Experimental and Adsorption Kinetics Study of Hg0 Removal from Flue Gas by Silver-Loaded Rice Husk Gasification Char
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. X-ray Diffraction
3.2. Hg0 Removal Performance of SRHGC under Different Conditions
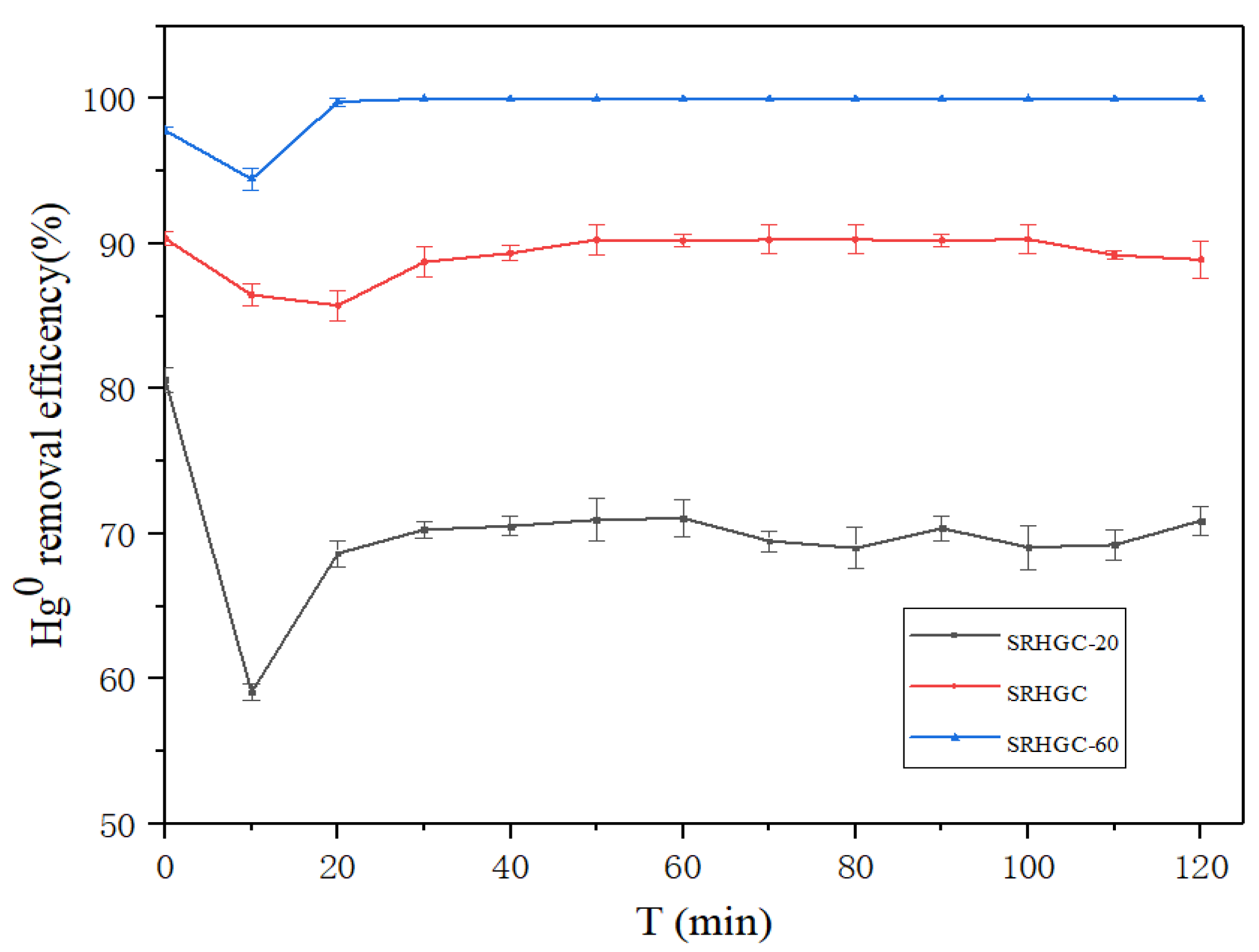
3.2.1. Hg0 Removal Performance of Different Adsorbents
3.2.2. Effect of Different Silver Loads on Hg0 Removal Performance of SRHGC
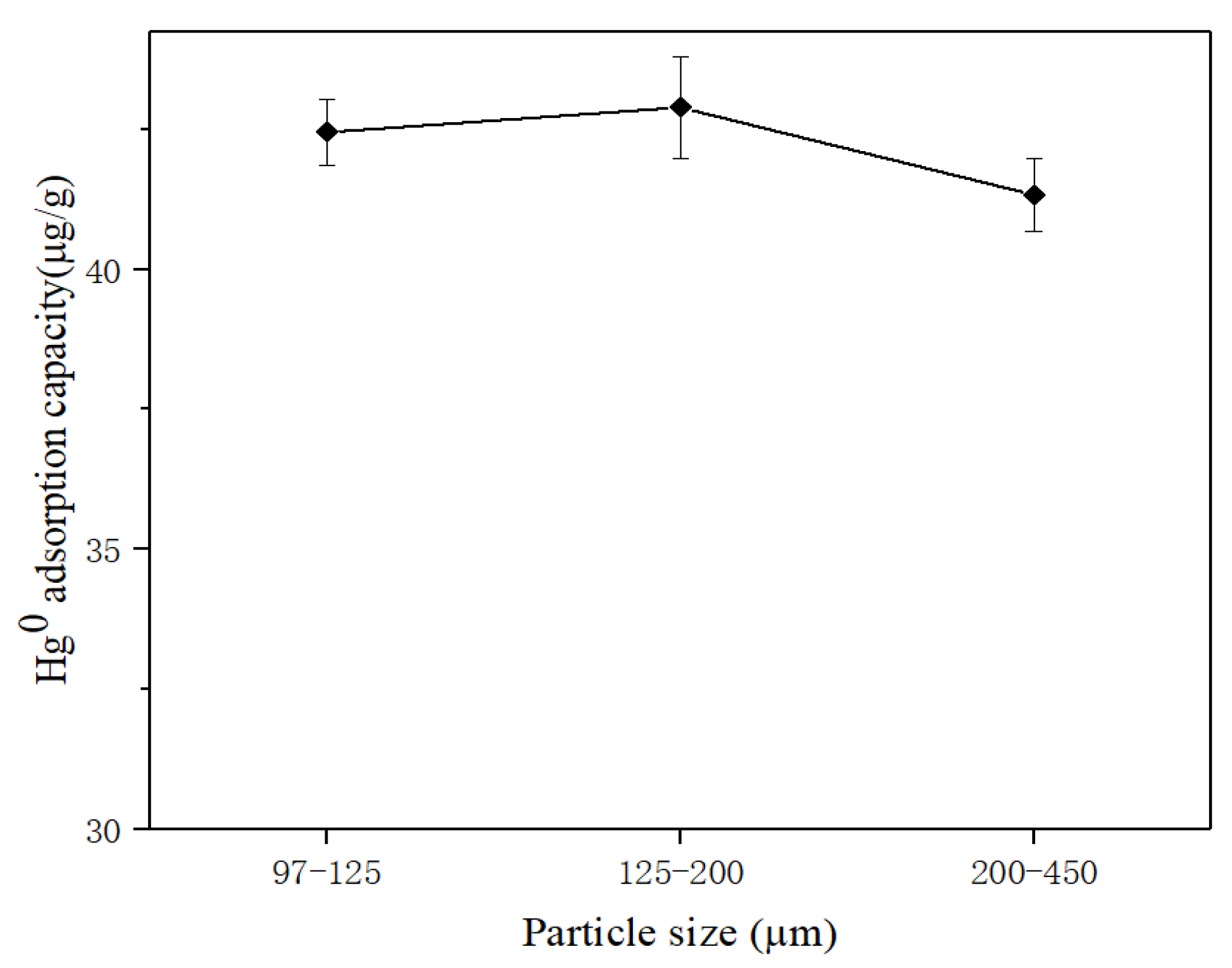
3.2.3. Effect of Different Particle Sizes on Hg0 Removal Performance of SRHGC
3.2.4. Effect of Different Temperatures on Hg0 Removal Performance of SRHGC
3.3. Adsorption Kinetics Study of Hg0 Removal by SRHGC
3.3.1. Quasi-First-Order Kinetic Equation Fitting
3.3.2. Quasi-Second-Order Kinetic Equation Fitting
3.3.3. In-Particle Diffusion Equation Fitting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Sun, Q.; Zhang, H.; Cheng, J.; Li, Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.; Li, D.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- Xie, K. Challenges and approaches of clean and efficient coal conversion in the context of new energy system development. J. Coal Sci. 2024, 1, 1–13. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Zhong, L.; Xiao, P.; Jiang, J.; Guo, T.; Guo, A. Coal-fired power plants of atmospheric mercury emission monitoring method analysis and test study. Proc. CSEE 2012, 32, 158–163. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Yang, J.; Tohidi, B.; Chuvilin, E.M.; Istomin, V.; Bukhanov, B.A.; Cheremisin, A. CO2 Capture by Injection of Flue Gas or CO2–N2 Mixtures into Hydrate Reservoirs: Dependence of CO2 Capture Efficiency on Gas Hydrate Reservoir Conditions. Environ. Sci. Technol. 