Effect of Temperature on the Structure and Tribological Properties of Ti, TiN and Ti/TiN Coatings Deposited by Cathodic Arc PVD
Abstract
:1. Introduction
2. Materials and Methods
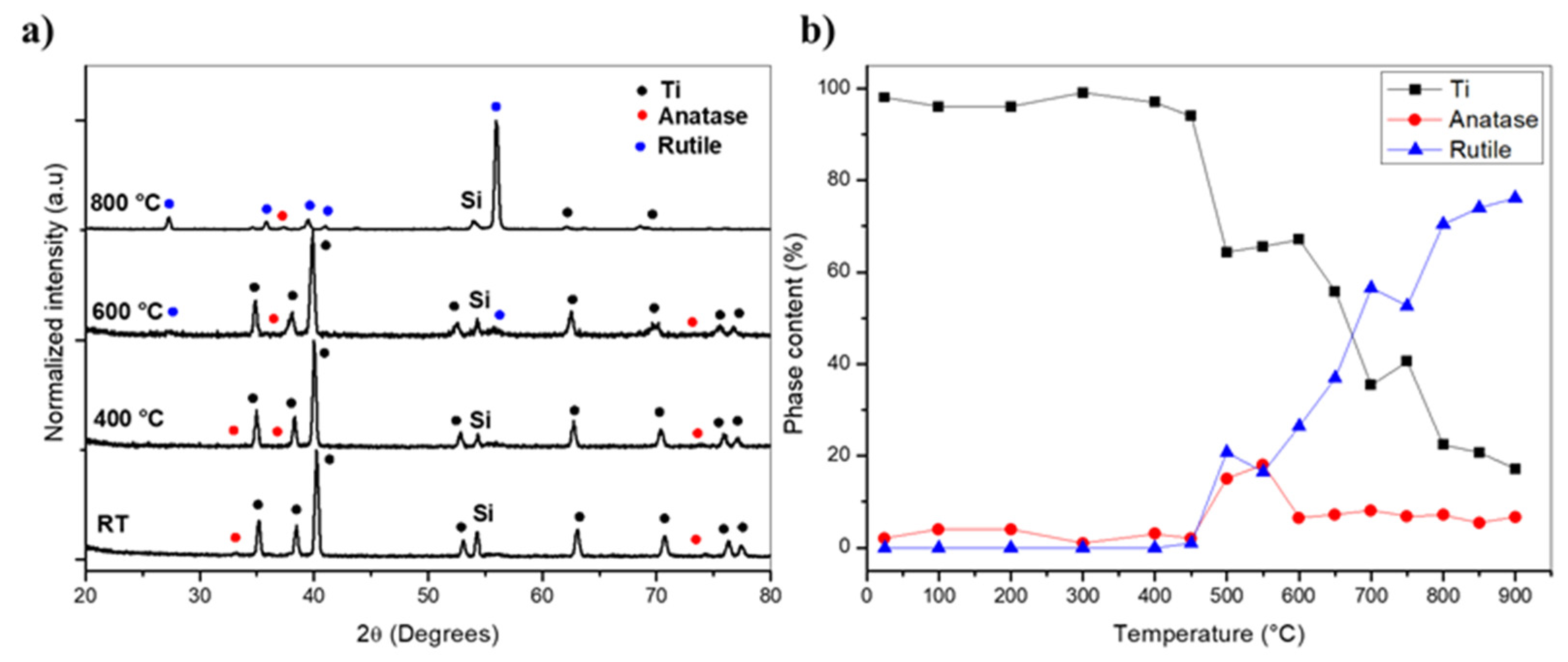
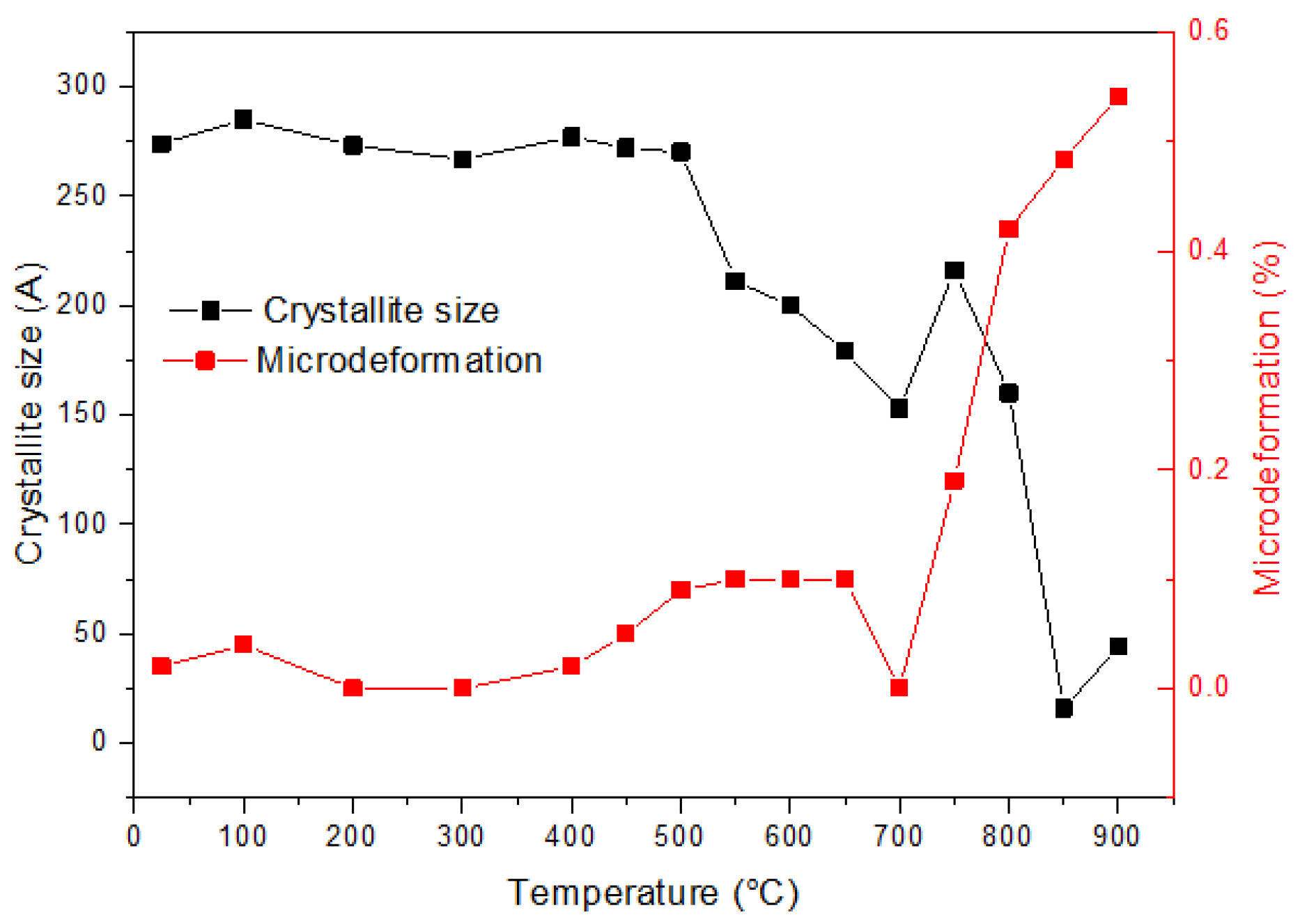
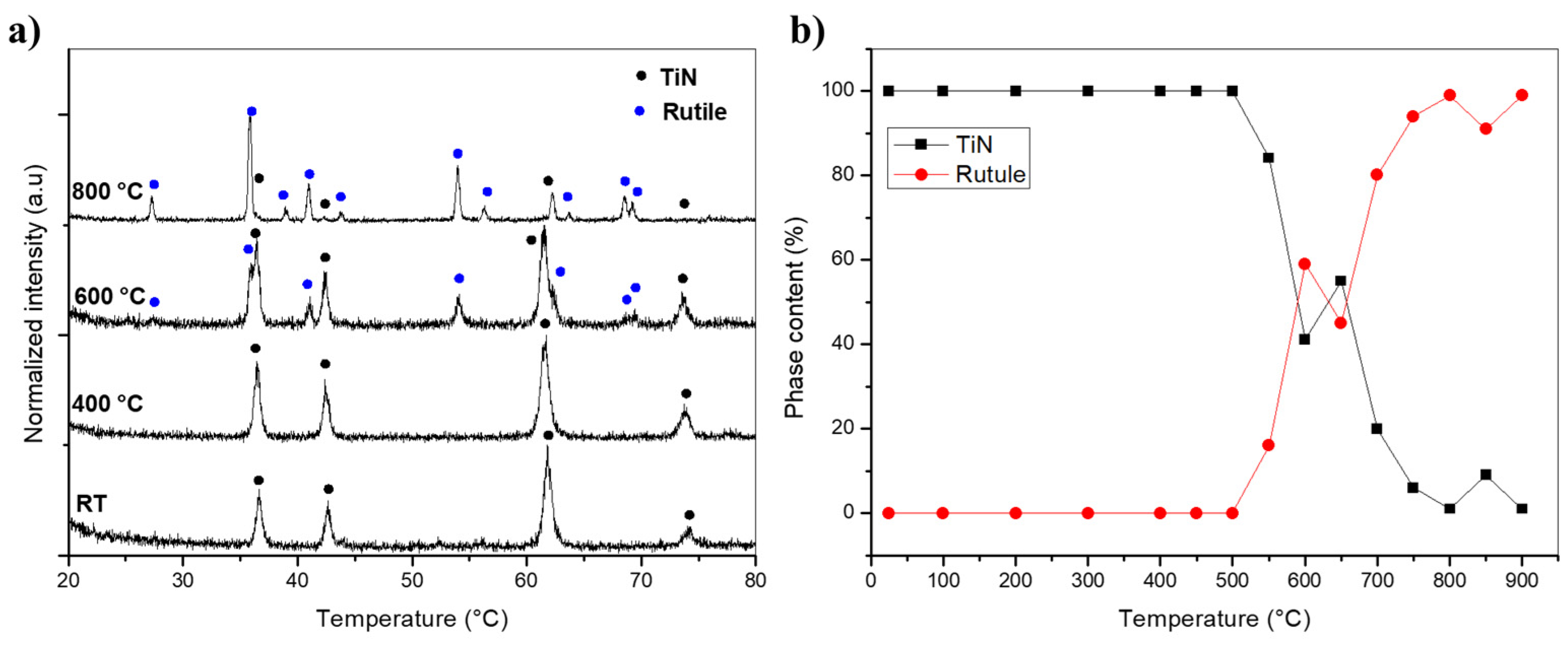
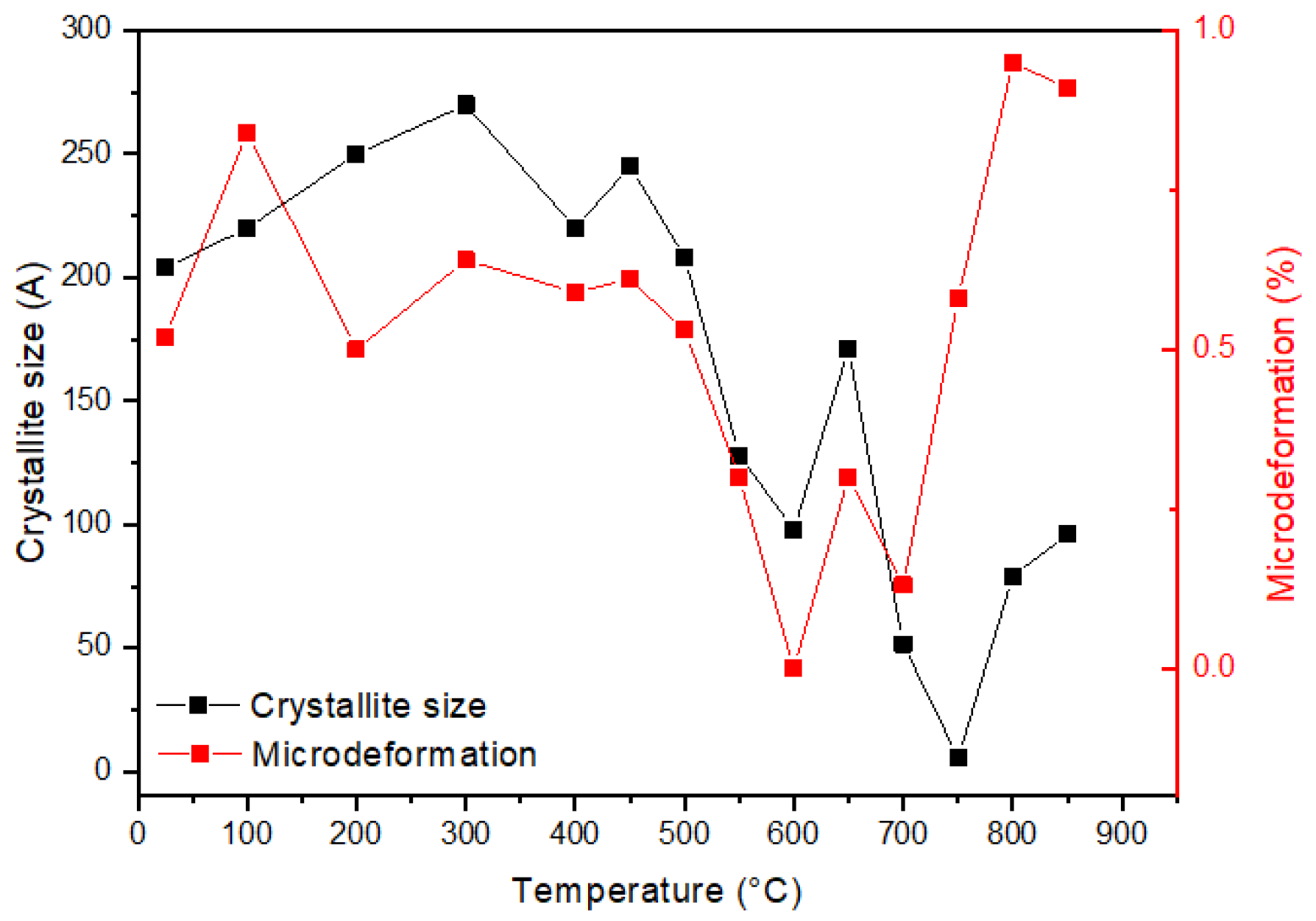
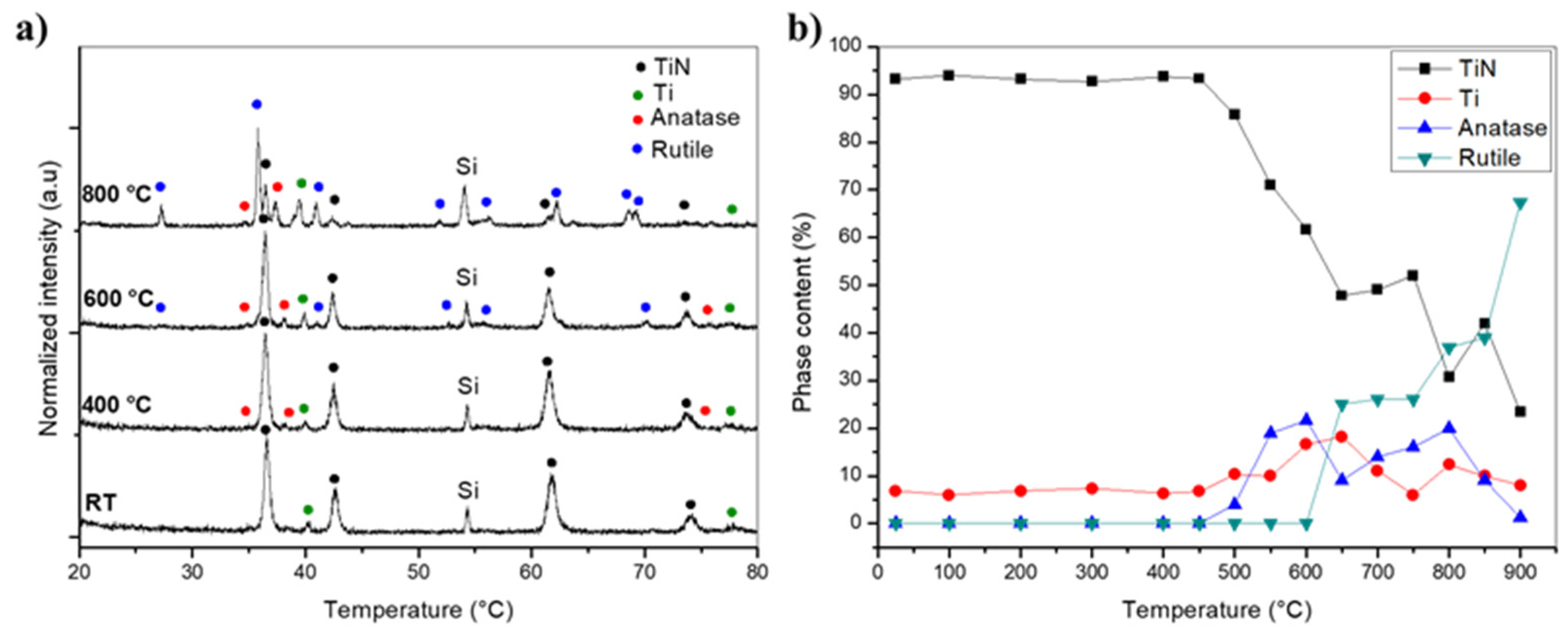
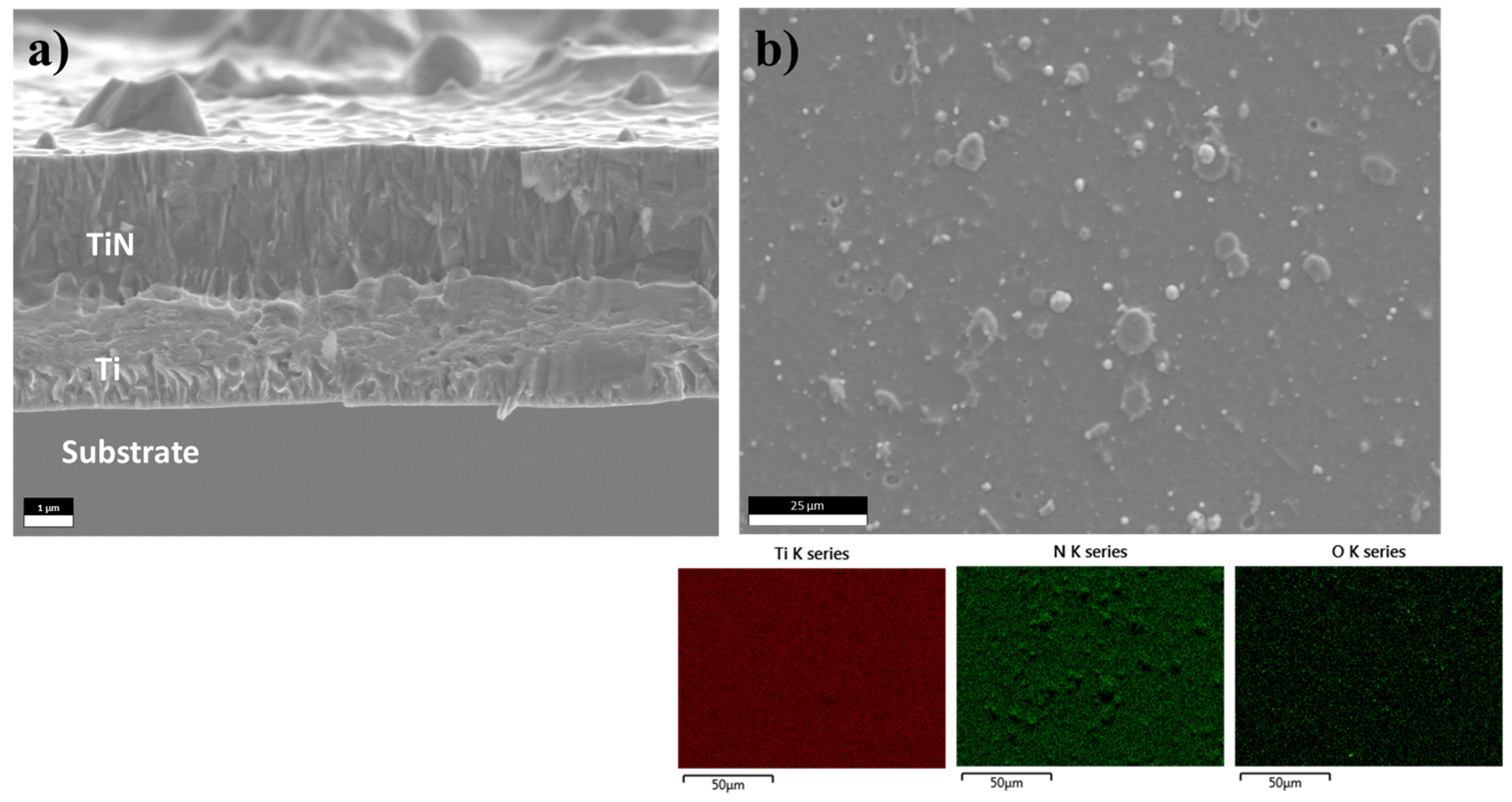
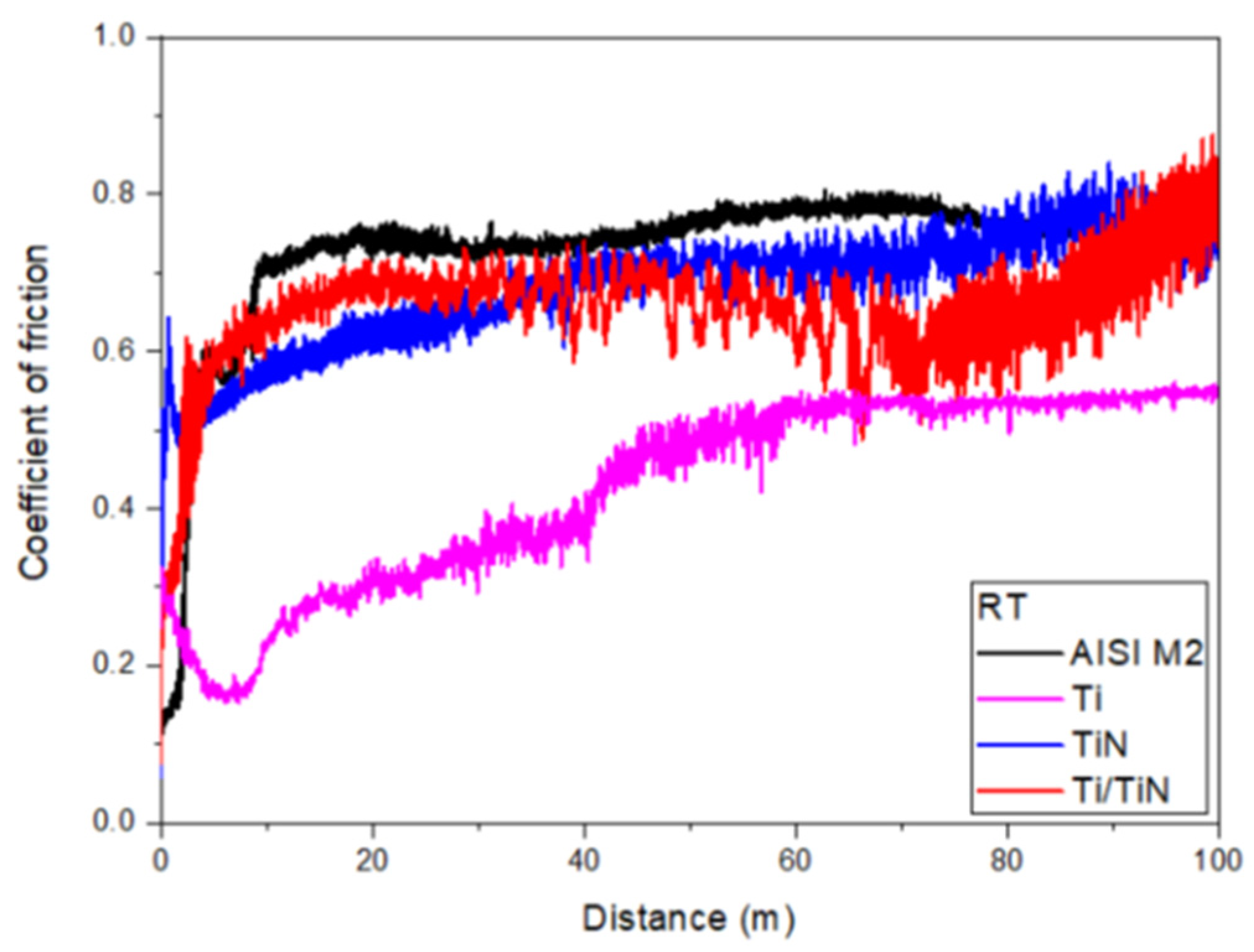
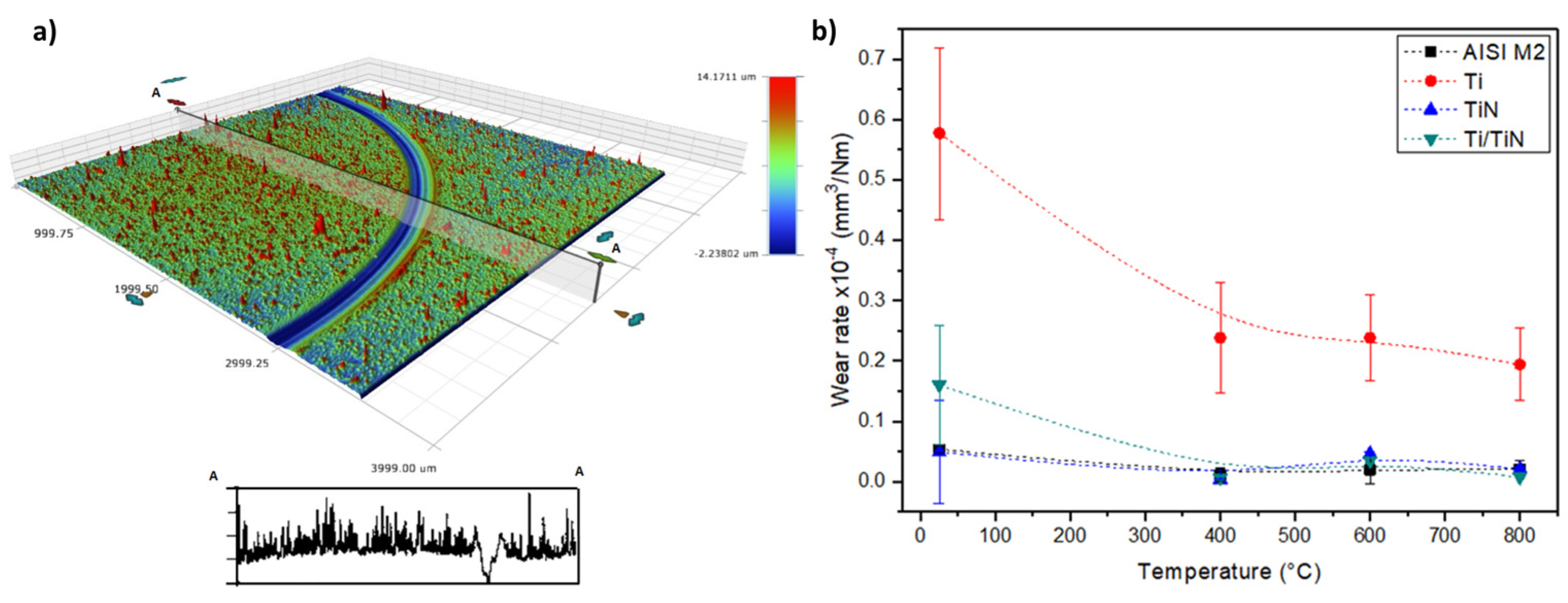
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, Y.; Niu, J.; Liu, S.; Lin, Y.; Liu, N.; Ma, J.; Zhang, Z.; Wang, J. Microstructure and mechanical properties of M2 high speed steel produced by electron beam melting. Mater. Sci. Eng. A 2023, 862, 144327. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, H.; Alfano, M. Multi-response optimization of M2 steel coatings deposited by co-axial laser cladding on A2 steel tool surfaces. J. Mater. Res. Technol. 2024, 29, 1102–1117. [Google Scholar] [CrossRef]
- Dang, M.N.; Singh, S.; King, H.J.; Navarro-Devia, J.H.; Le, H.; Pattison, T.G.; Hocking, R.K.; Wade, S.A.; Stephens, G.; Papageorgiou, A.; et al. Surface Enhancement of Titanium-Based Coatings on Commercial Hard Steel Cutting Tools. Crystals 2024, 14, 470. [Google Scholar] [CrossRef]
