Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
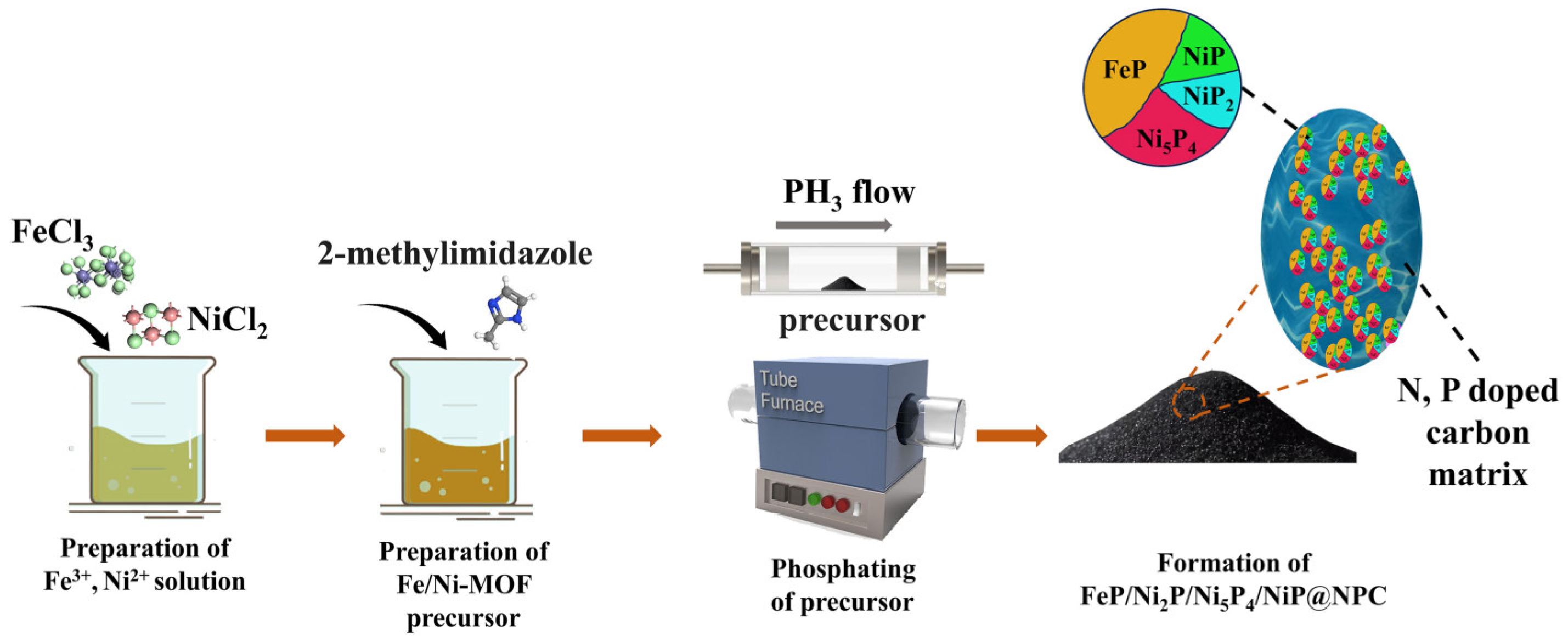
2.1. Sample Preparation
2.1.1. N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles
2.1.2. N, P-Doped Carbon-Shell-Encapsulated FeP Nanoparticles
2.1.3. N-Doped Carbon-Shell-Encapsulated Fe2O3/NiO Nanoparticles
2.2. Material Characterization
2.3. Electrochemical Measurements
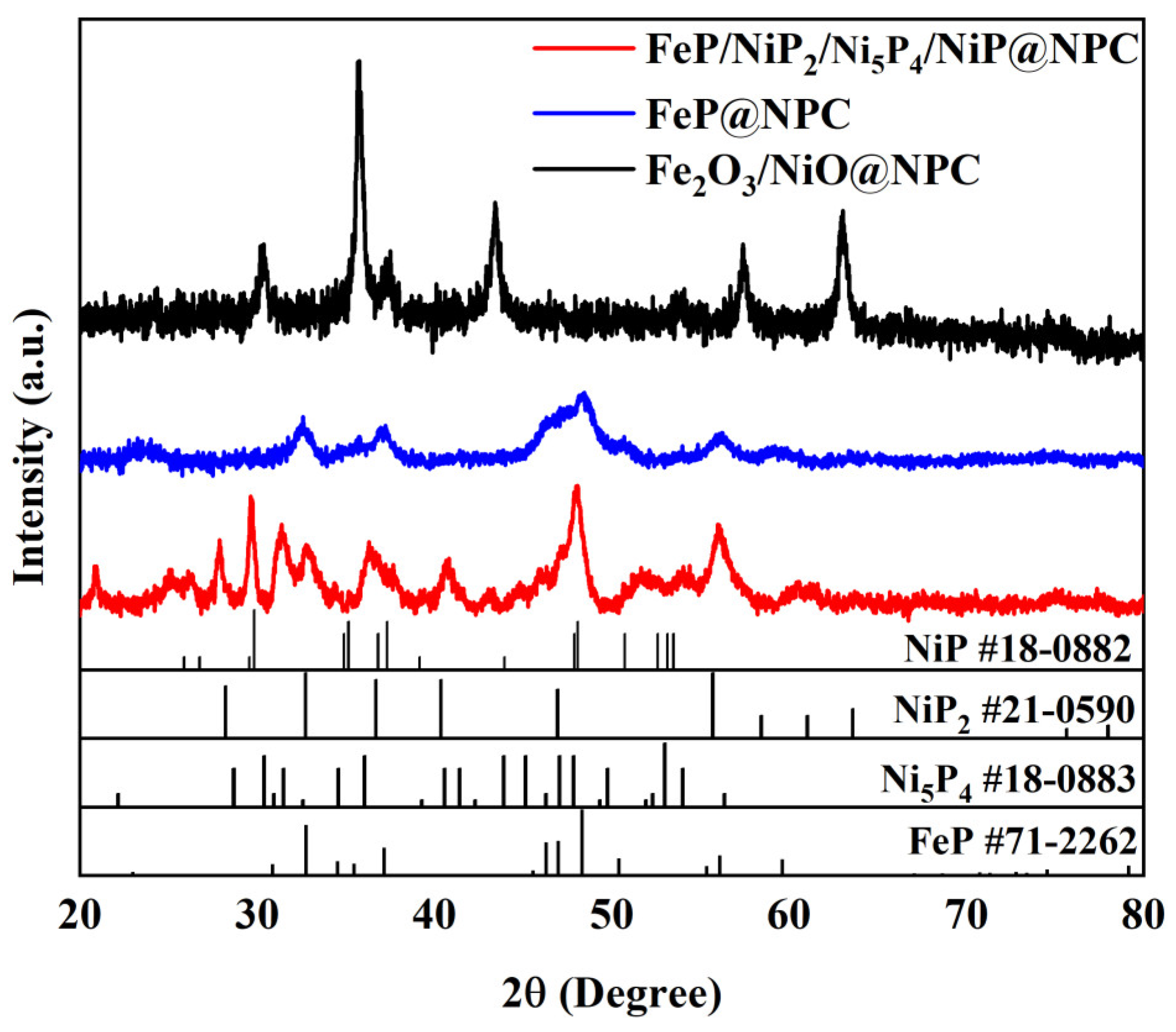
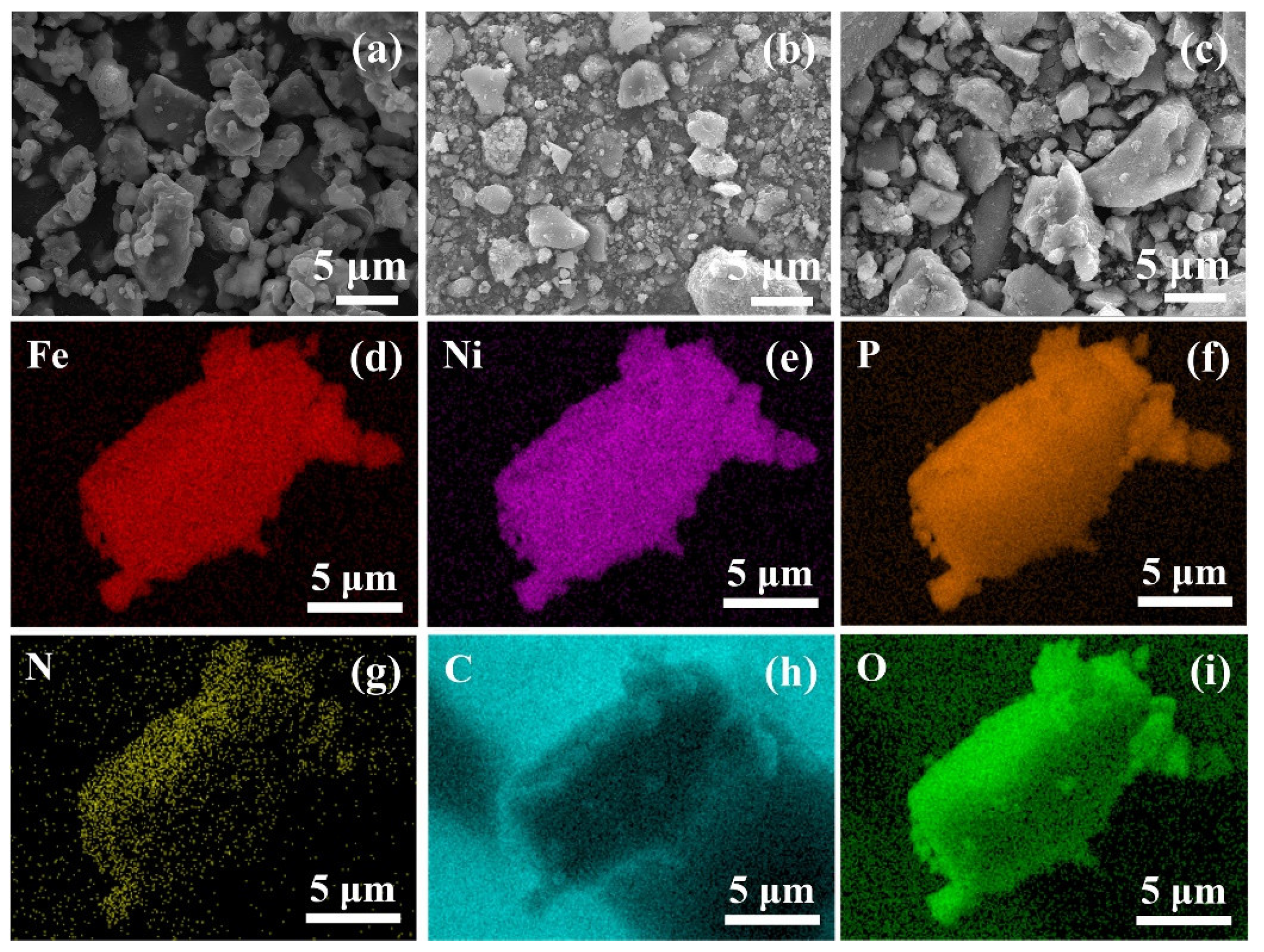
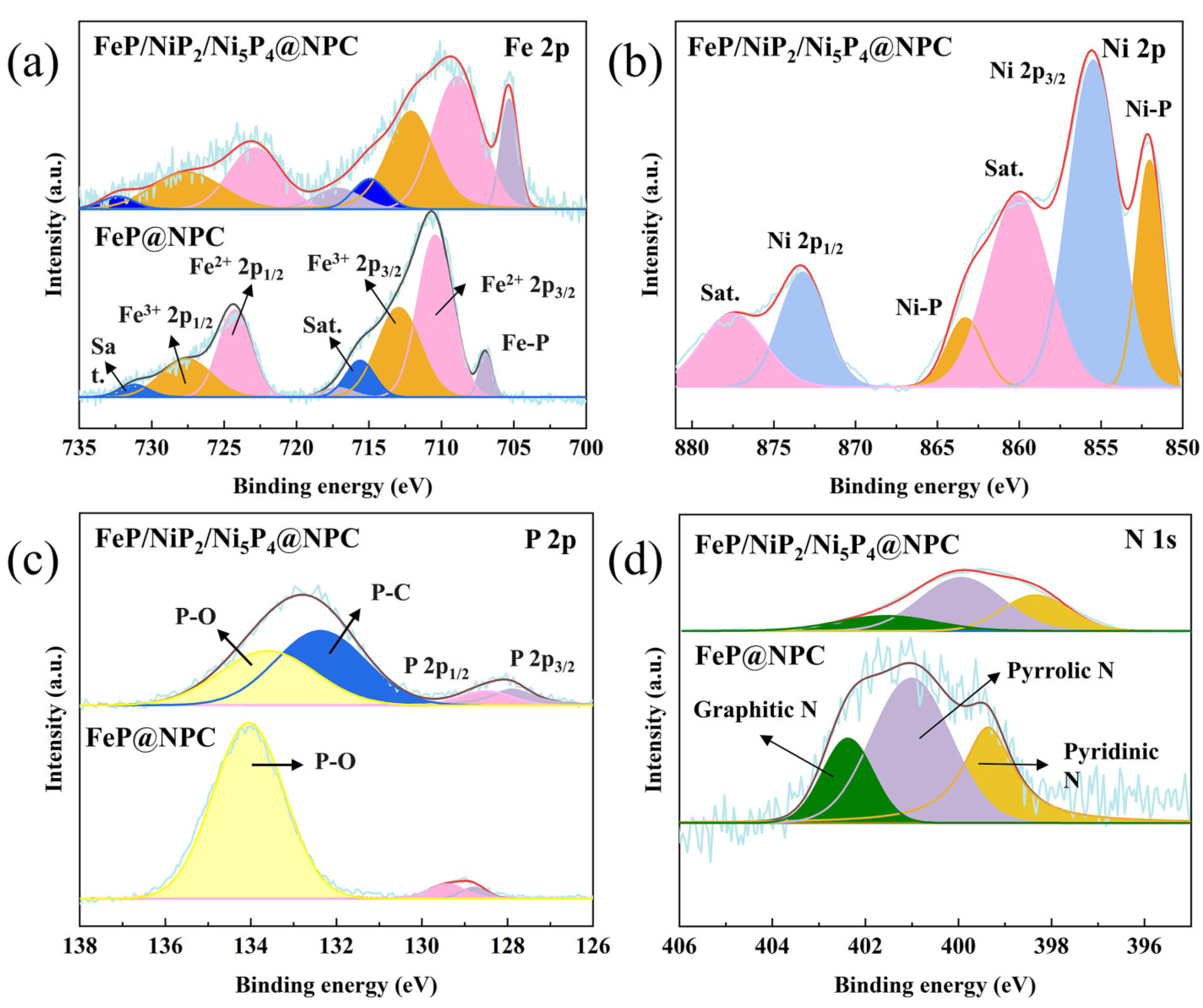
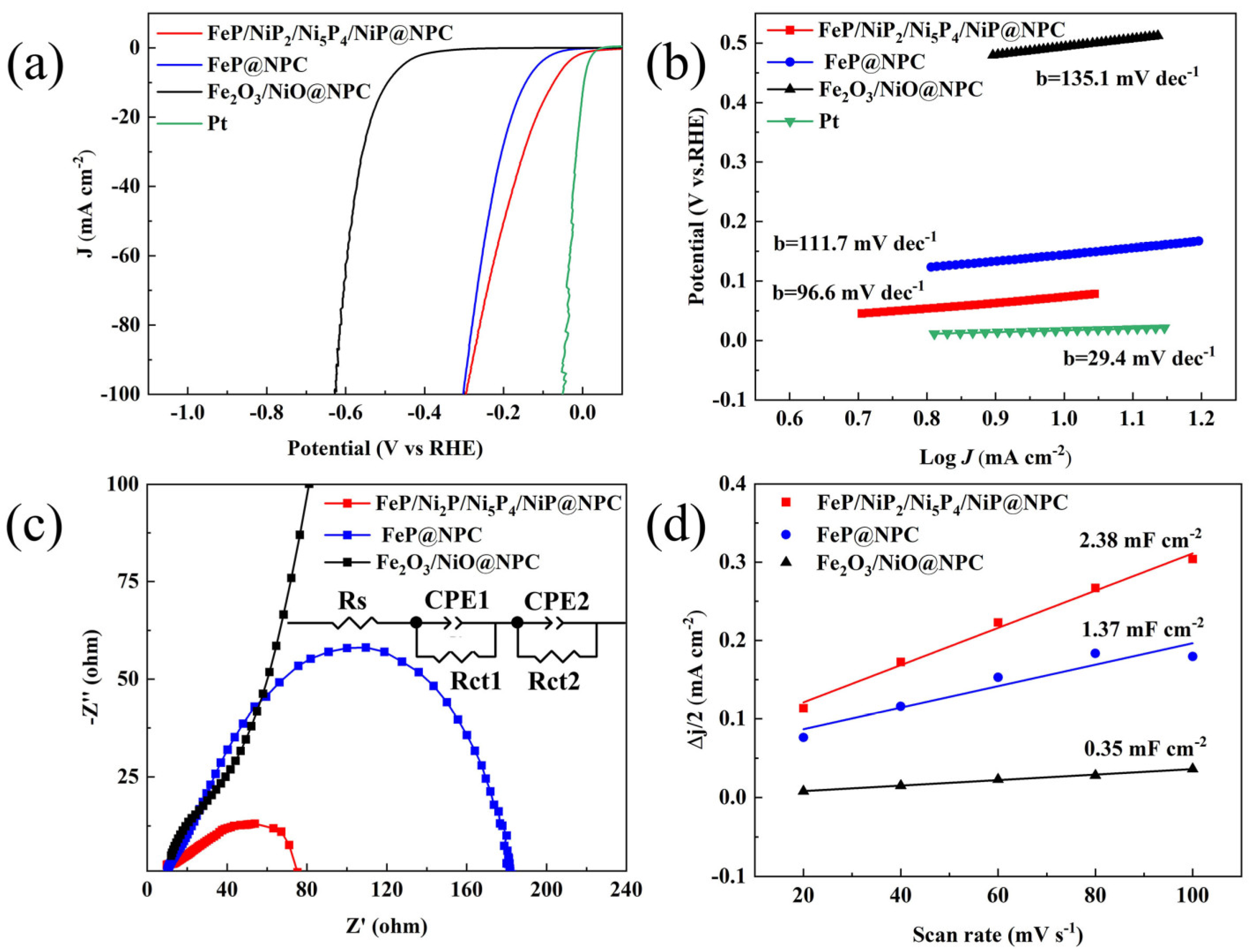
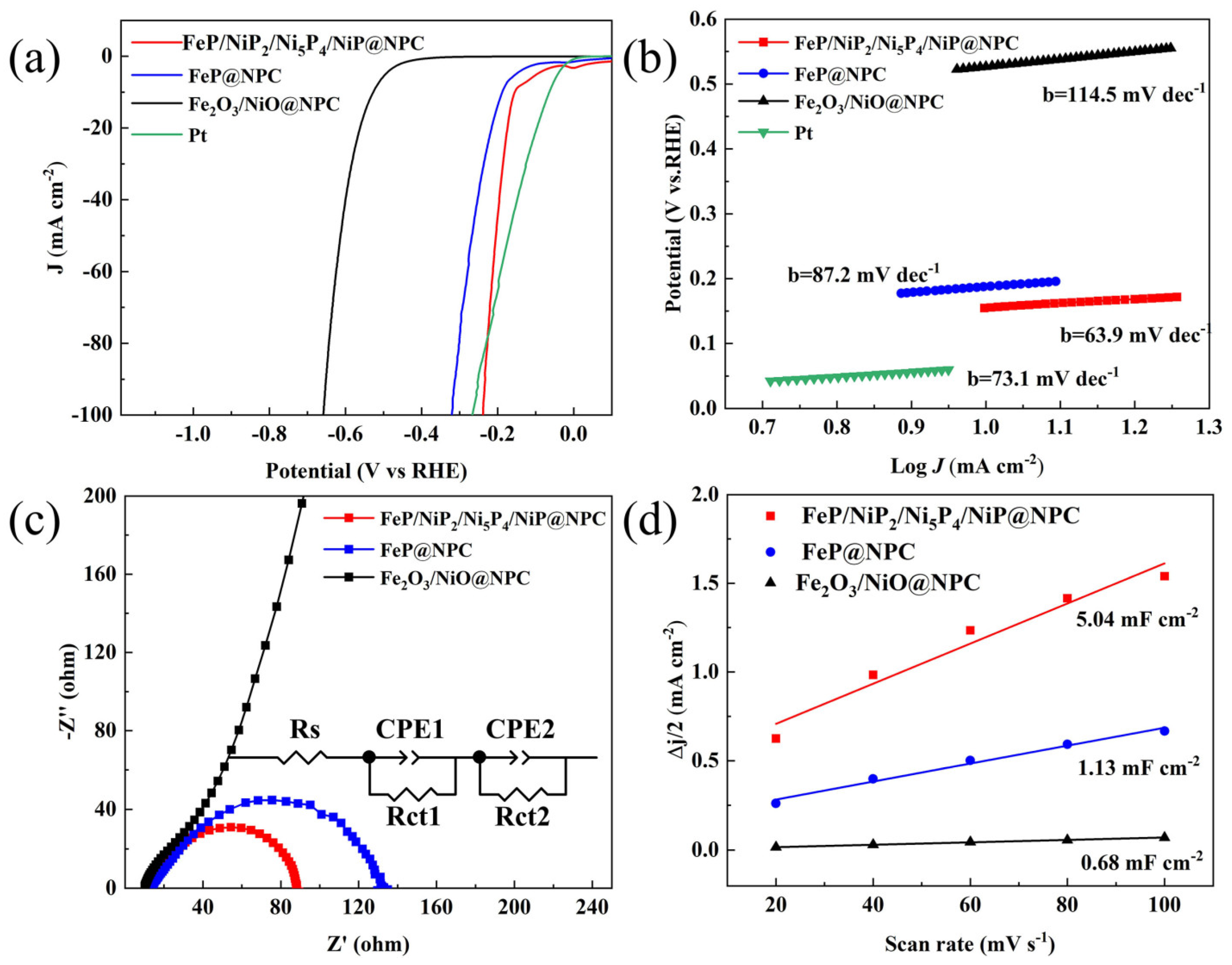
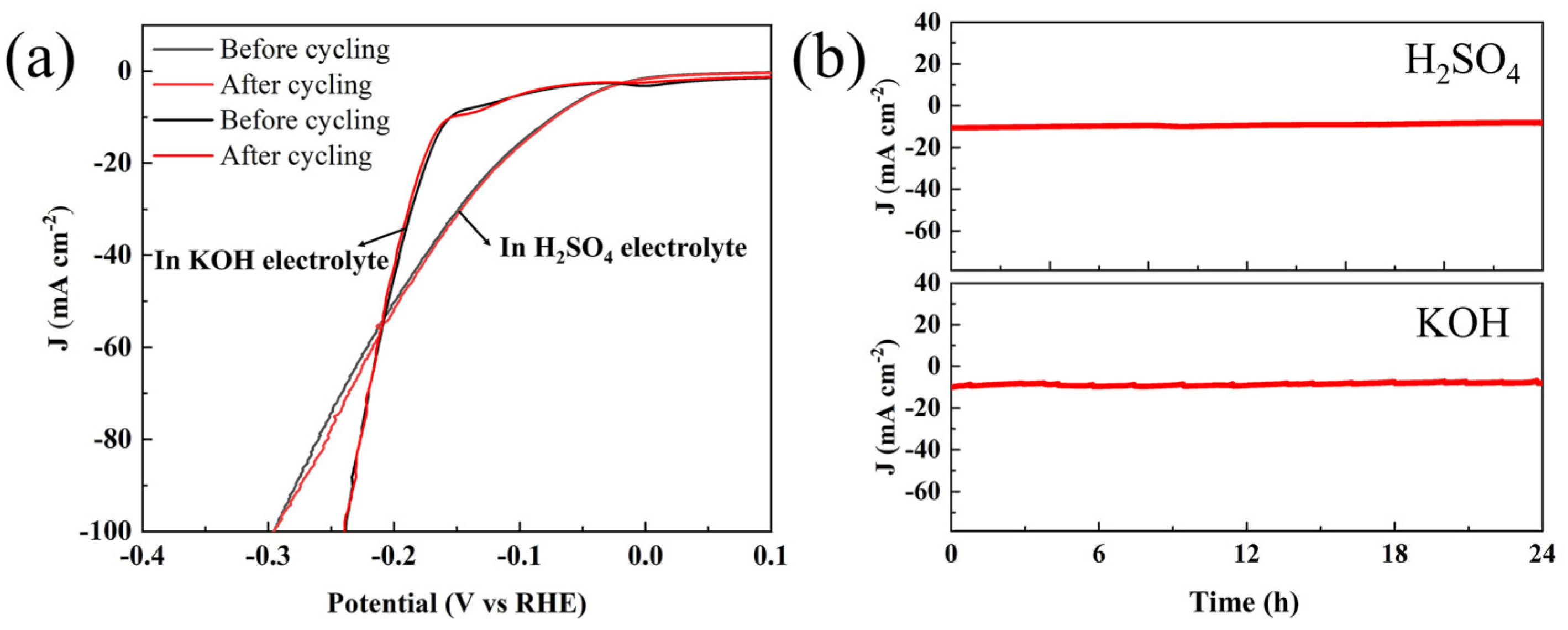
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-nitrogen-doped carbon materials as highly efficient catalysts: Progress and rational design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Logeshwaran, N.; Vijayapradeep, S.; Kim, A.R.; Sampath, P.; Ramakrishnan, S.; Poudel, M.B.; Kim, D.H.; Yoo, D.J. Study of engineering electronic structure modulated non-noble metal oxides for scaled-up alkaline blend seawater splitting. J. Energy Chem. 2023, 86, 167–179. [Google Scholar] [CrossRef]
- Vijayapradeep, S.; Kumar, R.S.; Karthikeyan, S.; Ramakrishnan, S.; Yoo, D.J. Constructing micro-nano rod-shaped iron-molybdenum oxide heterojunctions to enhance overall water electrolysis. Mater. Today Chem. 2024, 36, 101934. [Google Scholar] [CrossRef]
