Characterization of Materials Used in the Concrete Industry, from the Point of View of Corrosion Behavior
Abstract
1. Introduction
2. Materials and Methods
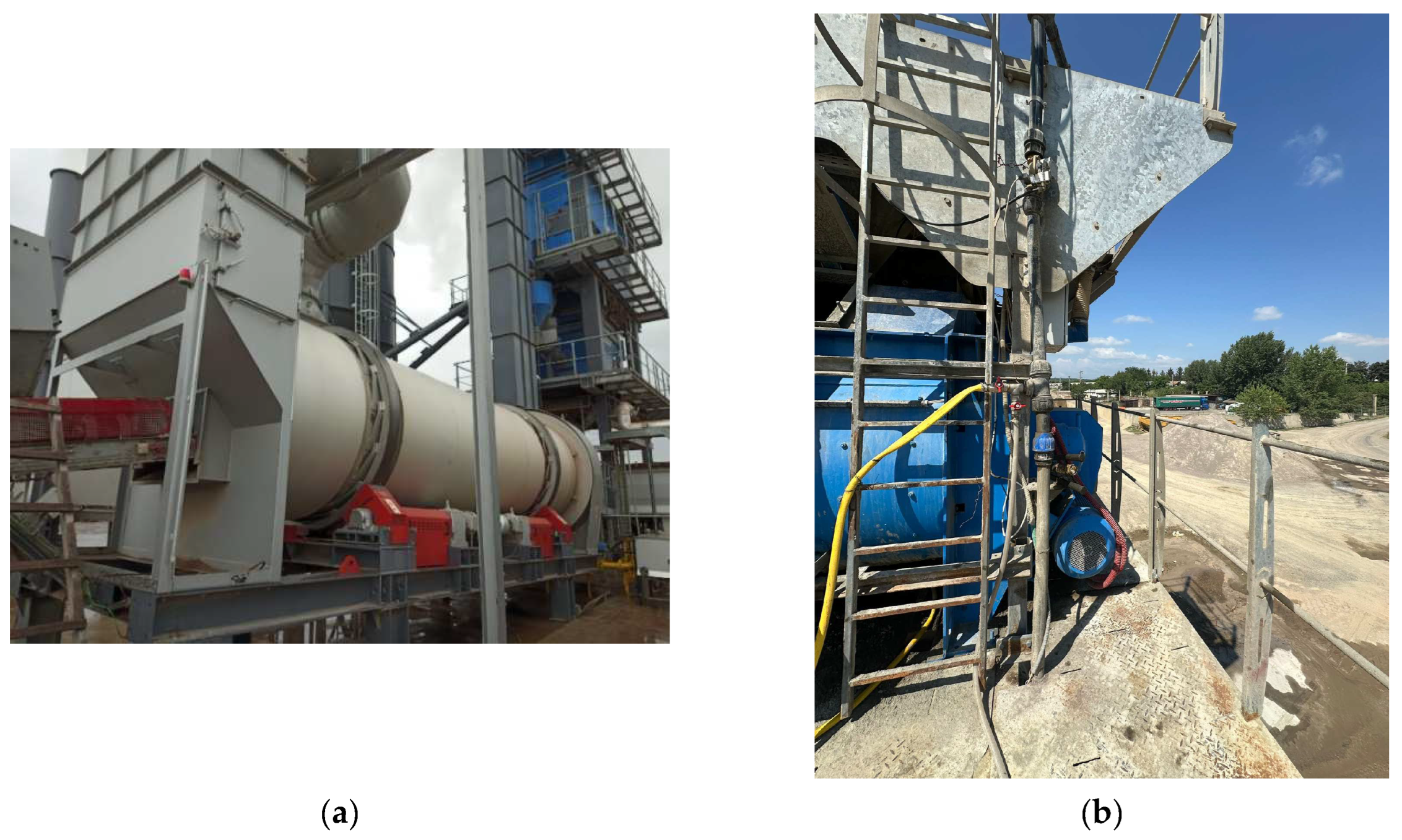
2.1. Equipment Used for the Electrochemical Corrosion Testing
2.2. Sample Selection and Preparation
2.3. Theoretical Considerations
3. Results and Discussion
3.1. Testing of Materials Used for Mixing Blades (in Cement Paste)
3.2. Testing of Materials Used for Rotary Dryers (in Water)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burlacu, A.; Gabriel Petrescu, M.; George Rîpeanu, R.; Dumitru, T.; Victor Laudacescu, E.; Naim Ramadana, I.; Niță, A. Experimental Investigations on Wear Phenomena Specific to Rotary Dryer Flights (Blades). Tribol. Ind. 2024, 46, 56–65. [Google Scholar] [CrossRef]
- Revol, D.; Briens, C.L.; Chabagno, J.M. The Design of Flights in Rotary Dryers. Powder Technol. 2001, 121, 230–238. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hasankhoei, A.; Parsapour, G.; Razi, E.; Banisi, S. A Combined Physical and DEM Modelling Approach to Improve Performance of Rotary Dryers by Modifying Flights Design. Dry. Technol. 2021, 39, 548–565. [Google Scholar] [CrossRef]
- Karali, M.A.; Specht, E.; Herz, F.; Mellmann, J.; Refaey, H.A. Unloading Characteristics of Flights in a Flighted Rotary Drum Operated at Optimum Loading. Powder Technol. 2018, 333, 347–352. [Google Scholar] [CrossRef]
- Nascimento, S.M.; Santos, D.A.; Barrozo, M.A.S.; Duarte, C.R. Solids Holdup in Flighted Rotating Drums: An Experimental and Simulation Study. Powder Technol. 2015, 280, 18–25. [Google Scholar] [CrossRef]
- Karali, M.A.; Sunkara, K.R.; Herz, F.; Specht, E. Experimental Analysis of a Flighted Rotary Drum to Assess the Optimum Loading. Chem. Eng. Sci. 2015, 138, 772–779. [Google Scholar] [CrossRef]
- Silveira, J.C.; Lima, R.M.; Brandao, R.J.; Duarte, C.R.; Barrozo, M.A.S. A Study of the Design and Arrangement of Flights in a Rotary Drum. Powder Technol. 2022, 395, 195–206. [Google Scholar] [CrossRef]
- Ilhan, E.; Findik, F.; Aslanlar, S. An Investigation of the Factors Affecting the Design of Drum Dryers. Mater. Des. 2003, 24, 503–507. [Google Scholar] [CrossRef]
- Fasano, J.; Janz, E.E.; Myers, K. Design Mixers to Minimize Effects of Erosion and Corrosion Erosion. Int. J. Chem. Eng. 2012, 2012, 1–8. [Google Scholar] [CrossRef][Green Version]