- Masuda, K.; Ishihara, S.; Shibata, H.; Sakamoto, Y.; Oguma, N.; Iwasaki, M. Effect of surface coating on fatigue life and fatigue crack growth behavior of AISI D2 tool steel. Int. J. Fatigue 2024, 183, 108230. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, Z.B.; Hu, S.Y.; Zhao, Y.H.; Ren, H.; Gong, F.; Xie, Z.W. Tailoring growth structure. wear and corrosion properties of TiN coatings via gradient bias and arc enhanced glow discharge. Surf. Coat. Technol. 2022, 450, 129015. [Google Scholar] [CrossRef]
- Siriprom, W.; Chananonnawathorn, C.; Kongsriprapan, S.; Teanchai, K.; Horprathum, M. Preparation and characterization of CrN thin film by DC reactive magnetron Sputtering. Mater. Today Proc. 2018, 5, 15224–15227. [Google Scholar] [CrossRef]
- Zambrano, D.; Hernández-Bravo, R.; Ruden, A.; Espinosa-Arbelaez, D.; González-Carmona, J.; Mujica, V. Mechanical, tribological and electrochemical behavior of Zr-based ceramic thin films for dental implants. Ceram. Int. 2023, 49, 2102–2114. [Google Scholar] [CrossRef]
- Gonzalez-Carmona, J.M.; Triviño, J.D.; Gómez-Ovalle, Á.; Ortega, C.; Alvarado-Orozco, J.M.; Sánchez-Sthepa, H.; Avila, A. Wear mechanisms identification using Kelvin probe force microscopy in TiN, ZrN and TiN/ZrN hard ceramic multilayers coatings. Ceram. Int. 2020, 46, 24592–24604. [Google Scholar] [CrossRef]
- Locks, E.; He, Q.; DePaiva, J.M.; Guimaraes, M.; Arif, A.F.; Veldhuis, S.C.; Kish, J.R. Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel. Coatings 2024, 14, 290. [Google Scholar] [CrossRef]