- Zhang, T.; He, Z.; Yin, L.; Gao, W.; Wang, Y. CoNi alloy nanoparticles confined by N-doped carbon matrix with tailored d-Band center for electrocatalytic hydrogen evolution. Fuel 2024, 365, 131176. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, T.; Qu, G.; Huang, S.; Cao, P.; Gao, W.J. Phase control and stabilization of 1T-MoS2 via black TiO2−x nanotube arrays supporting for electrocatalytic hydrogen evolution. J. Energy Chem. 2022, 68, 71–77. [Google Scholar] [CrossRef]
- Guo, T.; Fei, H.; Liu, R.; Liu, F.; Wang, D.; Wu, Z. Constructing molybdenum vacancy defect for MoP with optimized p-band center towards high-efficiency hydrogen evolution. Appl. Catal. B Environ. 2024, 343, 123480. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Yao, H.; Wei, Y.; Hu, J. Bifunctional CoxP-FeP@C for overall water splitting realized by manipulating the electronic states of Co via phosphorization. J. Colloid Interface Sci. 2024, 653, 857–866. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Tang, X.; Zhan, K.; Zhao, B.; Xia, B.Y.; Yan, Y. Metal–organic framework-derived hierarchical ultrathin CoP nanosheets for overall water splitting. J. Mater. Chem. 2020, 8, 19254–19261. [Google Scholar] [CrossRef]
- Suliman, M.H.; Baroud, T.N.; Siddiqui, M.N.; Qamar, M.; Giannelis, E.P. Confined growth and dispersion of FeP nanoparticles in highly mesoporous carbons as efficient electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 8507–8518. [Google Scholar] [CrossRef]