- Yao, Z.; Yang, R.; Yuan, W.; An, H. Mechanical analysis and optimal design of mixing paddlesforcsam Mixers. Acad. J. Manuf. Eng. 2019, 17, 80–86. [Google Scholar]
- Khidir, T.C. Designing, Remodeling and Analyzing the Blades of Portable Concrete Mixture. Int. J. Mech. Eng. Robot. Res. 2018, 7, 674–678. [Google Scholar] [CrossRef]
- Jungedal, M. Mild Impact Wear in a Concrete Mixer, an Evaluation of Wet Abrasive Wear. Master’s Thesis, Royal Institute of Technology Department of Material Science and Engineering SE–100 44, Stockholm, Sweden, 2012. [Google Scholar]
- Zhang, H.; Feng, P.; Ying, W. Abrasive Wear and Optimal Installation Angle of Concrete Double-Horizontal Shaft Mixer Stirring Blades. SN Appl. Sci. 2020, 2, 1067. [Google Scholar] [CrossRef]
- Valigi, M.C.; Logozzo, S.; Rinchi, M. Wear Resistance of Blades in Planetary Concrete Mixers. Part II: 3D Validation of a New Mixing Blade Design and Efficiency Evaluation. Tribol. Int. 2016, 103, 37–44. [Google Scholar] [CrossRef]
- Valigi, M.C.; Logozzo, S.; Rinchi, M. Wear Resistance of Blades in Planetary Concrete Mixers. Design of a New Improved Blade Shape and 2D Validation. Tribol. Int. 2016, 96, 191–201. [Google Scholar] [CrossRef]
- Niță, A.; Petrescu, M.G.; Dumitru, T.; Burlacu, A.; Tănase, M.; Laudacescu, E.; Ramadan, I. Experimental Research on the Wear Behavior of Materials Used in the Manufacture of Components for Cement Concrete Mixers. Materials 2023, 16, 2326. [Google Scholar] [CrossRef]
- Labiapari, W.S.; Gonçalves, R.J.; De Alcântara, C.M.; Pagani, V.; Di Cunto, J.C.; De Mello, J.D.B. Understanding Abrasion-Corrosion to Improve Concrete Mixer Drum Performance: A Laboratory and Field Approach. Wear 2021, 477, 203830. [Google Scholar] [CrossRef]
- Niță, A.; Laudacescu, E.; Petrescu, M.G.; Dumitru, T.; Burlacu, A.; Bădoiu, D.G.; Tănase, M. Experimental Research Regarding the Effect of Mineral Aggregates on the Wear of Mixing Blades of Concrete Mixers. Materials 2023, 16, 5047. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, H.R.; Ismail, M.E. Comparing Corrosion Measurement Methods to Assess the Corrosion Activity of Laboratory OPC and HPC Concrete Specimens. Cem. Concr. Res. 2004, 34, 2037–2044. [Google Scholar] [CrossRef]