- Aditharajan, A.; Radhika, N.; Saleh, B. Recent advances and challenges associated with thin film coatings of cutting tools: A critical review. Trans. IMF 2023, 101, 205–221. [Google Scholar] [CrossRef]
- Ariharan, N.; Sriram, C.G.; Radhika, N.; Aswin, S.; Haridas, S. A comprehensive review of vapour deposited coatings for cutting tools: Properties and recent advances. Trans. IMF 2022, 100, 262–275. [Google Scholar] [CrossRef]
- Martan, J.; Beneš, P. Thermal properties of cutting tool coatings at high temperatures. Thermochim. Acta 2012, 539, 51–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Li, C.; Piao, Y.; Hou, N.; Hu, K. Characterization of surface and subsurface defects induced by abrasive machining of optical crystals using grazing incidence X-ray diffraction and molecular dynamics. J. Adv. Res. 2022, 36, 51–61. [Google Scholar] [CrossRef]
- Gómez-Ortega, A.; Pinilla-Bedoya, J.A.; Ortega-Portilla, C.; Félix-Martínez, C.; Mondragón-Rodríguez, G.C.; Espinosa-Arbeláez, D.G.; Pérez-Barrera, J.; González-Carmona, J.M.; Franco Urquiza, E.A. The Scratch Resistance of a Plasma-Assisted DUPLEX-Treated 17-4 Precipitation-Hardened Stainless Steel Additively Manufactured by Laser Powder Bed Fusion. Coatings 2024, 14, 605. [Google Scholar] [CrossRef]
