Lifetime Extension of Atmospheric and Suspension Plasma-Sprayed Thermal Barrier Coatings in Burner Rig Tests by Pre-Oxidizing the CoNiCrAlY Bond Coats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Investigation of Specimens after Pre-Oxidation
2.3. Thermal Gradient Cycling Tests
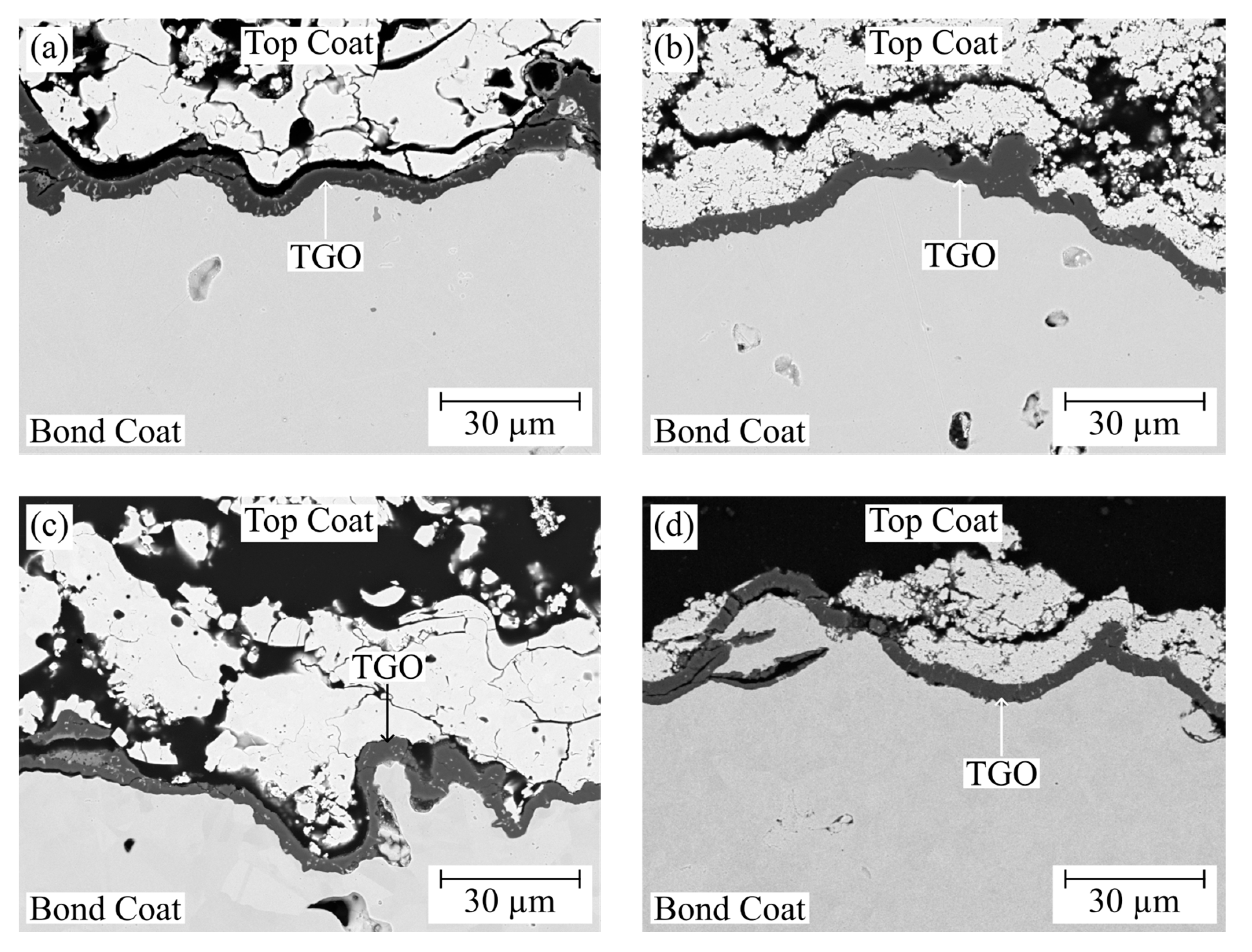
3. Results and Discussion
3.1. Oxide Formation during Heat Treatment
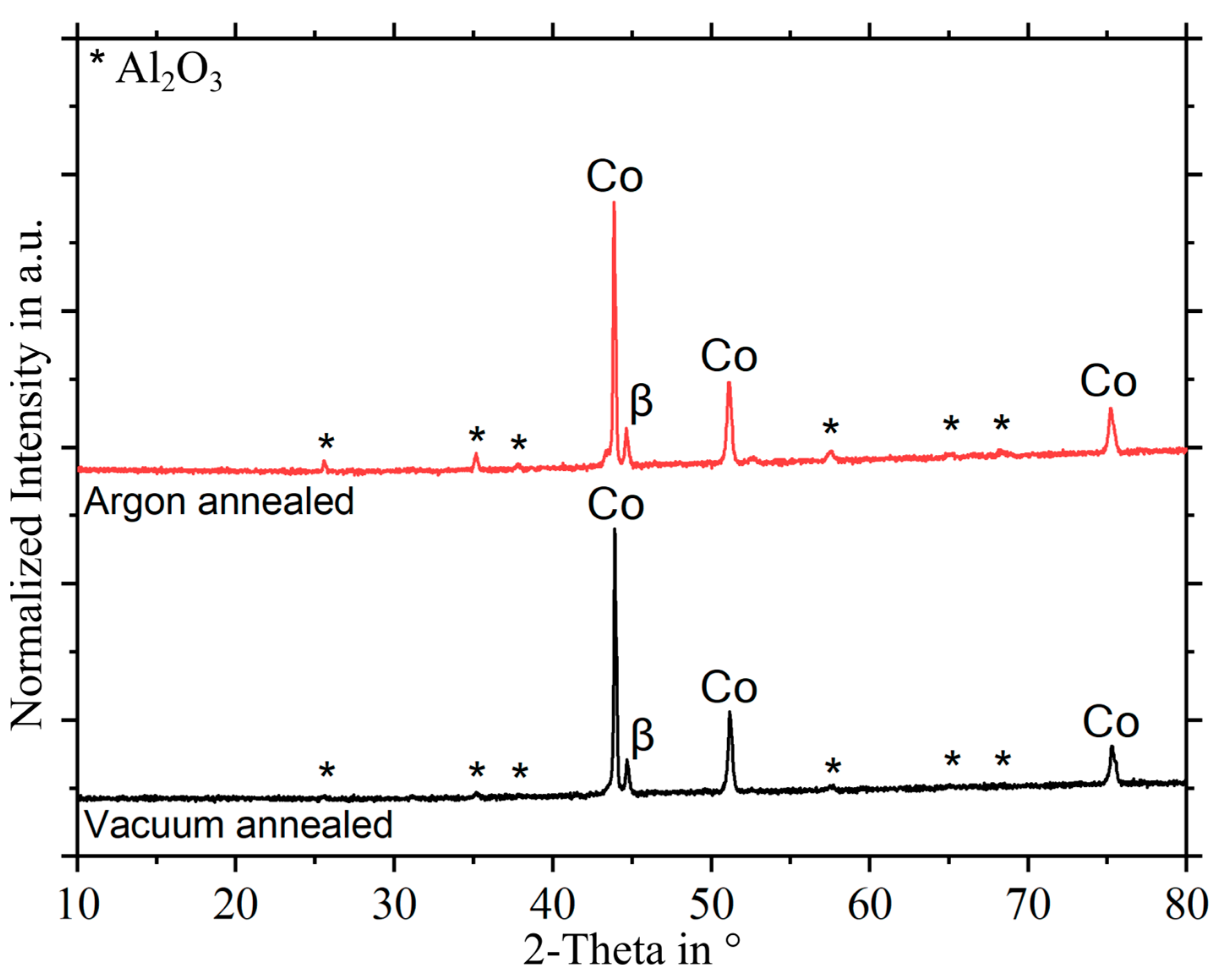
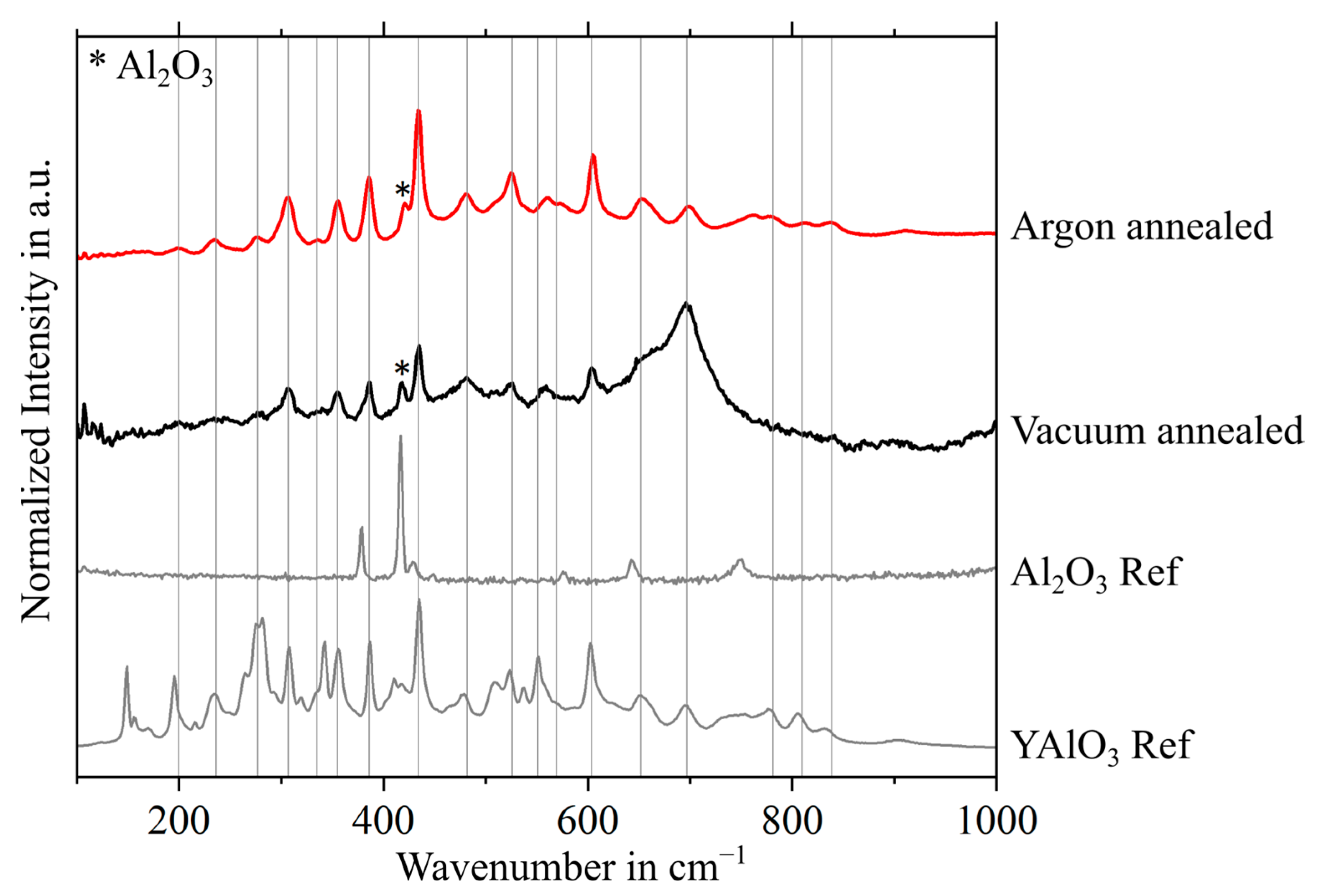
3.2. Phase Characterization of Oxides Formed during Heat Treatment
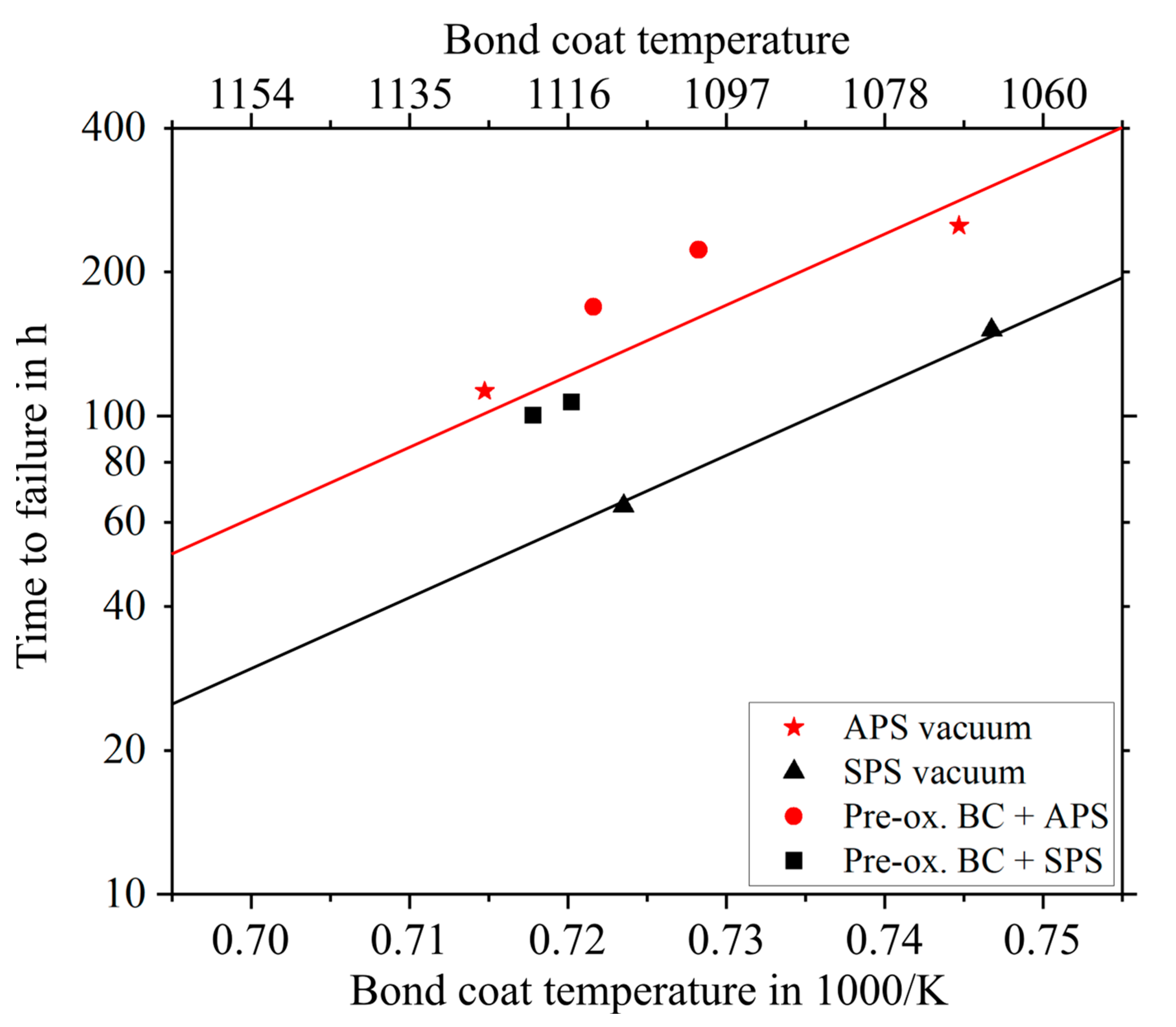
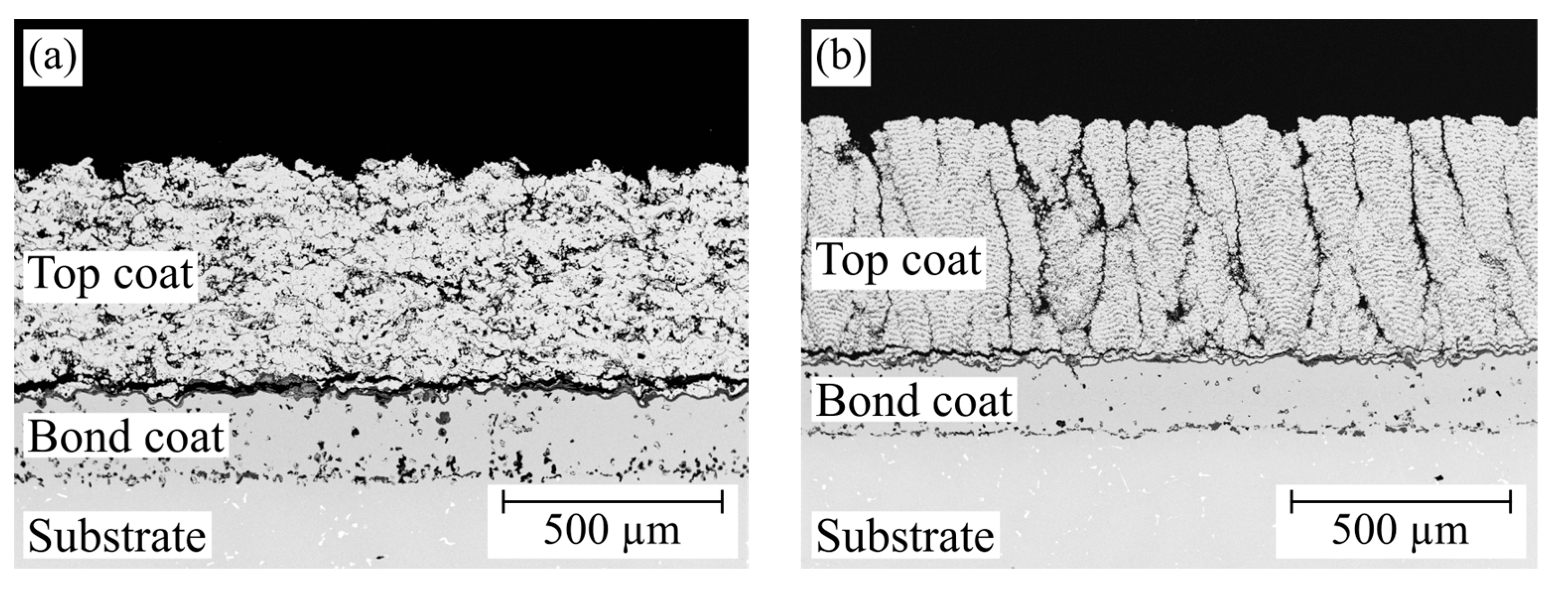
3.3. Burner Rig Cycling Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnault, V.; Mévrel, R.; Alpérine, S.; Jaslier, Y. Thermal barrier coatings for aircraft turbine airfoils thermal challenge and materials. Rev. Met. 1999, 96, 585–597. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Wellman, R.G.; Deakin, M.J.; Nicholls, J.R. The effect of TBC morphology on the erosion rate of EB PVD TBCs. Wear 2005, 258, 349–356. [Google Scholar] [CrossRef]
- Jones, R.L. Some Aspects of the Hot Corrosion of Thermal Barrier Coatings. Therm. Spray Technol. 1997, 6, 77–84. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G.; Johnson, C.A. Thermochemical Interaction of Thermal Barrier Coatings with Molten CaO–MgO–Al2O3–SiO2 (CMAS) Deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar] [CrossRef]
