Catalytic Activity Evaluation of Molten Salt-Treated Stainless Steel Electrodes for Hydrogen Evolution Reaction in Alkaline Medium
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
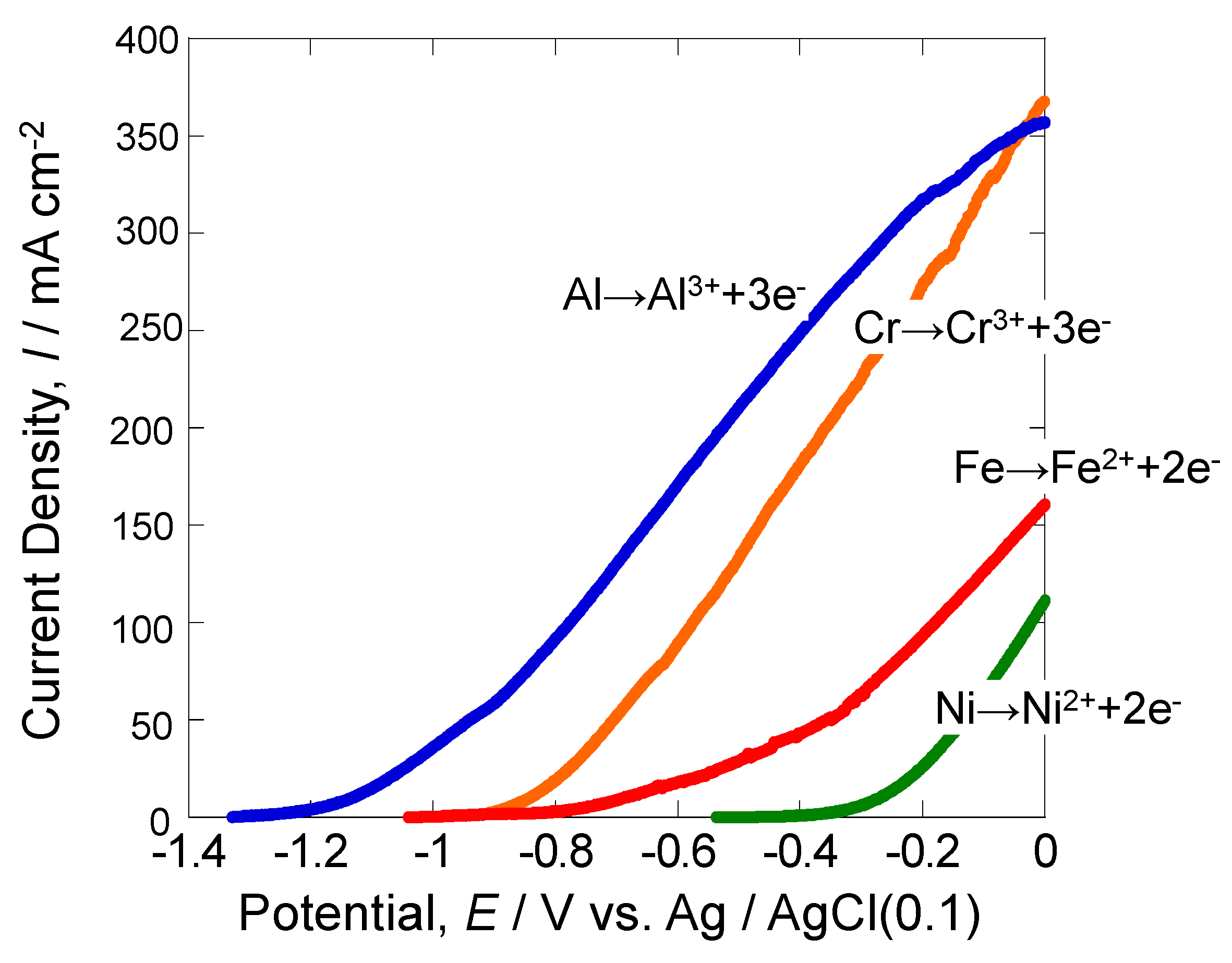
3.1. Anodic Polarization Curves of Various Metals
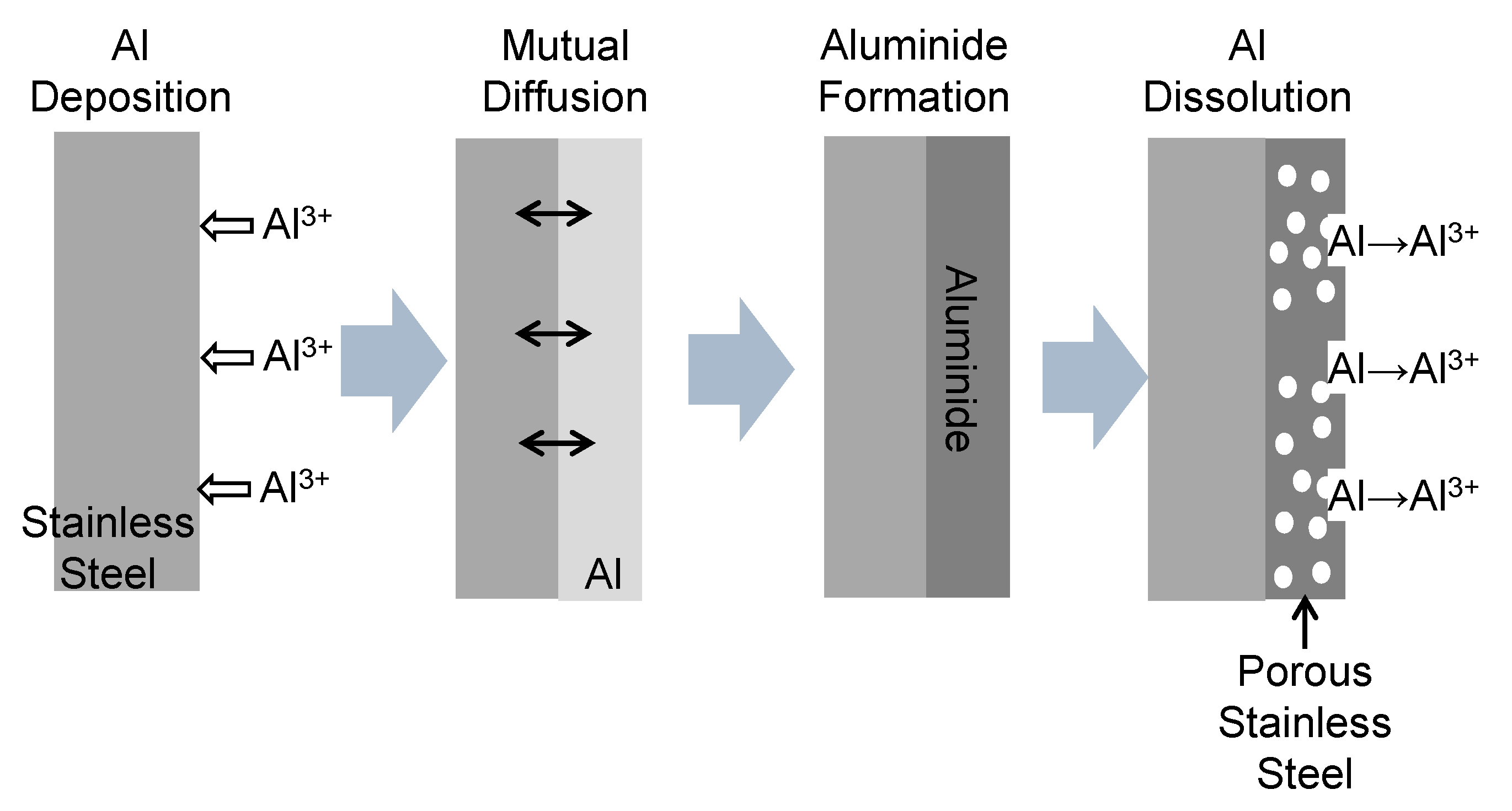
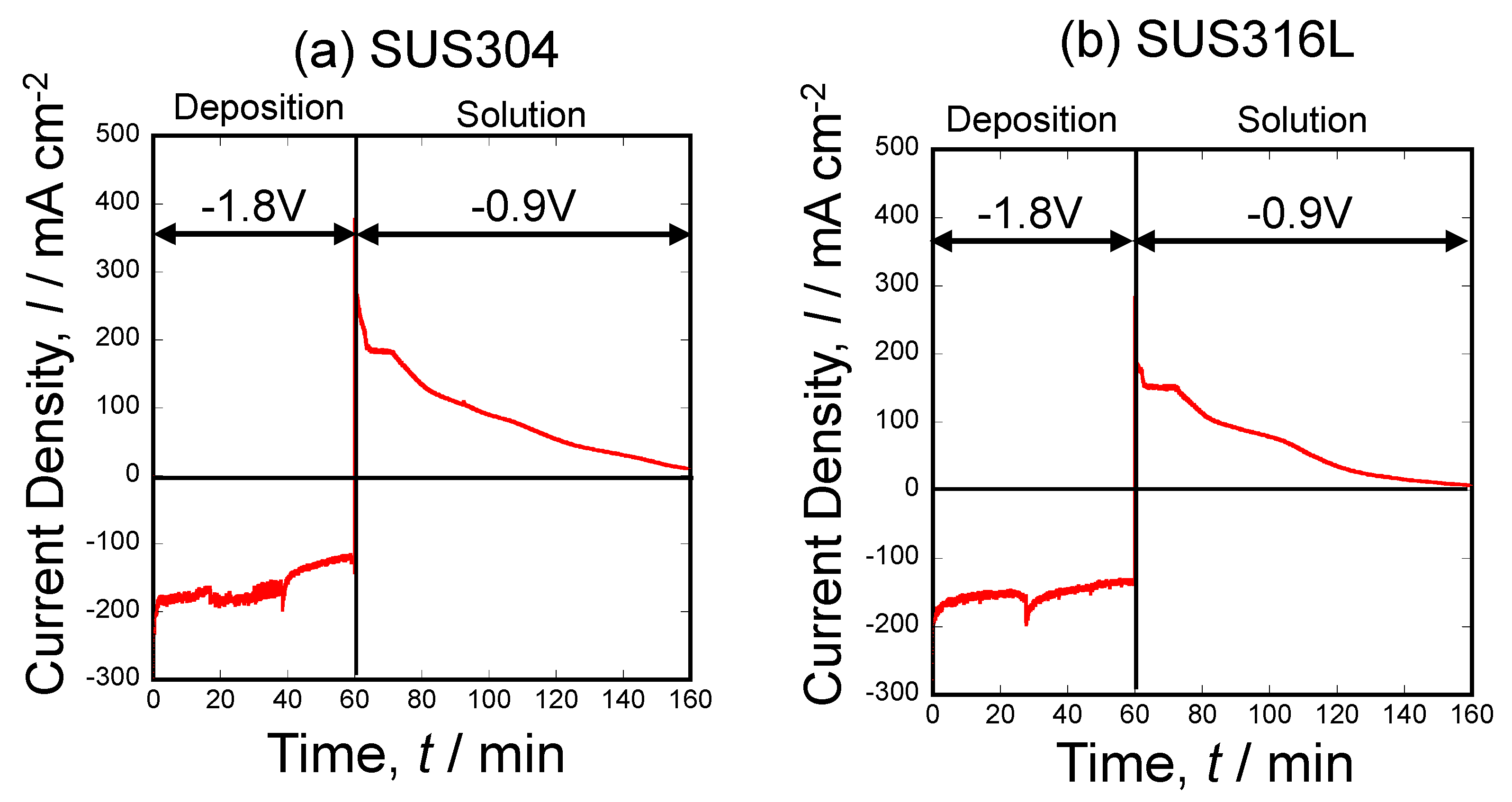