- ASTM A600-92a; Standard Specification for Tool Steel High Speed. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Mondragón-Rodríguez, G.; Hernández-Mendoza, J.; Gómez-Ovalle, A.; González-Carmona, J.; Ortega-Portilla, C.; Camacho, N.; Hurtado-Macías, A.; Espinosa-Arbeláez, D.; Alvarado-Orozco, J. High-temperature tribology of Hf doped c-Al0.64Ti0.36N cathodic arc PVD coatings deposited on M2 tool steel. Surf. Coat. Technol. 2021, 422, 127516. [Google Scholar] [CrossRef]
- ASTM G99-17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM: West Conshohocken, PA, USA, 2023. [CrossRef]
- Djerdj, I. Rietveld Refinement in the Characterization of Crystalline Materials; MDPI: Basel, Switzerland, 2019. [Google Scholar] [CrossRef]
- Mittireddi, R.T.; Makani, N.H.; Prajapati, D.G.; Gautam, A.R.S.; Banerjee, R.; Panda, E. Microstructure-induced functionality in titanium dioxide thin films. Mater. Charact. 2023, 199, 112818. [Google Scholar] [CrossRef]
- Inamdar, A.K.; Hulsure, N.R.; Kadam, A.S.; Rajenimbalkar, R.S.; Karpoormath, R.; Shelke, S.B.; Inamdar, S.N. Flame synthesized tetragonal TiO2 nanoparticles for Methylene Blue and Congo Red dye removal applications. Results Chem. 2023, 5, 100854. [Google Scholar] [CrossRef]
- Eroğlu, O.; Kizil, H. The effect of the iron doping on anatase TiO2 anode for electrochemical performance of sodium-ion batteries. Solid State Ionics 2023, 393, 116168. [Google Scholar] [CrossRef]
