nano-sized magnetic particles from spent pickling liquors by ultrasonic-assisted chemical co-precipitation. J. Hazard. Mater. 2009, 163, 1173–1178. [Google Scholar] [CrossRef]
- Tang, J.; Pei, Y.; Hu, Q.; Pei, D.; Xu, J. The recycling of ferric salt in steel pickling liquors: Preparation of nano-sized iron oxide. Procedia Environ. Sci. 2016, 31, 778–784. [Google Scholar] [CrossRef]
- Shi, C.H.; Zhang, Y.Q.; Zhou, S.; Jiang, J.C.; Huang, X.Y.; Hua, J. Status of research on the resource utilization of stainless steel pickling sludge in China: A review. Environ. Sci. Pollut. Res. 2023, 30, 90223–90242. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Tu, G.; Zhao, D.; Tsang, P.E.; Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 2019, 360, 212–220. [Google Scholar] [CrossRef]
- Sherif, E.S.M. Corrosion inhibition in 2.0 M sulfuric acid solutions of high strength maraging steel by aminophenyl tetrazole as a corrosion inhibitor. Appl. Surf. Sci. 2014, 292, 190–196. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Y.; Zhao, Y.; Chen, W. Recovery of arsenic oxide, harmless gypsum residue and clean water by lime neutralization and precipitation. Hydrometallurgy 2023, 215, 105996. [Google Scholar] [CrossRef]
- Aboul-Magd, A.A.S.; Al-Husain, S.A.R.; Al-Zahrani, S.A. Batch adsorptive removal of Fe (III), Cu (II) and Zn (II) ions in aqueous and aqueous organic–HCl media by Dowex HYRW2-Na Polisher resin as adsorbents. Arab. J. Chem. 2016, 9, S1–S8. [Google Scholar] [CrossRef]
- Lorenz, M.; Seitfudem, G.; Randazzo, S.; Gueccia, R.; Gehring, F.; Prenzel, T.M. Combining membrane and zero brine technologies in waste acid treatment for a circular economy in the hot-dip galvanizing industry: A life cycle perspective. J. Sustain. Metall. 2023, 9, 537–549. [Google Scholar] [CrossRef]
- Leonzio, G. Recovery of metal sulphates and hydrochloric acid from spent pickling liquors. J. Clean. Prod. 2016, 129, 417–426. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Wang, M.; Jiang, C.; Xiang, X.; Zhang, X. Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP. Hydrometallurgy 2015, 153, 38–45. [Google Scholar] [CrossRef]
- Arguillarena, A.; Margallo, M.; Irabien, Á.; Urtiaga, A. Life cycle assessment of zinc and iron recovery from spent pickling acids by membrane-based solvent extraction and electrowinning. J. Environ. Manag. 2022, 318, 115567. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yue, T.; Sun, W.; He, D.; Lu, C.; Fu, X. Acid recovering and iron recycling from pickling waste acid by extraction and spray pyrolysis techniques. J. Clean. Prod. 2021, 312, 127747. [Google Scholar] [CrossRef]
- Schiemann, M.; Wirtz, S.; Scherer, V.; Bärhold, F. Spray roasting of iron chloride FeCl2: Numerical modelling of industrial scale reactors. Powder Technol. 2013, 245, 70–79. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, F.; Li, Z.; Liao, J.; Huang, Y.; Lei, Y.; Li, N. Mixed-charge poly(2,6-dimethyl-phenylene oxide)anion exchange membrane for diffusion dialysis in acid recovery. J. Membr. Sci. 2018, 549, 543–549. [Google Scholar] [CrossRef]
- Yue, X.; Wu, W.; Chen, G.; Yang, C.; Liao, S.; Li, X. Influence of 2,2′,6,6′-tetramethyl biphenol-based anion-exchange membranes on the diffusion dialysis of hydrochloride acid. J. Appl. Polym. Sci. 2017, 134, 45333. [Google Scholar] [CrossRef]
- Chavan, V.; Agarwal, C.; Adya, V.C.; Pandey, A.K. Hybrid organic-inorganic anion-exchange pore-filled membranes for the recovery of nitric acid from highly acidic aqueous waste streams. Water Res. 2018, 133, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Abad, J.; García-Gabaldón, M.; Pérez-Herranz, V. Treatment of spent pickling baths coming from hot dip galvanizing by means of an electrochemical membrane reactor. Desalination 2014, 343, 38–47. [Google Scholar] [CrossRef][Green Version]
- Azizitorghabeh, A.; Rashchi, F.; Babakhani, A.; Noori, M. Synergistic extraction and separation of Fe(III) and Zn(II) using TBP and D2EHPA. Sep. Sci. Technol. 2016, 52, 476–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Cao, Y.; Zhou, M.; Miao, Z.; Yang, J.; Du, Z.; Lu, B.; Liu, G.; Li, J.; Chen, S. Efficient Recycling and Utilization Strategy for Steel Spent Pickling Solution. Coatings 2024, 14, 784. https://doi.org/10.3390/coatings14070784
Liu Q, Cao Y, Zhou M, Miao Z, Yang J, Du Z, Lu B, Liu G, Li J, Chen S. Efficient Recycling and Utilization Strategy for Steel Spent Pickling Solution. Coatings. 2024; 14(7):784. https://doi.org/10.3390/coatings14070784
Chicago/Turabian StyleLiu, Qi, Yuqing Cao, Meng Zhou, Zehao Miao, Jinkun Yang, Zhaokai Du, Baoyang Lu, Guiqun Liu, Jianhong Li, and Shuai Chen. 2024. "Efficient Recycling and Utilization Strategy for Steel Spent Pickling Solution" Coatings 14, no. 7: 784. https://doi.org/10.3390/coatings14070784
APA StyleLiu, Q., Cao, Y., Zhou, M., Miao, Z., Yang, J., Du, Z., Lu, B., Liu, G., Li, J., & Chen, S. (2024). Efficient Recycling and Utilization Strategy for Steel Spent Pickling Solution. Coatings, 14(7), 784. https://doi.org/10.3390/coatings14070784