- Yang, G.; Jiao, Y.; Yan, H.; Xie, Y.; Wu, A.; Dong, X.; Guo, D.; Tian, C.; Fu, H. Interfacial engineering of MoO2-FeP heterojunction for highly efficient hydrogen evolution coupled with biomass electrooxidation. Adv. Mater. 2020, 32, 2000455. [Google Scholar] [CrossRef]
- Sreekanth, T.; Kiran, G.; Kim, J.; Yoo, K. NiCo bimetallic metal-organic framework (NiCo-MOFs) with distinct morphologies for efficient HER activity. Inorg. Chem. Commun. 2024, 161, 112128. [Google Scholar] [CrossRef]
- Liu, S.-S.; Ma, L.-J.; Li, J.-S. Dual-metal-organic-framework derived CoP/MoP hybrid as an efficient electrocatalyst for acidic and alkaline hydrogen evolution reaction. J. Colloid. Interface Sci. 2023, 631, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, P.; Zhang, J.; Xia, H.; Cheng, F.; Zhou, M.; Li, J.; Qiao, Y.; Mu, S.; Xu, Q. Co2P–CoN double active centers confined in N-doped carbon nanotube: Heterostructural engineering for trifunctional catalysis toward HER, ORR, OER, and Zn–air batteries driven water splitting. Adv. Funct. Mater. 2018, 28, 1805641. [Google Scholar] [CrossRef]
- Chakrabartty, S.; Sahu, D.; Raj, C.R. General approach for the synthesis of nitrogen-doped carbon encapsulated Mo and W phosphide nanostructures for electrocatalytic hydrogen evolution. ACS Appl. Energy Mater. 2020, 3, 2811–2820. [Google Scholar] [CrossRef]