2018, 52, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Draseh, G.; O’Reilly, S.B.; Beinhoff, C.; Roider, G.; Maydl, S. The Mt. Diwata Study on the Philippines 1999 Assessing Mereury Intoxication of the Population by Small Seale Gold Mining. Sci. Total Environ. 2001, 267, 151–168. [Google Scholar] [CrossRef]
- Zeng, Z.F.; Liu, T.X.; Li, H.M.; Hao, R.L. Research progress of adsorbents for mercury removal from coal flue gas. Appl. Chem. Ind. 2023, 52, 1170–1174. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Hameed, M.U.; Nasim, N.; Ahmed, F.; Altaf, F.; Shahida, S.; Fayyaz, S.; Sabir, S.M.; Bocchetta, P. Plasmonic Nano Silver: An Efficient Colorimetric Sensor for the Selective Detection of Hg2+ Ions in Real Samples. Coatings 2022, 12, 763. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Liu, Y.; Huang, R.; Wang, Z.; Zhao, Y. A review on removal of mercury from flue gas utilizing existing air pollutant control devices (apcds). J. Hazard. Mater. 2022, 427, 128132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, J.; Xu, L.; Liu, X.; Li, L.; Wang, H. Removal of Elemental Mercury from Simulated Flue Gas by a Copper-Based ZSM-5 Molecular Sieve. Coatings 2022, 12, 772. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Huang, T.; Sheng, P.; Zhang, J.; Tian, H.; Zhao, X.; Zhao, L.; He, P.; Ren, J.; et al. Removal of elemental mercury by Ce-Mn co-modified activated carbon catalyst. Catal. Commun. 2017, 93, 62–66. [Google Scholar] [CrossRef]