3.2. Current Density–Time Curve during Al Deposition and Al Dissolution onto SUS304 and SUS316L Stainless Steel Substrates
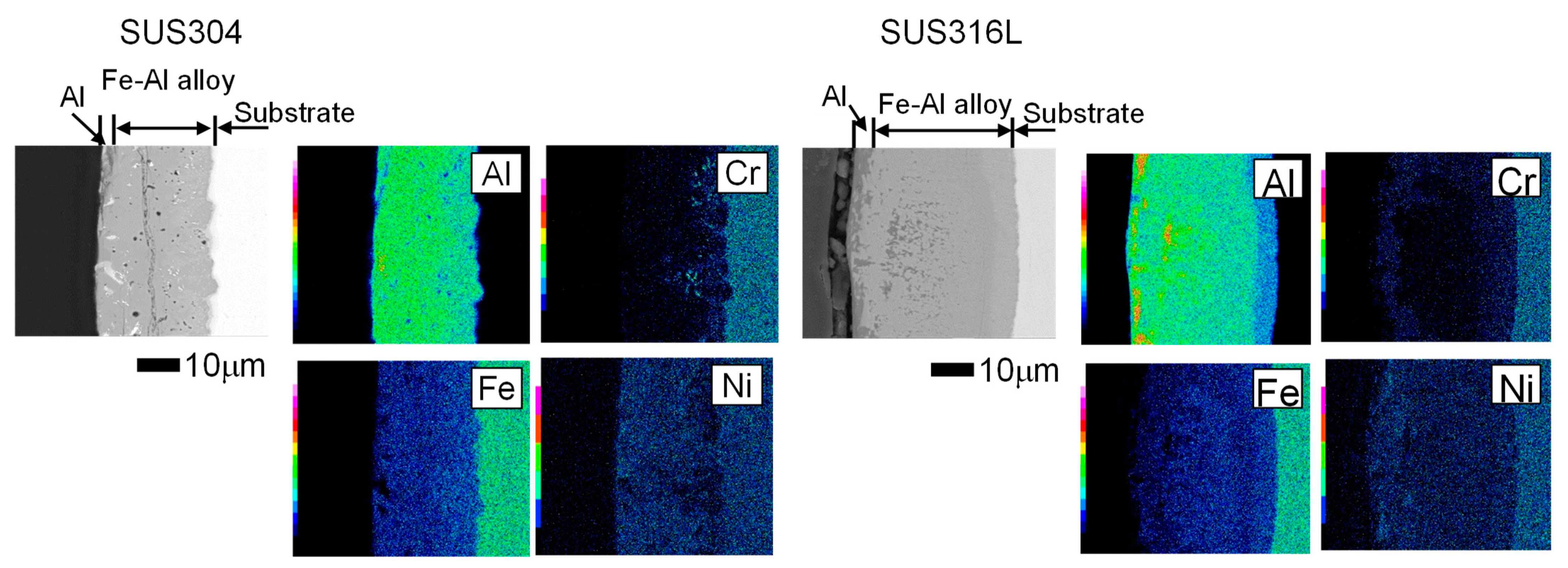
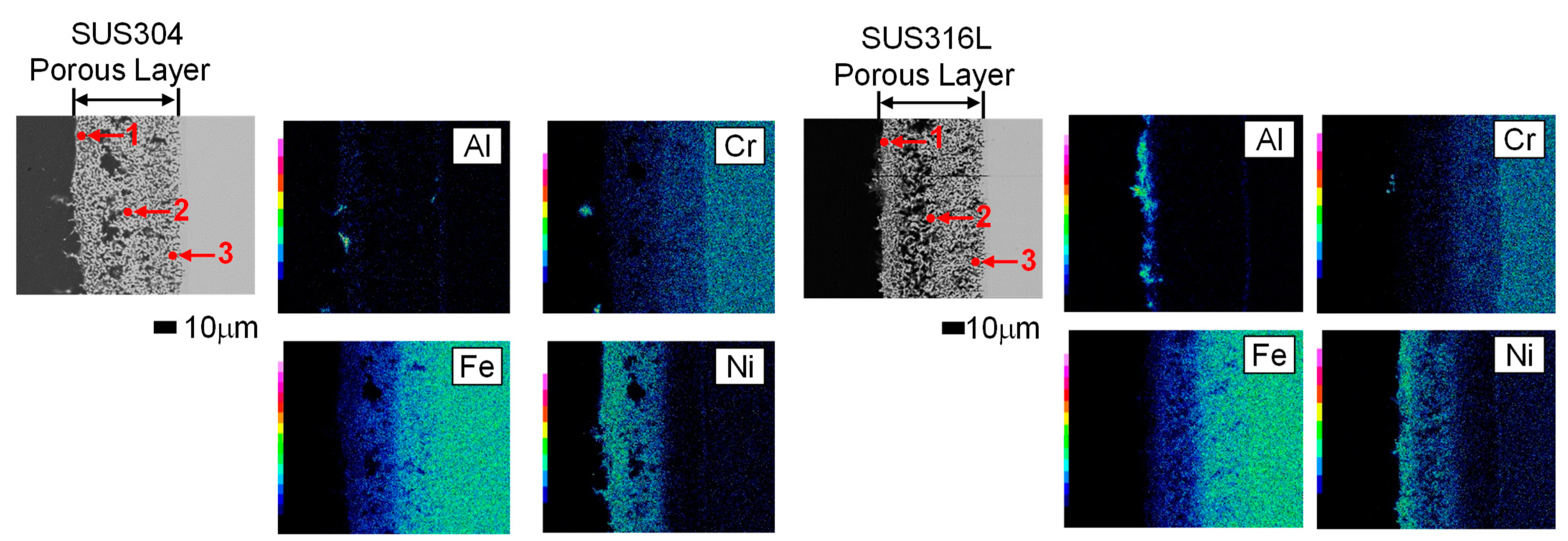