- Garcia, E.; Torres, J.; Rebolledo, N.; Arrabal, R.; Sanchez, J. Corrosion of Steel Rebars in Anoxic Environments. Part I: Electrochemical Measurements. Materials 2021, 14, 2491. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion Rate of Steel in Concrete—Measurements beyond the Tafel Law. Corros. Sci. 2005, 47, 3019–3033. [Google Scholar] [CrossRef]
- Sanusi, M.S.; Shamsudin, S.R.; Rahmat, A.; Wardan, R. Electrochemical Corrosion Behaviours of AISI 304 Austenitic Stainless Steel in NaCl Solutions at Different pH; AIP Publishing: Ho Chi Minh, Vietnam, 2018; p. 020116. [Google Scholar]
- Fahim, A.; Dean, A.E.; Thomas, M.D.A.; Moffatt, E.G. Corrosion Resistance of Chromium-steel and Stainless Steel Reinforcement in Concrete. Mater. Corros. 2019, 70, 328–344. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test Methods for On-Site Corrosion Rate Measurement of Steel Reinforcement in Concrete by Means of the Polarization Resistance Method. Mat. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Dubuc, B.; Ebrahimkhanlou, A.; Salamone, S. Corrosion Monitoring of Prestressed Concrete Structures by Using Topological Analysis of Acoustic Emission Data. Smart Mater. Struct. 2019, 28, 055001. [Google Scholar] [CrossRef]
- Ming, J.; Shi, J. Distribution of Corrosion Products at the Steel-Concrete Interface: Influence of Mill Scale Properties, Reinforcing Steel Type and Corrosion Inducing Method. Constr. Build. Mater. 2019, 229, 116854. [Google Scholar] [CrossRef]
- Chang, Z.-T.; Cherry, B.; Marosszeky, M. Polarisation Behaviour of Steel Bar Samples in Concrete in Seawater. Part 2: A Polarisation Model for Corrosion Evaluation of Steel in Concrete. Corros. Sci. 2008, 50, 3078–3086. [Google Scholar] [CrossRef]
- Otieno, M.; Ikotun, J.; Ballim, Y. Experimental Investigations on the Influence of Cover Depth and Concrete Quality on Time to Cover Cracking Due to Carbonation-Induced Corrosion of Steel in RC Structures in an Urban, Inland Environment. Constr. Build. Mater. 2019, 198, 172–181. [Google Scholar] [CrossRef]
- Lambert, P.; Page, C.L.; Vassie, P.R.W. Investigations of Reinforcement Corrosion. 2. Electrochemical Monitoring of Steel in Chloride-Contaminated Concrete. Mater. Struct. 1991, 24, 351–358. [Google Scholar] [CrossRef]
- González, J.A.; Miranda, J.M.; Feliu, S. Considerations on Reproducibility of Potential and Corrosion Rate Measurements in Reinforced Concrete. Corros. Sci. 2004, 46, 2467–2485. [Google Scholar] [CrossRef]