- Lévy, F.; Hones, P.; Schmid, P.; Sanjinés, R.; Diserens, M.; Wiemer, C. Electronic states and mechanical properties in transition metal nitrides. Surf. Coat. Technol. 1999, 120–121, 284–290. [Google Scholar] [CrossRef]
- Restrepo-Parra, E.; Amaya-Roncancio, S.; Bedoya-Hincapie, C.; Riaño-Rojas, J. Simulation of band structure for CrN lattices by using a 3D array of range-limited circularly symmetric attractive potential. Superlattices Microstruct. 2008, 43, 559–563. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Chen, X.; Hosseini, S.N.; van Huis, M.A. Heating-Induced Transformation of Anatase TiO2 Nanorods into Rock-Salt TiO Nanoparticles: Implications for Photocatalytic and Gas-Sensing Applications. ACS Appl. Nano Mater. 2022, 5, 1600–1606. [Google Scholar] [CrossRef]
- Dubey, R.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by sol-gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Cavalheiro, A.A.; De Oliveira, L.C.; Santos, S.A.D. Structural Aspects of Anatase to Rutile Phase Transition in Titanium Dioxide Powders Elucidated by the Rietveld Method. Alberto Adriano Cavalheiro, Lincoln Carlos Silva de Oliveira and Silvanice Aparecida Lopes dos Santos. In Titanium Dioxide; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Klimenko, I.O.; Belous, V.A.; Podhurska, V.Y.; Ostash, O.P.; Ovcharenko, V.D.; Tolmachova, G.N.; Kolodiy, I.V.; Ishchenko, M.G.; Babayev, I.M.; Kuprin, O.S. Tribological