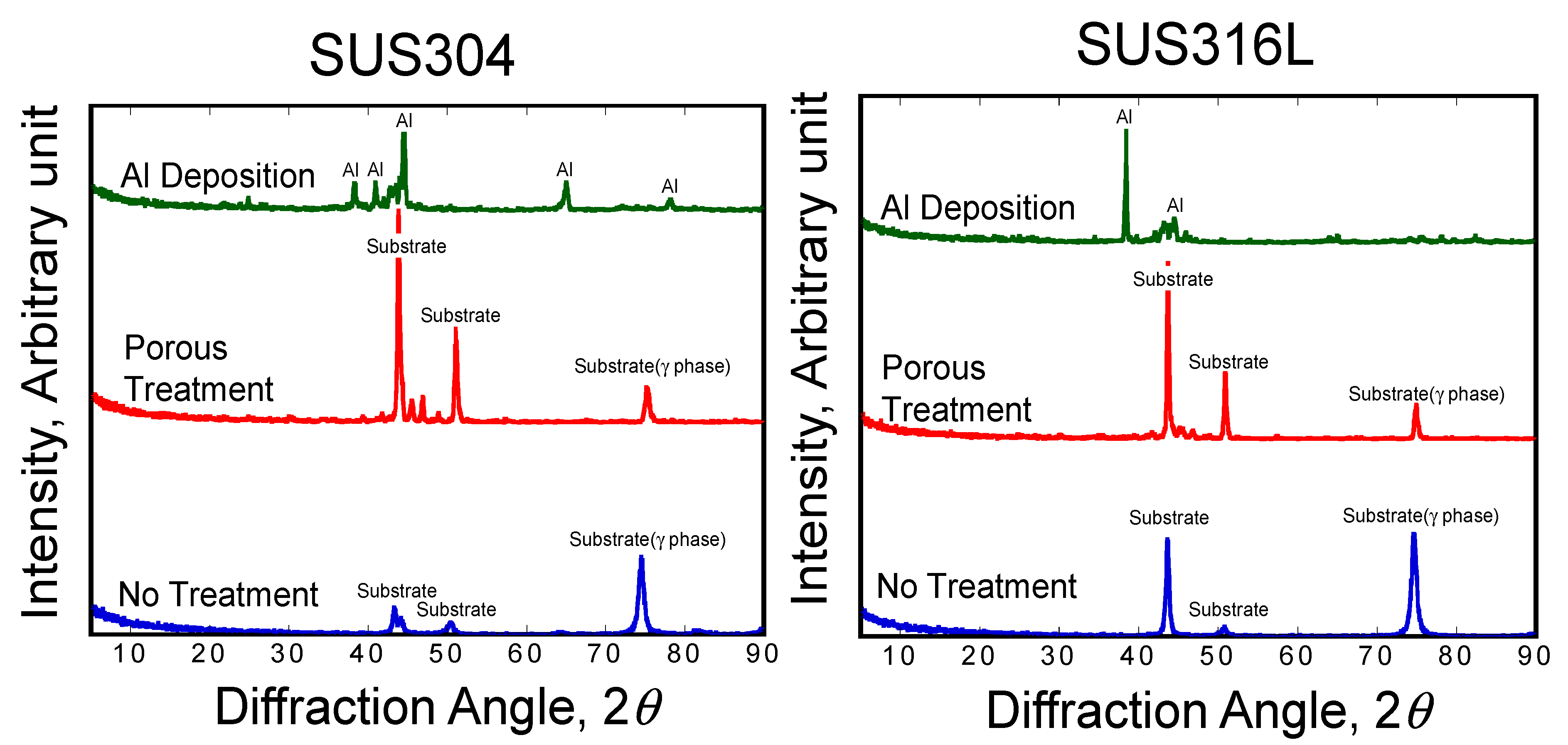
3.3. Morphology of Stainless Steel Samples after Al Molten Salts Electrodeposition
3.4. Morphology and Composition of Porous Steel Samples after Molten Salts Treatment and Dissolution of Al