- Rungnim, C.; Promarak, V.; Hannongbua, S.; Kungwan, N.; Namuangruk, S. Complete reaction mechanisms of mercury oxidation on halogenated activated carbon. J. Hazard. Mater. 2016, 310, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, B.; Zeng, X.; Luo, G.; Li, X.; Yao, H.; Zheng, C. Deep study on effects of activated carbon’s oxygen functional groups for elemental mercury adsorption using temperature programmed desorption method. Fuel 2017, 200, 100–106. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, S. A high proportion reuse of RAP in plant-mixed cold recycling technology and its benefits analysis. Coatings 2022, 12, 1283. [Google Scholar] [CrossRef]
- Sun, H. Study on Controllable Synthesis of Molecular Sieve Loaded Silver Composite and Performance in Removing Hg0 from Natural Gas. Master’s Thesis, Shandong University of Science and Technology, Qingdao, China, 2021. [Google Scholar]
- Xu, H.; Qu, Z.; Huang, W.; Mei, J.; Chen, W.; Zhao, S.; Yan, N. Regenerable Ag/graphene sorbent for elemental mercury capture at ambient temperature. Colloids Surf. A Physicochem. Eng. Asp. 2015, 476, 47683–47689. [Google Scholar] [CrossRef]
- Lee, T.G. Study of Mercury Kinetics and Control Methodogies in Simulates Combustion Flue Gases. Bachelor’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 1999. [Google Scholar]
- Yang, R.; Diao, Y.; Befkadu, A. Removal of Hg0 from simulated flue gas over silver-loaded rice husk gasification char. R. Soc. Open Sci. 2018, 5, 180248. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.F.; Radisav, D.V.; Wei, L.; Robert, C.T. Modeling Powdered Activated Carbon Injection for the Uptake of Elemental Mercury Vapors. J. Air Waste Manag. Assoc. 1998, 48, 1051–1059. [Google Scholar] [CrossRef]
- Yan, R.; Liang, D.T.; Tsen, L.; Wong, Y.P.; Lee, Y.K. Bench-scale experimental evaluation of carbon performance on mercury vapour adsorption. Fuel 2004, 83, 2401–2409. [Google Scholar] [CrossRef]