- Balasubramanian, P.; He, S.-B.; Jansirani, A.; Deng, H.-H.; Peng, H.-P.; Xia, X.-H.; Chen, W. Engineering of oxygen vacancies regulated core-shell N-doped carbon@ NiFe2O4 nanospheres: A superior bifunctional electrocatalyst for boosting the kinetics of oxygen and hydrogen evaluation reactions. J. Chem. Eng. 2021, 405, 126732. [Google Scholar] [CrossRef]
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Senthil Raja, D.; Cheng, C.-C.; Ting, Y.-C.; Lu, S.-Y. NiMo-MOF-derived carbon-armored Ni4Mo alloy of an interwoven nanosheet structure as an outstanding pH-universal catalyst for hydrogen evolution reaction at high current densities. ACS Appl. Mater. Interfaces 2023, 15, 20130–20140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jia, J.; Yang, E.; Qi, S.; Tian, H.; Chen, J.; Li, J.; Lou, Y.; Guo, Y. Work-function-induced electron rearrangement of in-plane FeP@CoP heterojunction enhances all pH range and alkaline seawater hydrogen evolution reaction. Nano Energy 2023, 114, 108601. [Google Scholar] [CrossRef]
- Wei, S.; Xing, P.; Tang, Z.; Wang, Y.; Dai, L. Spindle-shaped cobalt-doped iron phosphide anchored on three-dimensional graphene electrocatalysis for hydrogen evolution reactions in both acidic and alkaline media. J. Power Sources 2023, 555, 232414. [Google Scholar] [CrossRef]
- Hong, W.; Kitta, M.; Xu, Q. Bimetallic MOF-derived FeCo-P/C nanocomposites as efficient catalysts for oxygen evolution reaction. Small 2018, 2, 1800214. [Google Scholar] [CrossRef]