- Rabiei, A. Failure mechanisms associated with the thermally grown oxide in plasma-sprayed thermal barrier coatings. Acta Mater. 2000, 48, 3963–3976. [Google Scholar] [CrossRef]
- Thompson, J.A.; Clyne, T.W. The Stiffness of Plasma Sprayed Zirconia Top Coats in TBCs. In Proceedings of the UTSC 1999, UTSC’99, Dusseldorf, Germany, 17–19 March 1999. [Google Scholar]
- Scott, H.G. Phase relationships in the zirconia-yttria system. J. Mater. Sci. 1975, 10, 1527–1535. [Google Scholar] [CrossRef]
- Chang, G.C.; Phucharoen, W.; Miller, R.A. Behavior of thermal barrier coatings for advanced gas turbine blades. Surf. Coat. Technol. 1987, 30, 13–28. [Google Scholar] [CrossRef]
- Traeger, F.; Ahrens, M.; Vaßen, R.; Stöver, D. A life time model for ceramic thermal barrier coatings. Mater. Sci. Eng. A 2003, 358, 255–265. [Google Scholar] [CrossRef]
- Vaßen, R.; Giesen, S.; Stöver, D. Lifetime of Plasma-Sprayed Thermal Barrier Coatings: Comparison of Numerical and Experimental Results. J. Therm. Spray Technol. 2009, 18, 835–845. [Google Scholar] [CrossRef]