Properties at 20 and 500 °C of TiN and CrN Cathodic ARC Coatings Deposited on Ti-6Al-4V Alloy. East Eur. J. Phys. 2024, 1, 380–385. [Google Scholar] [CrossRef]
- Han, C.-S.; Park, S.-S.; Chun, M.-G. Investigation of the characteristics of TiN-coating film deposited by arc ion plating method and the feasibility of measuring residual stress according to heat treatment. J. Korean Phys. Soc. 2024, 84, 779–792. [Google Scholar] [CrossRef]
- Devia, D.M.; Restrepo, J.; Ruden, A.; González, J.M.; Sequeda, F.; Arango, P.J. The Tribological Characteristics of TiN. TiC. TiC/TiN Films Prepared by Reactive Pulsed Arc Evaporation Technique. In Proceedings of the 52nd Annual Technical Conference Proceedings, Santa Clara, CA, USA, 9–14 May 2009; pp. 32–36. [Google Scholar]
- Glaser, A.; Surnev, S.; Netzer, F.; Fateh, N.; Fontalvo, G.; Mitterer, C. Oxidation of vanadium nitride and titanium nitride coatings. Surf. Sci. 2007, 601, 1153–1159. [Google Scholar] [CrossRef]
- Hou, X.; Chou, K.; Zhang, M. The Model for Oxidation Kinetics of Titanium Nitride Coatings. Int. J. Appl. Ceram. Technol. 2010, 7, 248–255. [Google Scholar] [CrossRef]
- Joshi, A.; Hu, H. Oxidation behavior of titanium-aluminium nitrides. Surf. Coat. Technol. 1995, 76–77, 499–507. [Google Scholar] [CrossRef]