- Huang, H.; Cho, A.; Kim, S.; Jun, H.; Lee, A.; Han, J.W.; Lee, J. Structural design of amorphous CoMoPx with abundant active sites and synergistic catalysis effect for effective water splitting. Adv. Funct. Mater. 2020, 30, 2003889. [Google Scholar] [CrossRef]
- Amulya, M.S.; Nagaswarupa, H.; Kumar, M.A.; Ravikumar, C.; Prashantha, S.; Kusuma, K. Sonochemical synthesis of NiFe2O4 nanoparticles: Characterization and their photocatalytic and electrochemical applications. Appl. Surf. Sci. 2020, 1, 100023. [Google Scholar] [CrossRef]
- Cho, G.; Park, Y.; Kang, H.; Hong, Y.-k.; Lee, T.; Ha, D.-H. Transition metal-doped FeP nanoparticles for hydrogen evolution reaction catalysis. Appl. Surf. Sci. 2020, 510, 145427. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Sun, X. NiP2 nanosheet arrays supported on carbon cloth: An efficient 3D hydrogen evolution cathode in both acidic and alkaline solutions. Nanoscale 2014, 6, 13440–13445. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Cheng, H.; Cui, Y.; Xu, Y.; Yang, T.; Zhang, D.; Xu, X. Porous flower-like Ni5P4 for non-enzymatic electrochemical detection of glucose. Mater. Chem. Phys. 2020, 240, 122202. [Google Scholar] [CrossRef]
- Zheng, Y.; Tan, Y.; Yu, X.; Yao, H.; Hu, S.; Hu, J.; Chen, Z.; Guo, X. Optimized Intermediates Adsorption Configuration on Co-Doped Fe2P@NiP2 Heterojunction Interface for Enhanced Electrocatalytic Nitrate-To-Ammonia Conversion. Small 2024, 2312136. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, E.; Ramakrishnan, S.; Sathiskumar, C.; Yoo, D.J.; Balamurugan, J.; Noh, H.S.; Kwon, D.; Kim, Y.H.; Lee, H. MOF-derived CoP-nitrogen-doped carbon@ NiFeP nanoflakes as an efficient and durable electrocatalyst with multiple catalytically active sites for OER, HER, ORR and rechargeable zinc-air batteries. J. Chem. Eng. 2022, 428, 131115. [Google Scholar] [CrossRef]
- Shen, S.; Wang, Z.; Lin, Z.; Song, K.; Zhang, Q.; Meng, F.; Gu, L.; Zhong, W. Crystalline-amorphous interfaces coupling of CoSe2/CoP with optimized d-band center and boosted electrocatalytic hydrogen evolution. Adv. Mater. 2022, 34, 2110631. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, J.; Ding, Y.; Gao, W.; Wang, Y. Mo-doped FeP nanoparticles encapsulated in N, P doped carbon matrix with optimized d-band center for highly efficient and stable hydrogen evolution reaction. New J. Chem. 2024, 48, 11302–11309. [Google Scholar] [CrossRef]
- Lv, Q.; Li, M.; Li, X.; Yan, X.; Hou, Z.; Huang, C. Introducing hydroxyl groups to tailor the d-band center of Ir atom through side anchoring for boosted ORR and HER. J. Energy Chem. 2024, 90, 144–151. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, K.; Wang, X.; Zheng, H.; Cao, J.; Mi, L.; Huo, Q.; Yang, H.; Liu, J.; He, C. Subnanometric Ru clusters with upshifted D band center improve performance for alkaline hydrogen evolution reaction. Nat. Commun. 