- Skodras, G.; Diamantopoulou, I.; Pantoleontos, G.; Sakellaropoulos, G.P. Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J. Hazard. Mater. 2008, 158, 1–13. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zhou, Q. Experimental and Mechanism Study of Mercury Removal by Injection of Modified Adsorbent. Doctoral Thesis, Southeast University, Nanjing, China, 2016. [Google Scholar]



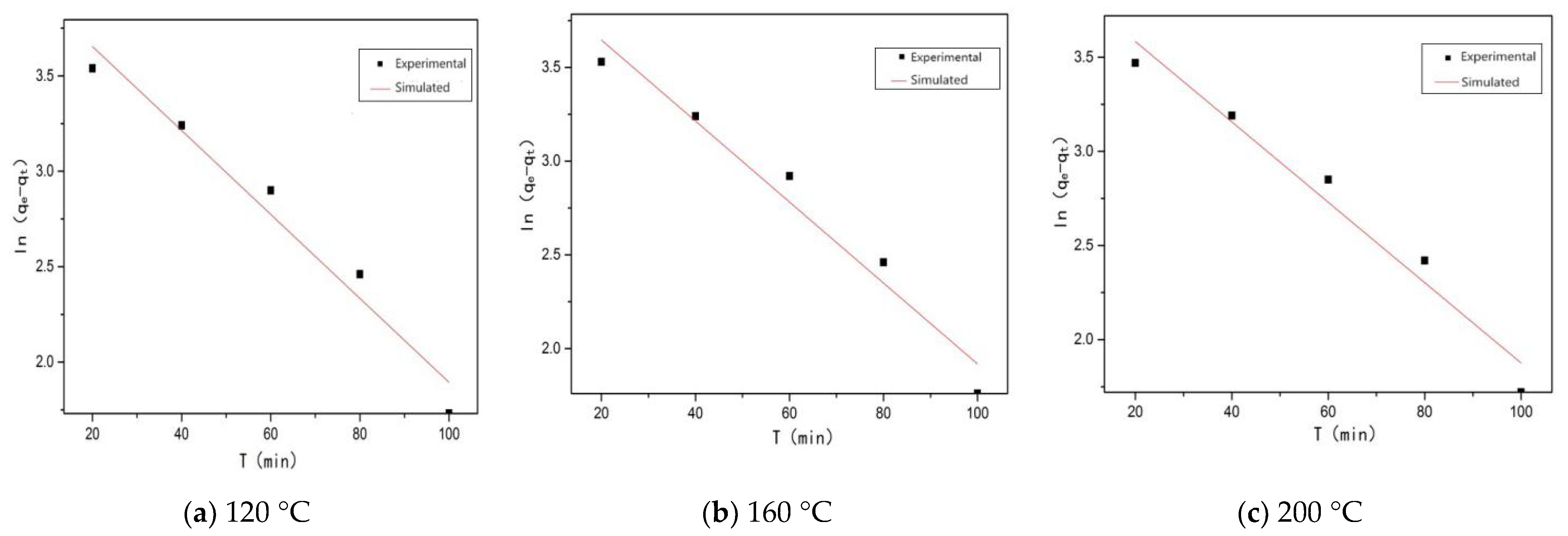


| Sample | qe | K1 | R2 |
|---|---|---|---|
| SRHGC-120 | 44.09 | 0.022 | 0.95199 |
| SRHGC-160 | 43.54 | 0.0216 | 0.95164 |
| SRHGC-200 | 41.15 | 0.02135 | 0.95307 |
| Sample | h | K2 | R2 |
|---|---|---|---|
| SRHGC-120 | 0.517 | 2.22 × 10−5 | 0.99348 |
| SRHGC-160 | 0.504 | 2.15 × 10−5 | 0.99623 |
| SRHGC-200 | 0.475 | 2.11 × 10−5 | 0.99713 |
| Sample | C | K1 | R2 |
|---|---|---|---|
| SRHGC-120 | −15.85088 | 5.36538 | 0.99063 |
| SRHGC-160 | −14.69875 | 5.23916 | 0.99233 |
| SRHGC-200 | −14.05459 | 4.938 | 0.99103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Diao, Y.; Liu, H.; Lu, Y. Experimental and Adsorption Kinetics Study of Hg0 Removal from Flue Gas by Silver-Loaded Rice Husk Gasification Char. Coatings 2024, 14, 797. https://doi.org/10.3390/coatings14070797
Yang R, Diao Y, Liu H, Lu Y. Experimental and Adsorption Kinetics Study of Hg0 Removal from Flue Gas by Silver-Loaded Rice Husk Gasification Char. Coatings. 2024; 14(7):797. https://doi.org/10.3390/coatings14070797
Chicago/Turabian StyleYang, Ru, Yongfa Diao, Hongbin Liu, and Yihang Lu. 2024. "Experimental and Adsorption Kinetics Study of Hg0 Removal from Flue Gas by Silver-Loaded Rice Husk Gasification Char" Coatings 14, no. 7: 797. https://doi.org/10.3390/coatings14070797