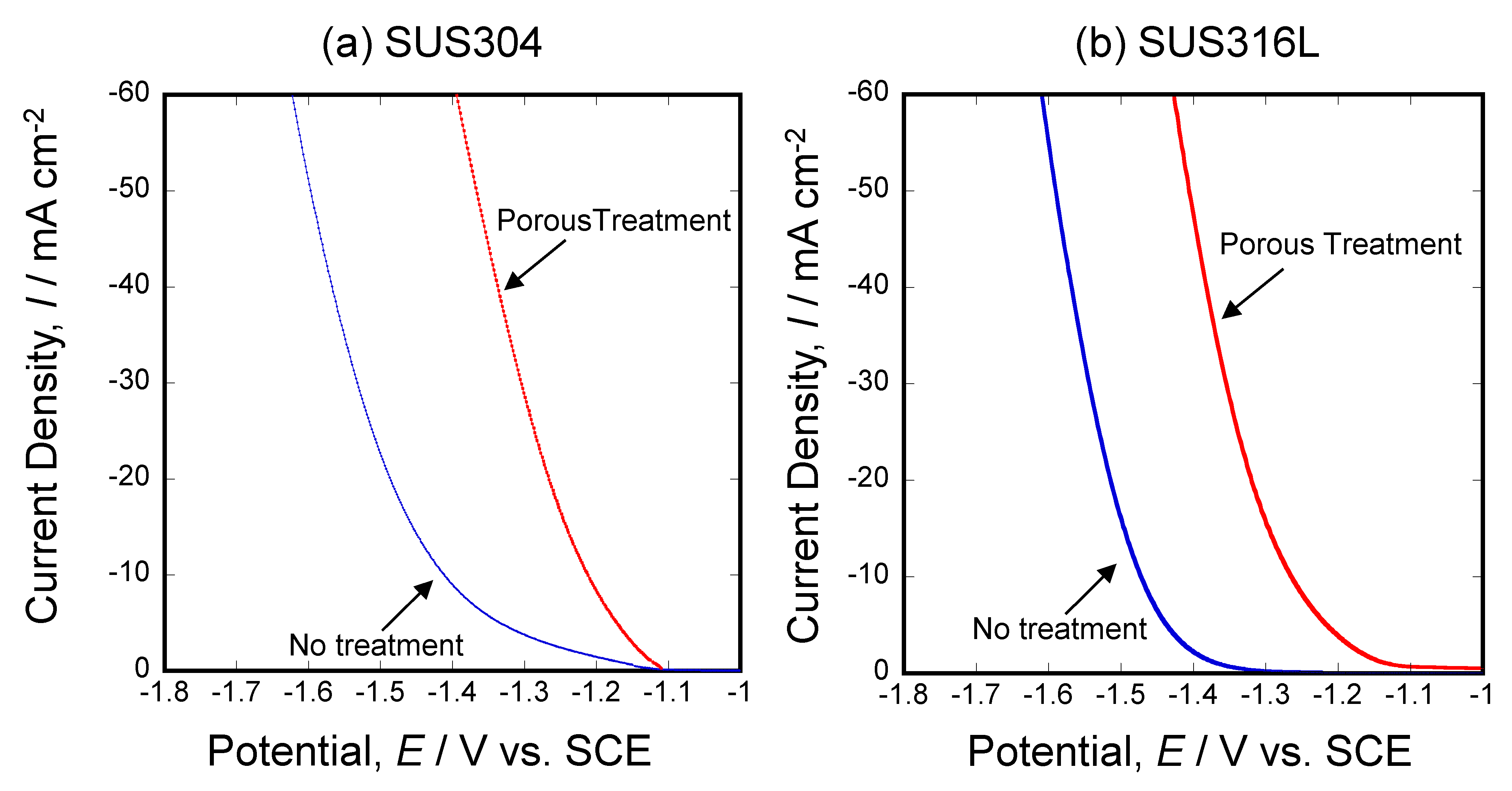
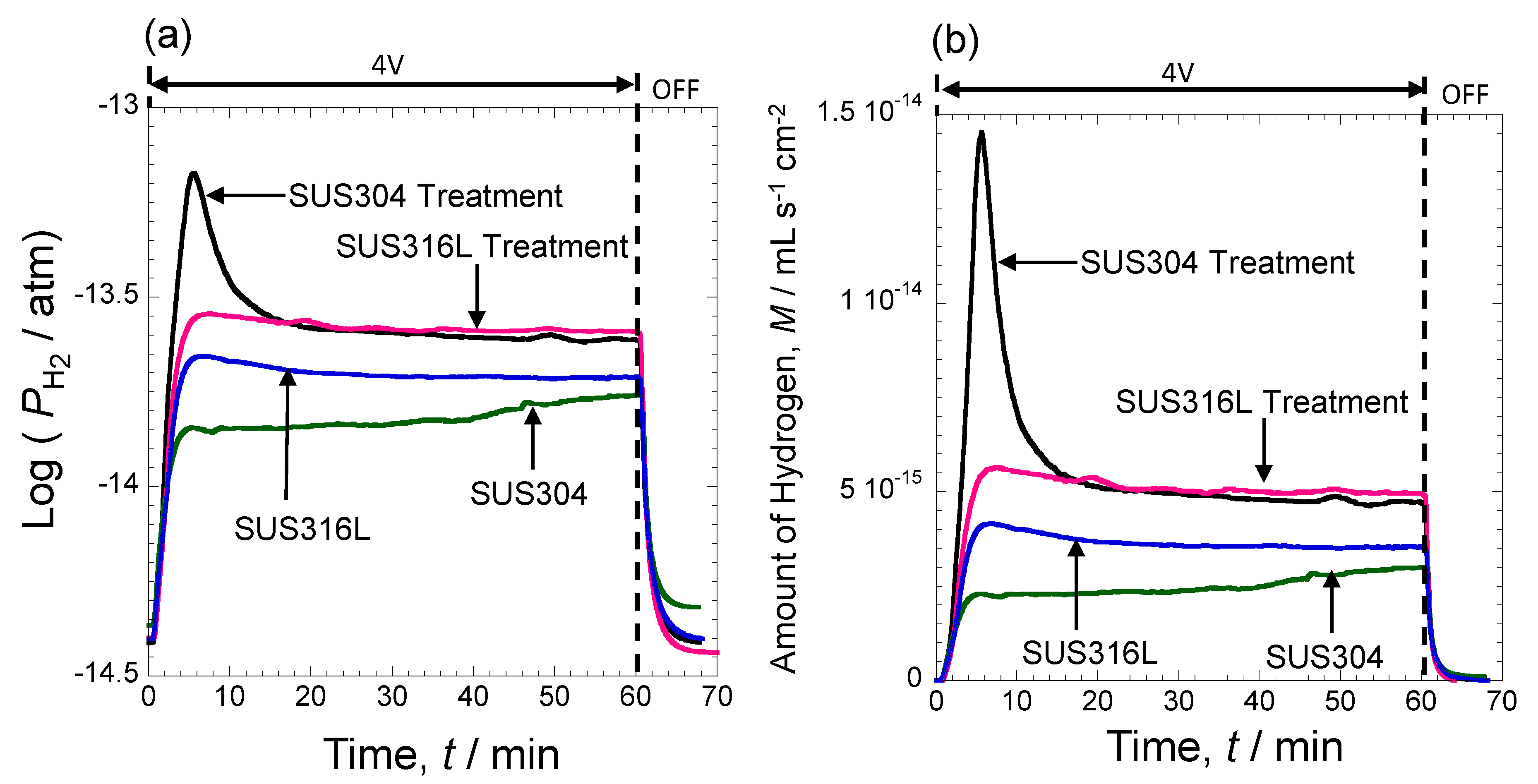
3.5. Electrochemical Activity Evaluation of Porous Stainless Steel Electrodes Obtained by Molten Salts Treatment in Hydrogen Evolution Reaction
4. Conclusions
- A porous stainless steel surface was successfully fabricated through Al electrodeposition and Al dissolution experiments.