- Niranatlumpong, P.; Ponton, C.B.; Evans, H.E. The Failure of Protective Oxides on Plasma-Sprayed NiCrAlY Overlay Coatings. Oxid. Met. 2000, 53, 241–258. [Google Scholar] [CrossRef]
- Chang, W.L.E.; Wu, B.C.; Chao, C.H. Effects of bond coat preoxidation on the properties of ZrO2-8wt.% Y2O3/Ni-22Cr-10Al-1Y thermal-barrier coatings. Oxid. Met. 1991, 36, 221–238. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hayakawa, K.; Kitaoka, S.; Matsubara, H.; Takayama, H.; Kagiya, Y.; Sugita, Y. The effect of preoxidation atmosphere on oxidation behavior and thermal cycle life of thermal barrier coatings. Mater. Sci. Eng. A 2006, 441, 119–125. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R.; Murphy, K.S. The effect of grit blasting on the oxidation behavior of a platinum-modified nickel-aluminide coating. Met. Mater. Trans. A 2001, 32, 1467–1478. [Google Scholar] [CrossRef]
- Prescott, R.; Graham, M.J. The formation of aluminum oxide scales on high-temperature alloys. Oxid. Met. 1992, 38, 233–254. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Sloof, W.G. Combined pre-annealing and pre-oxidation treatment for the processing of thermal barrier coatings on NiCoCrAlY bond coatings. Surf. Coat. Technol. 2006, 201, 3894–3900. [Google Scholar] [CrossRef]