2022, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Huang, C.; Yang, Y.; Song, H.; Zhang, X.; Zheng, Y.; Gao, B.; Fu, J.; Chu, P.K.; Huo, K. In situ formation of N-doped carbon-coated porous MoP nanowires: A highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range. Appl. Catal. B Environ. 2020, 263, 118358. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, F.; Li, X.; Chen, X.; Xiong, S.; Xi, B. Ultrafine PtMo Nanocrystals Confined on N-Doped Carbon Toward Efficient pH-Universal Hydrogen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2306340. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Zhang, W.; Gao, B.; Yan, T.; Zhang, Y.; Cao, X.; Gao, Q.; Tang, Y. Heterostructured MoP/CoMoP2 embedded in an N, P-doped carbon matrix as a highly efficient cooperative catalyst for pH-universal overall water splitting. J. Mater. Chem. 2024, 12, 1243–1252. [Google Scholar] [CrossRef]
- Gao, P.; Gao, M.; Lei, T.; Ren, Z.; Luo, J.; Huang, Z.; Wu, A. Hollow urchin-like FeP as highly efficient hydrogen evolution catalyst. Inorg. Chem. Commun. 2023, 156, 111143. [Google Scholar] [CrossRef]
- Chen, K.; Kim, G.-C.; Yadav, S.; Lee, I.-H. Engineering core-shell hollow-sphere Fe3O4@FeP@nitrogen-doped-carbon as an advanced bi-functional electrocatalyst for highly-efficient water splitting. J. Colloid Interface Sci. 2024, 657, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; An, X.; Wang, D.; Guo, X.; Sun, D.; Wang, Q.; Yang, Z. Facile synthesis of three-dimensional wrinkled cobalt-doped MoP/FeP4 nanospheres for boosted hydrogen evolution reaction. New J. Chem. 2023, 47, 19832–19837. [Google Scholar] [CrossRef]