- Ren, J.; Xu, L.; Luo, J.; Li, Z.; Li, B.; Shi, X.; Xu, L.; Bang, L.T.; Fu, Q. Hydrothermal oxidation of titanium nitride coating for enhanced corrosion resistance in fluoride-containing acidic solution. Mater. Lett. 2023, 335, 133790. [Google Scholar] [CrossRef]
- Ovchinnikov, S.V.; Korotaev, A.D.; Pinzhin, Y.P. Modification of the microstructure of TiN-based columnar coatings in indentation zones. Russ. Metall. 2015, 2015, 278–284. [Google Scholar] [CrossRef]
- Budinski, K.G. Tribological properties of titanium alloys. Wear 1991, 151, 203–217. [Google Scholar] [CrossRef]
- Lee, H.-J.; Huang, X.; Mohanchandra, K.; Carman, G.; Ramirez, A. Effects of crystallization temperature on the stress of NiTi thin films. Scr. Mater. 2009, 60, 1133–1136. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nishikata, A.; Tanase, T.; Konno, M. Size Effect on Crystal Structures of Barium Titanate Nanoparticles Prepared by a Sol-Gel Method. J. Sol-Gel Sci. Technol. 2004, 29, 49–55. [Google Scholar] [CrossRef]
- Dong, H.; Bell, T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment. Wear 2000, 238, 131–137. [Google Scholar] [CrossRef]
- Saier, A.; Esen, I.; Ahlatci, H.; Keskin, E. Effect of Oxidation Process on Mechanical and Tribological Behaviour of Titanium Grade 5 Alloy. Materials 2024, 17, 776. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Lin, Y.-T.; Chan, A.; Chang, J.-T. High Temperature Wear Behavior of Titanium Nitride Coating Deposited Using High Power Impulse Magnetron Sputtering. Coatings 2019, 9, 555. [Google Scholar] [CrossRef]