- Meng, D.; Lin, S.; Azari, H. Nondestructive Corrosion Evaluation of Reinforced Concrete Bridge Decks with Overlays: An Experimental Study. J. Test. Eval. 2020, 48, 516–537. [Google Scholar] [CrossRef]
- Gulikers, J. Statistical Interpretation of Results of Potential Mapping on Reinforced Concrete Structures. Eur. J. Environ. Civ. Eng. 2010, 14, 441–466. [Google Scholar] [CrossRef]
- Valipour, M.; Shekarchi, M.; Ghods, P. Comparative Studies of Experimental and Numerical Techniques in Measurement of Corrosion Rate and Time-to-Corrosion-Initiation of Rebar in Concrete in Marine Environments. Cem. Concr. Compos. 2014, 48, 98–107. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.B.; Pour-Ghaz, M. A Practical Method for Calculating the Corrosion Rate of Uniformly Depassivated Reinforcing Bars in Concrete. Mater. Corros. 2007, 58, 265–272. [Google Scholar] [CrossRef]
- Zou, Z.H.; Wu, J.; Wang, Z.; Wang, Z. Relationship between Half-Cell Potential and Corrosion Level of Rebar in Concrete. Corros. Eng. Sci. Technol. 2016, 51, 588–595. [Google Scholar] [CrossRef]
- Li, C.; Chen, Q.; Wang, R.; Wu, M.; Jiang, Z. Corrosion Assessment of Reinforced Concrete Structures Exposed to Chloride Environments in Underground Tunnels: Theoretical Insights and Practical Data Interpretations. Cem. Concr. Compos. 2020, 112, 103652. [Google Scholar] [CrossRef]
- ASTM G5-94; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM G1-90; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 1999.
- EN 10028-2:2003; Flat Products Made of Steels for Pressure Purposes—Part 2: Non-Alloy and Alloy Steels with Specified Elevated Temperature Properties. European Committee for Standardization: Brussels, Belgium, 2003.
- ASME SA-516; Specification for Pressure Vessel Plates, Carbon Steel, for Moderate- and Lower Temperature Service. ASTM International: New York, NY, USA, 2017.
- EN 10088-2:2005; Stainless Steels—Part 2: Technical Delivery Conditions for Sheet/Plate and Strip of Corrosion Resisting Steels for General Purposes. European Committee for Standardization: Brussels, Belgium, 2005.
- ASTM A29/A29M-23; Standard Specification for General Requirements for Steel Bars, Carbon and Alloy, Hot-Wrought. ASTM International: West Conshohocken, PA, USA, 2023.
- EN 10083; Steels for Quenching and Tempering. European Committee for Standardization: Brussels, Belgium, 2006.
- EN 10025; Hot Rolled Products of Structural Steels. European Committee for Standardization: Brussels, Belgium, 2019.