- Kitaoka, S.; Kuroyama, T.; Matsumoto, M.; Kitazawa, R.; Kagawa, Y. Control of polymorphism in Al2O3 scale formed by oxidation of alumina-forming alloys. Corros. Sci. 2010, 52, 429–434. [Google Scholar] [CrossRef]
- Joeris, J.; Scheld, W.S.; Uhlenbruck, S.; Sohn, Y.J.; Sebold, D.; Guillon, O.; Vaßen, R. Preparation of Highly Durable Columnar Suspension Plasma Spray (SPS) Coatings by Pre-Oxidation of the CoNiCrAlY Bondcoat. Coatings 2023, 13, 1575. [Google Scholar] [CrossRef]
- Zhou, D.; Guillon, O.; Vaßen, R. Development of YSZ Thermal Barrier Coatings Using Axial Suspension Plasma Spraying. Coatings 2017, 7, 120. [Google Scholar] [CrossRef]
- Negami, M.; Hibino, S.; Kawano, A.; Nomura, Y.; Tanaka, R.; Igashira, K. Development of Highly Durable Thermal Barrier Coating by Suppression of Thermally Grown Oxide. J. Eng. Gas Turbines Power 2018, 140, 082101. [Google Scholar] [CrossRef]
- Negami, M.; Kyuma, K.; Azuma, M.; Taniguchi, T.; Yamabe-Mitarai, Y. Improvement of the durability of thermal barrier coating by pre-oxidation. Corros. Sci. 2024, 227, 111806. [Google Scholar] [CrossRef]
- Negami, M.; Yamabe-Mitarai, Y. The Oxidation Behaviors of NiCoCrAlY Coatings After Pre-Oxidation Treatment During High-Temperature Oxidation at 800 °C and 900 °C. High Temp. Corros. Mater 2024, 101, 511–527. [Google Scholar] [CrossRef]