- It was observed that porous treatment allows to obtain a compact surface with numerous voids.
- The porous stainless steel alloy exhibited a higher current density at lower potentials in the cathodic polarization curve compared to the untreated sample
- In a hydrogen generation experiment using constant voltage electrolysis, the steel samples after porous treatment produced more hydrogen, especially SUS304 which generated the highest amount of hydrogen and produced 4 × 10−11 mL of hydrogen on a surface area of 2 cm2 in 1 h of electrolysis time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolutuion reaction in alkaline solutions—A Review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Chen, L.; Lasia, A. Study of the kinetics of hydrogen evolution reaction on nickel-zinc alloy electrodes. J. Electrochem. Soc. 1991, 138, 3321–3328. [Google Scholar] [CrossRef]
- Chen, L.; Lasia, A. Study of the kinetics of hydrogen evolution reaction on nickel-zinc powder electrodes. J. Electrochem. Soc. 1992, 139, 3214–3219. [Google Scholar] [CrossRef]
- Rami, A.; Lasia, A. Kinetics of hydrogen evolution on Ni-Al alloy electrodes. J. Appl. Electrochem. 1992, 22, 376–382. [Google Scholar] [CrossRef]
- Birry, L.; Lasia, A. Studies of the hydrogen evolution reaction on Raney nickel-molybdenum electrodes. J. Appl. Electrochem. 2004, 34, 735–749. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Mauer, A.E.; Kirk, D.W.; Thorpe, S.J. The role of iron in the prevention of nickel electrode deactivation in alkaline electrolysis. Electrochim. Acta 2007, 52, 3505–3509. [Google Scholar] [CrossRef]
- Dong, H.; Lei, T.; He, Y.; Xu, N.; Huang, B.; Liu, C.T. Electrochemical performance of porous Ni3Al electrodes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2011, 36, 12112–12120. [Google Scholar] [CrossRef]
- Mullis, A.M.; Bigg, T.D.; Adkins, N.J. A microstructural investigation of gas atomized Raney type Al-27.5 at.% Ni catalyst precursor alloys. J. Alloys Compd. 2015, 648, 139–148. [Google Scholar] [CrossRef]
- Yu, L.; Lei, T.; Nao, B.; Jiang, Y.; He, Y.; Liu, C.T. Characteristics of a sintered porous Ni-Cu alloy cathode for hydrogen production in a potassium hydroxide solution. Energy 2016, 97, 498–505. [Google Scholar] [CrossRef]
- Brown, I.J.; Sotiropoulos, S. Preparation and characterization of microporous Ni coatings as hydrogen evolving cathodes. J. Appl. Electrochem. 2000, 30, 107–111. [Google Scholar] [CrossRef]
- Tanaka, S.; Hirose, N.; Tanaki, T.; Ogata, Y.H. The effect of tin ingredients on electrocatalytic activity of Raney-Ni prepared by mechanical alloying. Int. J. Hydrogen Energy 2001, 26, 47–53. [Google Scholar] [CrossRef]
- Yüce, A.O.; Döner, A.; Kardaş, G. NiMn composite electrodes as cathode material for hydrogen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2013, 38, 4466–4473. [Google Scholar] [CrossRef]
- Chade, D.; Berlouis, L.; Infield, D.; Cruden, A.; Nielsen, P.N.; Mathiesen, T. Evaluation of Raney nickel electrodes prepared by atmos pheric plasma spraying for alkaline water electrolysers. Int. J. Hydrogen Energy 2013, 38, 14380–14390. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel-cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni-Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Liu, Z.; Li BZhao, W.; Zhang, C.; Jiang, S.; Wang, J.; Liu, K.; He, S. CoO supported NiFe layered double hydroxide sandwich-like nanosheets on hierarchical carbon framework for efficient electrocatalytic oxygen evolution. ChemSusChem 2024, 17, e202301703. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Springer, M.A.; Geng, W.; Zhu, X.; Li, T.; Li, M.; Jing, Y.; Heine, T. Rational Design of Two-Dimensional Binary Polymers from Heterotriangulenes for Photocatalytic Water Splitting. J. Phys. Chem. Lett. 2021, 33, 8134–8140. [Google Scholar] [CrossRef]