- Mansfeld, F. Simultaneous Determination of Instantaneous Corrosion Rates and Tafel Slopes from Polarization Resistance Measurements. J. Electrochem. Soc. 1973, 120, 515. [Google Scholar] [CrossRef]
- von Baeckmann, W.; Schwenk, W.; Prinz, W.; von Baeckmann, W. (Eds.) Handbook of Cathodic Corrosion Protection: Theory and Practice of Electrochemical Protection Processes, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; ISBN 978-0-88415-056-5. [Google Scholar]
- Ripeanu, R.G.; Ispas, V.; Ispas, D. Austenitic Stainless Steel Type AISI 316L Corrosive Behavior in Hair Shampoo Medium. J. Balk. Tribol. Assoc. 2012, 18, 36–43. [Google Scholar]
- Dudu, C.; Drumeanu, A.C.; Ripeanu, R.G.; Dinita, A. Some Considerations Regarding the Influence of Working Conditions on the Corrosion Wear of the Injection Water Treatment Plant Equipment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 724, 012033. [Google Scholar] [CrossRef]
- Eškinja, M.; Moshtaghi, M.; Hönig, S.; Zehethofer, G.; Mori, G. Investigation of the Effects of Temperature and Exposure Time on the Corrosion Behavior of a Ferritic Steel in CO2 Environment Using the Optimized Linear Polarization Resistance Method. Results Mater. 2022, 14, 100282. [Google Scholar] [CrossRef]
- Ahlström, J. Corrosion of Steel in Concrete at Various Moisture and Chloride Levels. Ph.D. Thesis, Division of Building Materials. LTH, Lund University, Lund, Sweden, 2014. [Google Scholar]




| Sample | Chemical Composition, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | P | S | Cr | Mn | Fe | Ni | Mo | |
| F1 | 3.28 | 0.76 | 0.07 | 0.03 | 3.83 | 1.10 | 89.86 | 0.89 | 0.20 |
| F2 | 3.72 | 0.74 | 0.02 | 0.04 | 25.65 | 0.87 | 68.19 | 0.040 | 0.35 |