- Yang, M.; Xie, J.-Y.; Lin, Z.-Y.; Dong, B.; Chen, Y.; Ma, X.; Wen, M.-L.; Zhou, Y.-N.; Wang, L.; Chai, Y.-M. N-doped FeP nanorods derived from Fe-MOFs as bifunctional electrocatalysts for overall water splitting. Appl. Surf. Sci. 2019, 507, 145096. [Google Scholar] [CrossRef]
- Yao, X.; Wang, H.; Yang, K.; Shu, Y.; He, J. Facile synthesis of Fe-MoS2/NRGO composite material as effective electrocatalyst for high-efficiency hydrogen evolution reaction. Appl. Surf. Sci. 2022, 587, 152842. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhong, J.; Gao, W.; Wang, Y. Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction. Coatings 2024, 14, 817. https://doi.org/10.3390/coatings14070817
Zhang T, Zhong J, Gao W, Wang Y. Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction. Coatings. 2024; 14(7):817. https://doi.org/10.3390/coatings14070817
Chicago/Turabian StyleZhang, Ting, Jianguo Zhong, Wei Gao, and Yuxin Wang. 2024. "Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction" Coatings 14, no. 7: 817. https://doi.org/10.3390/coatings14070817
APA StyleZhang, T., Zhong, J., Gao, W., & Wang, Y. (2024). Interface Engineering Induced N, P-Doped Carbon-Shell-Encapsulated FeP/NiP2/Ni5P4/NiP Nanoparticles for Highly Efficient Hydrogen Evolution Reaction. Coatings, 14(7), 817. https://doi.org/10.3390/coatings14070817