- Dong, B.; Yu, N.; Wang, Q.Y.; Ren, J.K.; Zhang, X.Y.; Zhang, Z.J.; Fan, R.Y.; Liu, D.P.; Chai, Y.M. Double active sites promoting hydrogen evolution activity and stability of CoRuOH/Co2P by rapid hydrolysis. Chin. Chem. Lett. 2024, 35, 109221. [Google Scholar] [CrossRef]
- Lavorante, M.J.; Franco, J.I. Performance of stainless steel 316L electrode with modified surface to be use in alkaline water electrolyers. Int. J. Hydrogen Energy 2016, 41, 9731–9737. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Pena, R. Advance in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Moureaux, F.; Stevens, P.; Toussaint, G. Timely-activated 316L stainless steel: A low cost, durable and active electrode for oxygen evolution reaction in concentrated alkaline environments. Appl. Catal. B Environ. 2019, 258, 117963. [Google Scholar] [CrossRef]
- Zamanizadeh, H.R.; Sunde, S.; Pollet, B.G.; Seland, F. Tailoring the oxide surface composition of stainless steel for improved OER performance in alkaline water electrolysis. Electrochim. Acta 2022, 424, 140561. [Google Scholar] [CrossRef]
- Zamanizadeh, H.R.; Barnett, A.O.; Sunde, S.; Pollet, B.G.; Seland, F. Performance of activated stainless steel and nickel-based anodes in alkaline water electrolyser. J. Power Sources 2023, 564, 232828. [Google Scholar] [CrossRef]
- Fukumoto, M.; Sugiuchi, K.; Nakajima, K. Formation of porous Ni surface by electrodeposition and dissolution in molten salt. Int. J. Hydrogen Energy 2020, 45, 28252–28259. [Google Scholar] [CrossRef]
- Nakajima, K.; Fukumoto, M. Porous Ni-Co surface formation and analysis of hydrogen generation by gas sensor. Int. J. Hydrogen Energy 2021, 46, 26263–26271. [Google Scholar] [CrossRef]
- Fukumoto, M.; Nakajima, K.; Kawamori, Y. Investigation of Alumina Formation and Oxidation Rate of Ni-5wt%Al-Xwt%Cr Alloy Using Hydrogen Sensor and Oxygen Pump Sensor. Oxid. Met. 2020, 94, 191–204. [Google Scholar] [CrossRef]
- Fukumoto, M.; Takahashi, H.; Kutyla, D.; Wojnicki, M.; Zabinski, P. Morphological investigation and electrochemical performance evaluation of novel porous Ni-Pt produced by Al-deposition/dissolution in molten salts for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 49, 754–765. [Google Scholar] [CrossRef]
- Huang, L.; Hou, Y.; Yu, Z.; Peng, Z.; Wang, L.; Huang, J.; Zhang, B.; Qian, L.; Wu, L.; Li, Z. Pt/Fe-NF electrode with high double-layer capacitance for efficient hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2017, 42, 9458–9466. [Google Scholar] [CrossRef]


| Cr | Ni | Mn | Si | Mo | Fe | |
|---|---|---|---|---|---|---|
| SUS304 | 17%–19% | 8%–11% | <2% | <1% | Balance | |
| SUS316L | 16%–18% | 10%–14% | <2% | <1% | 2%–3% | Balance |
| SUS304 (at.%) | SUS316L (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| Cr | Fe | Ni | Cr | Fe | Ni | ||
| 1 | 6.81 | 21.90 | 71.29 | 1 | 1.78 | 11.09 | 87.14 |
| 2 | 13.2 | 55.4 | 26.4 | 2 | 10.86 | 53.51 | 35.63 |
| 3 | 13.91 | 58.38 | 27.70 | 3 | 14.96 | 72.31 | 12.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumoto, M.; Takahashi, H.; Kutyła, D.; Wojnicki, M.; Żabiński, P. Catalytic Activity Evaluation of Molten Salt-Treated Stainless Steel Electrodes for Hydrogen Evolution Reaction in Alkaline Medium. Coatings 2024, 14, 796. https://doi.org/10.3390/coatings14070796
Fukumoto M, Takahashi H, Kutyła D, Wojnicki M, Żabiński P. Catalytic Activity Evaluation of Molten Salt-Treated Stainless Steel Electrodes for Hydrogen Evolution Reaction in Alkaline Medium. Coatings. 2024; 14(7):796. https://doi.org/10.3390/coatings14070796
Chicago/Turabian StyleFukumoto, Michihisa, Hiroki Takahashi, Dawid Kutyła, Marek Wojnicki, and Piotr Żabiński. 2024. "Catalytic Activity Evaluation of Molten Salt-Treated Stainless Steel Electrodes for Hydrogen Evolution Reaction in Alkaline Medium" Coatings 14, no. 7: 796. https://doi.org/10.3390/coatings14070796