| F3 | 3.08 | 0.96 | 0.04 | 0.03 | 9.77 | 1.14 | 84.12 | 0.35 | 0.49 |
| Sample | Sample Microstructure | Hardness HV0.2 (Average of Three Measurements) | Occupied Surface by the Hard Constituents, % | Hardness HV0.2, (Weighted Average) | |
|---|---|---|---|---|---|
| Matrix | Carbide | ||||
| 1 |  Low cast iron alloyed with Cr (4%), pearlite, ledeburites, and secondary cementites | 318 | 644 | 44.986 | 464.654 |
| 2 | 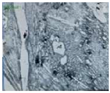 High cast iron alloyed with Cr (25%), austenitic structure with carbides | 718 | 1632 | 55.854 | 1228.505 |
| 3 |  Medium cast iron alloyed with Cr (9%), pearlite, secondary cementite, and ledeburite | 462 | 799 | 50.739 | 632.990 |
| Steel | Chemical Composition, wt.% | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Mo | Ni | Al | Co | Cu | Nb | Ti | V | W | |
| A P355GH: EN10028 [39] (ASME SA 516 GRADE 70 [40]) | 97.384 | 0.264 | 0.175 | 1.229 | 0.013 | 0.005 | 0.362 | 0.040 | 0.181 | 0.020 | 0.010 | 0.256 | 0.002 | 0.044 | 0.005 | 0.010 |
| B X5CrNi18-10 (1.4301): EN 10088 [41] (AISI 304) | 72.151 | 0.08 | 0.110 | 1.260 | 0.006 | 0.003 | 18.010 | 0.020 | 8.030 | - | 0.130 | 0.090 | 0.010 | 0.090 | 0.010 | - |
| B′ P265GH (1.0425): EN 10028 [39] | 98.659 | 0.072 | 0.098 | 0.51 | 0.007 | 0.004 | 0.12 | 0.03 | 0.09 | - | 0.14 | 0.08 | 0.01 | 0.12 | 0.06 | - |
| C (AISI 4140 ASTM A29 [42]/42Cr4Mo2 EN 10083 [43]; rolled semi-finished product, thickness s = 4 mm | 98.291 | 0.289 | 0.252 | 0.468 | 0.011 | 0.003 | 0.340 | 0.020 | 0.030 | 0.035 | 0.007 | 0.026 | 0.040 | 0.110 | 0.058 | 0.020 |
| D AISI 4140 ASTM A29 [42]/42Cr4Mo2 EN 10083 [43]; rolled semi-finished product, thickness s = 3 mm) | 97.775 | 0.174 | 0.034 | 1.390 | 0.010 | 0.003 | 0.07 | 0.0017 | 0.06 | 0.021 | - | 0.23 | 0.0004 | 0.14 | 0.09 | - |