- Igel, J.; Mauer, G.; Guillon, O.; Vaßen, R. Systematic Approach to Optimize Technological and Economical Aspects of Atmospheric Plasma Sprayed Thermal Barrier Coatings. Adv. Eng. Mater. 2023, 26, 2300623. [Google Scholar] [CrossRef]
- Traeger, F.; Vaßen, R.; Rauwald, K.-H.; Stöver, D. Thermal Cycling Setup for Testing Thermal Barrier Coatings. Adv. Eng. Mater. 2003, 5, 429–432. [Google Scholar] [CrossRef]
- Nowak, W.; Naumenko, D.; Mor, G.; Mor, F.; Mack, D.E.; Vassen, R.; Singheiser, L.; Quadakkers, W. Effect of processing parameters on MCrAlY bondcoat roughness and lifetime of APS–TBC systems. Surf. Coat. Technol. 2014, 260, 82–89. [Google Scholar] [CrossRef]
- Vande Put, A.; Oquab, D.; Péré, E.; Raffaitin, A.; Monceau, D. Beneficial Effect of Pt and of Pre-Oxidation on the Oxidation Behaviour of an NiCoCrAlYTa Bond-Coating for Thermal Barrier Coating Systems. Oxid. Met. 2011, 75, 247–279. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Monteseguro, V.; Lozano-Gorrín, A.D.; Manjón, F.J.; González-Platas, J.; Rodríguez-Hernández, P.; Muñoz, A.; Lavín, V.; Martín, I.R.; Rodríguez-Mendoza, U.R. Structural, Vibrational, and Elastic Properties of Yttrium Orthoaluminate Nanoperovskite at High Pressures. J. Phys. Chem. C 2017, 121, 15353–15367. [Google Scholar] [CrossRef]