| E (S275 EN 10025 [44]; laminated semi-finished product, thickness s = 2 mm) | 98.721 | 0.075 | 0.004 | 0.268 | 0.021 | 0.011 | 0.170 | 0.040 | 0.130 | - | 0.100 | - | - | 0.380 | 0.050 | 0.030 |
| F (S185 EN 10025 [44]; laminated semi-finished product, thickness s = 2 mm) | 98.403 | 0.051 | 0.005 | 0.300 | 0.018 | 0.013 | 0.200 | 0.060 | 0.270 | - | 0.340 | - | - | 0.220 | 0.110 | 0.010 |
| G G (S355 JR SR EN 10025 [44] laminated semi-finished product, thickness s = 4 mm | 98.390 | 0.090 | 0.040 | 0.760 | 0.014 | 0.011 | 0.130 | 0.040 | 0.140 | 0.015 | 0.100 | 0.100 | 0.010 | 0.050 | 0.100 | 0.010 |
| S -flight material (S235 EN 10025 [44]; Rolled semi-finished products, thickness s = 4 mm) | 98.380 | 0.1710 | 0.0320 | 1.370 | 0.0106 | 0.0032 | 0.0279 | 0.0017 | 0.0.0205 | - | - | - | 0.0005 | - | 0.0029 | - |
| Steel Sample | A | B | B′ | C | D | E | F | G | S |
|---|---|---|---|---|---|---|---|---|---|
| Environment | Cement paste | Cement paste/water | Cement paste/water | Cement paste | Water | Water | Water/ Cement paste | water | water |
| Sample | Parameter | ||||
|---|---|---|---|---|---|
| icor, μA/cm2 | Ecor, mV | βA, mV | βC, mV | CR, μm/year | |
| F1 | 0.685 | −383.1 | 55.7 | −56.8 | 7.96 |
| F2 | 0.603 | −249.6 | 57.2 | −63.4 | 7.00 |
| F3 | 2.198 | −439 | 62.1 | −60 | 25.55 |
| A | 1.3804 | −449.3 | 57.3 | −65.3 | 16.04 |
| B | 0.7639 | −468 | 85 | −38 | 8.87 |
| B′ (cement paste) | 0.5525 | −565.7 | 18.3 | −41.9 | 6.42 |
| C | 0.3442 | −325.7 | 43.3 | −37.9 | 3.99 |
| D (cement paste) | 0.6121 | −504.2 | 25.3 | −86 | 7.11 |
| F (cement paste) | 0.7167 | −500 | 58.3 | −55 | 8.32 |
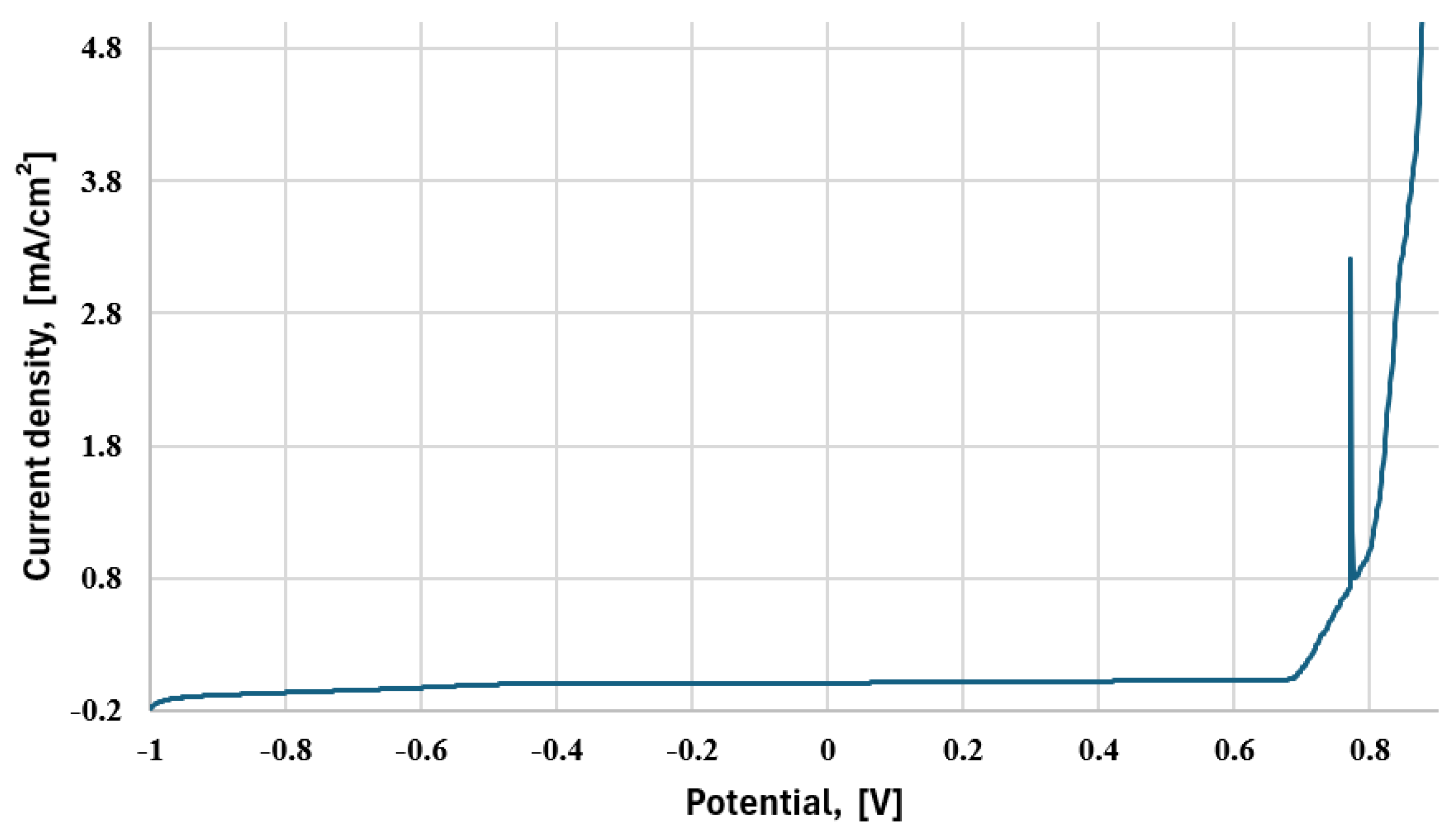
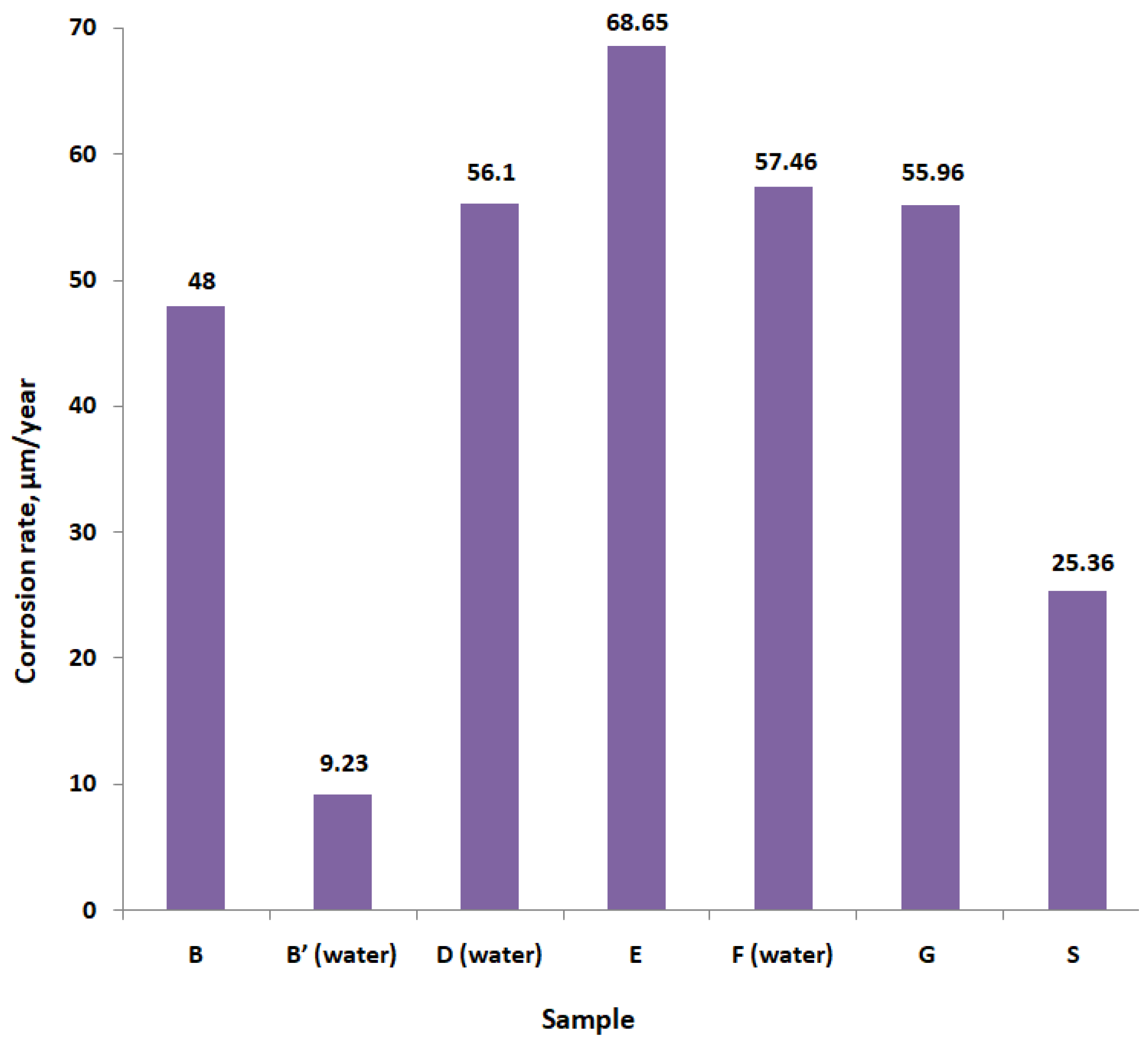
| Sample | Parameter | ||||
|---|---|---|---|---|---|
| icor, μA/cm2 | Ecor, mV | βA, mV | βC, mV | CR, μm/Year | |
| B | 4.1307 | −111.1 | 48.4 | −96.8 | 48.00 |
| B′ (water) | 0.7942 | −378.3 | 58.7 | −53 | 9.23 |
| D (water) | 4.8271 | −521.1 | 45.4 | −59 | 56.1 |
| E | 5.9076 | −527.5 | 36.6 | −58.3 | 68.65 |
| F (water) | 4.9443 | −567.3 | 40.4 | −55.7 | 57.46 |
| G | 4.8157 | −576.4 | 43.5 | −64.4 | 55.96 |
| S | 2.1827 | −205.9 | 45.5 | −58.9 | 25.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrescu, M.G.; Ripeanu, R.G.; Laudacescu, E.; Tanase, M.; Niță, A.; Burlacu, A. Characterization of Materials Used in the Concrete Industry, from the Point of View of Corrosion Behavior. Coatings 2024, 14, 800. https://doi.org/10.3390/coatings14070800
Petrescu MG, Ripeanu RG, Laudacescu E, Tanase M, Niță A, Burlacu A. Characterization of Materials Used in the Concrete Industry, from the Point of View of Corrosion Behavior. Coatings. 2024; 14(7):800. https://doi.org/10.3390/coatings14070800
Chicago/Turabian StylePetrescu, Marius Gabriel, Razvan George Ripeanu, Eugen Laudacescu, Maria Tanase, Adrian Niță, and Andrei Burlacu. 2024. "Characterization of Materials Used in the Concrete Industry, from the Point of View of Corrosion Behavior" Coatings 14, no. 7: 800. https://doi.org/10.3390/coatings14070800
APA StylePetrescu, M. G., Ripeanu, R. G., Laudacescu, E., Tanase, M., Niță, A., & Burlacu, A. (2024). Characterization of Materials Used in the Concrete Industry, from the Point of View of Corrosion Behavior. Coatings, 14(7), 800. https://doi.org/10.3390/coatings14070800