- Thapa, J.; Liu, B.; Woodruff, S.D.; Chorpening, B.T.; Buric, M.P. Raman scattering in single-crystal sapphire at elevated temperatures. Appl. Opt. 2017, 56, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Bolelli, G.; Vorkötter, C.; Lusvarghi, L.; Morelli, S.; Testa, V.; Vaßen, R. Performance of wear resistant MCrAlY coatings with oxide dispersion strengthening. Wear 2020, 444–445, 203116. [Google Scholar] [CrossRef]

| Chemical Composition in wt.-% | Particle Size Distribution in µm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metco | ZrO2 | HfO2 * | Y2O3 | SiO2 | TiO2 | Al2O3 | Fe2O3 | Others | d10 | d50 | d90 |
| 233C | Bal. | <2.0 | 7.0–9.0 | <0.5 | <0.2 | <0.2 | <0.2 | <0.8 | 57.8 | 77.2 | 101.9 |
| 6608 | Bal | <2.5 | 7.0–8.0 | <0.05 | <0.05 | <0.05 | <0.05 | - | 0.4 | 1.5 | 4.9FZJ |
| Plasma Gases | Powder | Standoff | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Specimen | BC Heat Treatment | Topcoat | Current | Ar | H2 | Feed Rate | Distance | Robot Speed | Step Size |
| [A] | [nlpm] | [nlpm] | [g/min] | [mm] | [mm/s] | [mm] | |||
| APSv | Vacuum | APS | 411 | 52 | 6.2 | 100 | 161 | 1000 | 4 |
| SPSv | Vacuum | SPS | 550 | 64 | 8 | 12 * | 70 | 1000 | 2 |
| APSpre-ox. | Argon | APS | 411 | 52 | 6.2 | 100 | 161 | 1000 | 4 |
| SPSpre-ox. | Argon | SPS | 550 | 64 | 8 | 12 * | 70 | 1000 | 2 |
| BC Heat Treatment | TGO Thickness | Ra | Rz |
|---|---|---|---|
| [µm] | [µm] | [µm] | |
| Vacuum | 0.1–0.3 (partially no oxide layer) | 7.0 ± 0.6 | 49.8 ± 2.8 |
| Argon | 0.8 ± 0.2 | 8.3 ± 0.7 | 60.4 ± 5.0 |
| Specimen | TGO Thickness [µm] |
|---|---|
| APSv | 3.9 ± 0.8 |
| SPSv | 3.6 ± 0.8 |
| APSpre-ox. | 3.2 ± 1.0 |
| SPSpre-ox. | 3.3 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igel, J.; Scheld, W.S.; Mack, D.E.; Guillon, O.; Vaßen, R. Lifetime Extension of Atmospheric and Suspension Plasma-Sprayed Thermal Barrier Coatings in Burner Rig Tests by Pre-Oxidizing the CoNiCrAlY Bond Coats. Coatings 2024, 14, 793. https://doi.org/10.3390/coatings14070793
Igel J, Scheld WS, Mack DE, Guillon O, Vaßen R. Lifetime Extension of Atmospheric and Suspension Plasma-Sprayed Thermal Barrier Coatings in Burner Rig Tests by Pre-Oxidizing the CoNiCrAlY Bond Coats. Coatings. 2024; 14(7):793. https://doi.org/10.3390/coatings14070793
Chicago/Turabian StyleIgel, Jens, Walter Sebastian Scheld, Daniel Emil Mack, Olivier Guillon, and Robert Vaßen. 2024. "Lifetime Extension of Atmospheric and Suspension Plasma-Sprayed Thermal Barrier Coatings in Burner Rig Tests by Pre-Oxidizing the CoNiCrAlY Bond Coats" Coatings 14, no. 7: 793. https://doi.org/10.3390/coatings14070793