- Liu, A.H.; Zhou, S.X. Tribological Properties of TiN Coating at Elevated Temperature up to 600 °C. Appl. Mech. Mater. 2014, 644–650, 4784–4787. [Google Scholar] [CrossRef]

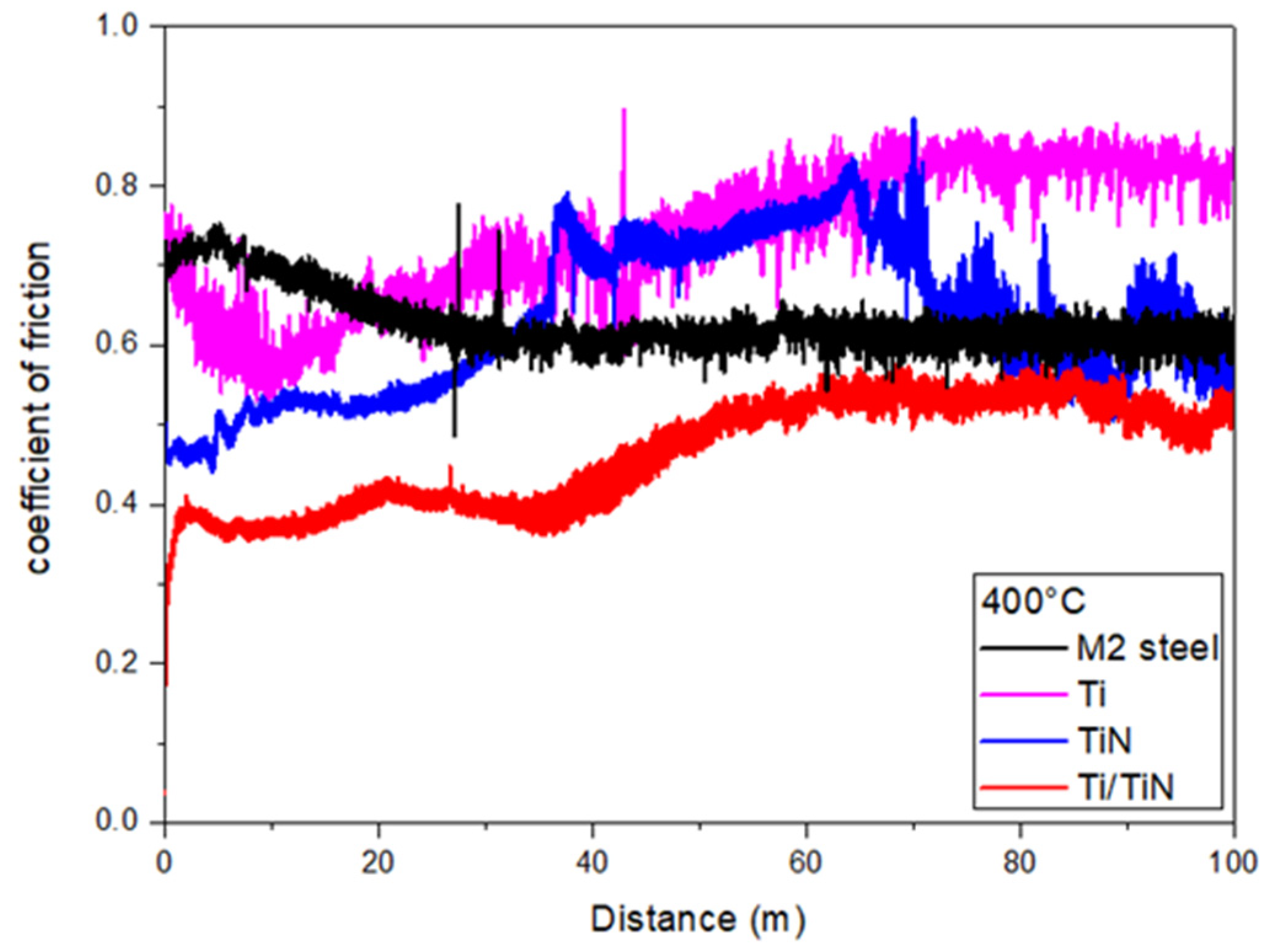
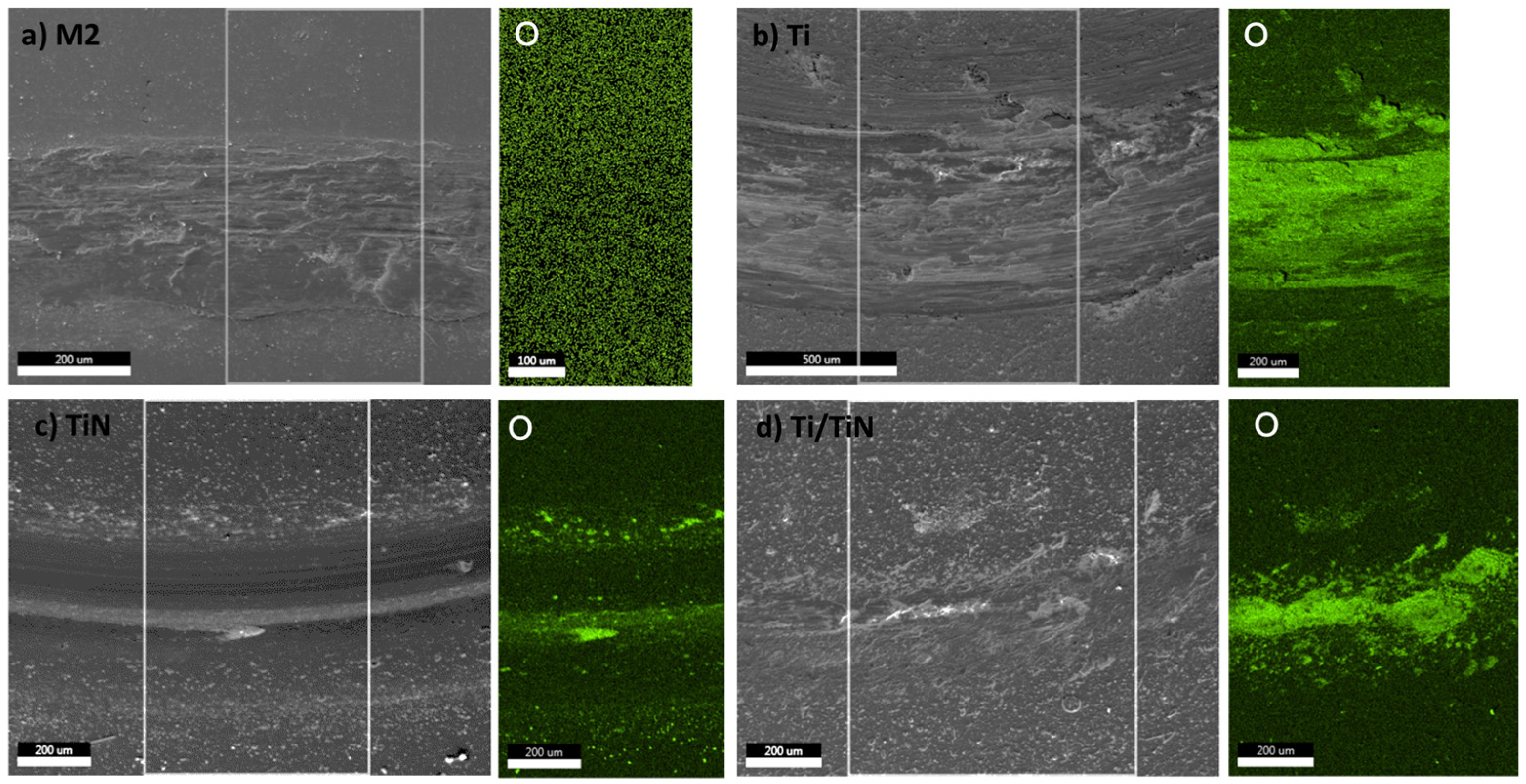

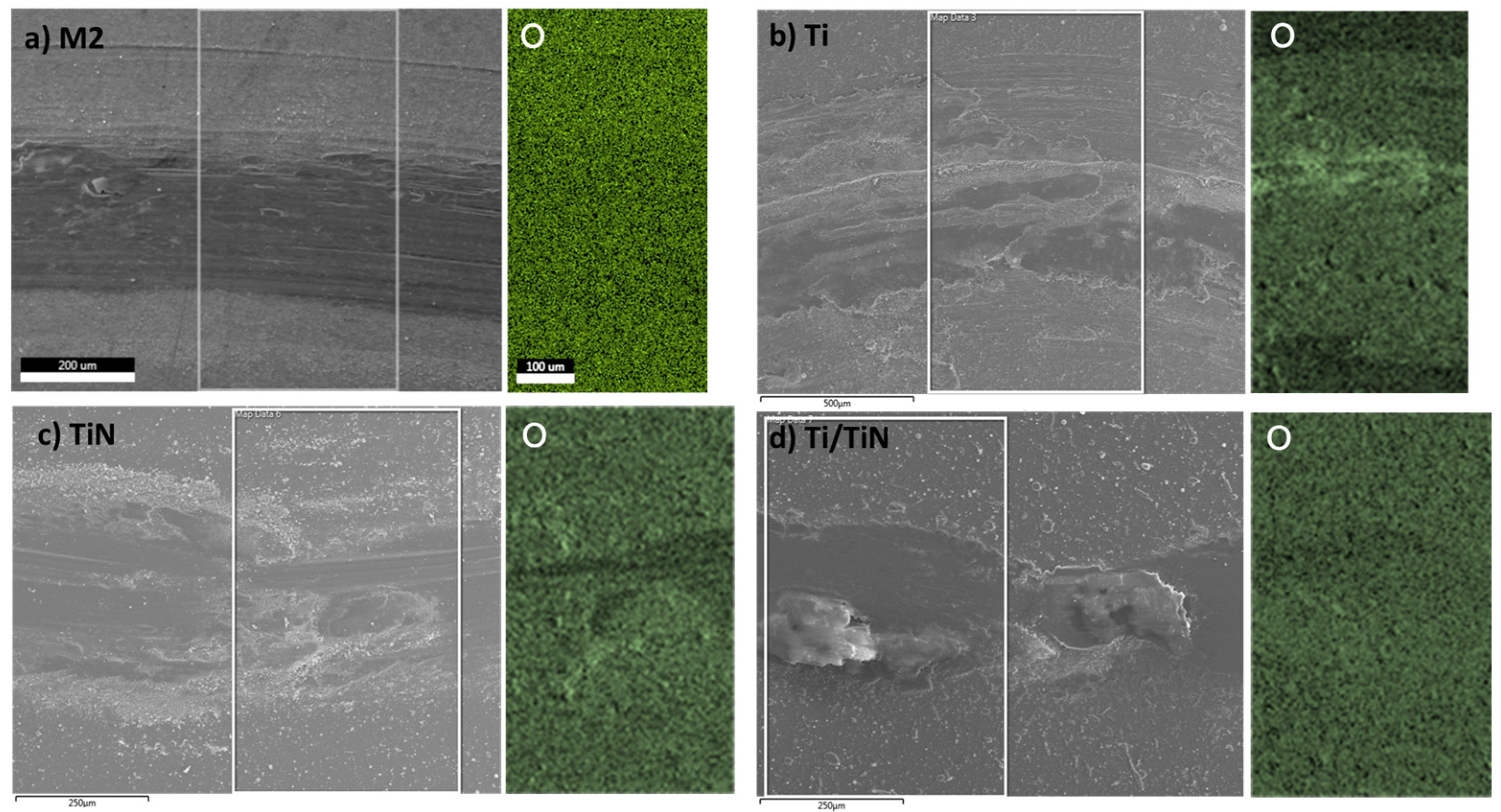
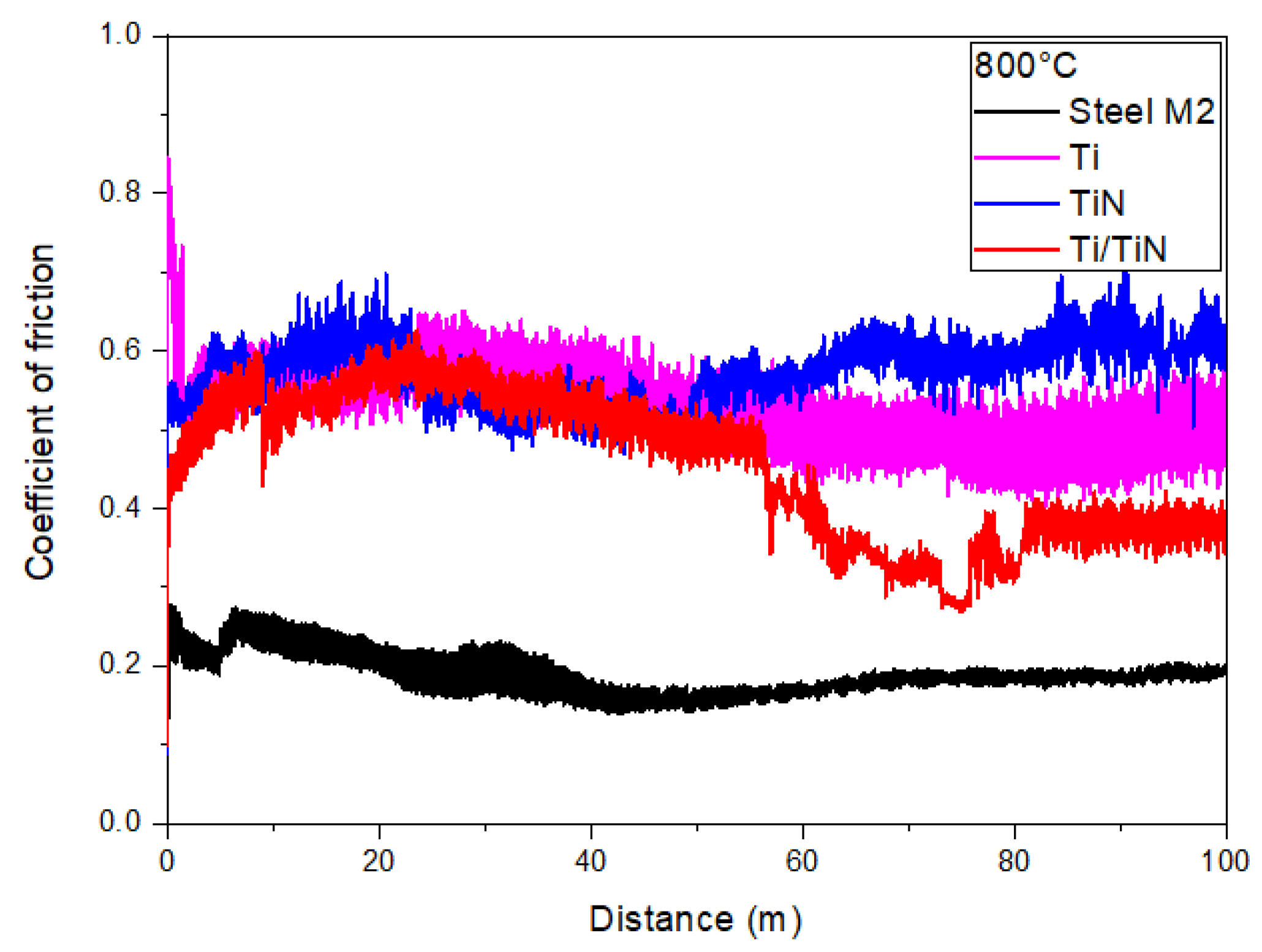

| Element | C | Mo | V | Cr | W | Co | Si | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt. % | 0.9 ± 0.2 | 3.83 ± 0.5 | 1.43 ± 0.1 | 3.97 ± 0.4 | 4.17 ± 0.3 | 0.445 ± 0.04 | 0.39 ± 0.06 | 0.286 ± 0.01 | Balance |
| Thickness (μm) | Roughness Ra (μm) | |
|---|---|---|
| AISI M2 | -- | 65.3 ± 11.70 |
| Ti | 4.11 ± 0.73 | 102.4 ± 18.43 |
| TiN | 5.3 ± 0.54 | 98.6 ± 17.74 |
| Ti/TiN | 5.2 ± 0.96 | 110.1 ± 19.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Portilla, C.; Giraldo, A.; Cardona, J.A.; Ruden, A.; Mondragón, G.C.; Trujillo, J.P.; Gómez Ortega, A.; González-Carmona, J.M.; Franco Urquiza, E.A. Effect of Temperature on the Structure and Tribological Properties of Ti, TiN and Ti/TiN Coatings Deposited by Cathodic Arc PVD. Coatings 2024, 14, 823. https://doi.org/10.3390/coatings14070823
Ortega-Portilla C, Giraldo A, Cardona JA, Ruden A, Mondragón GC, Trujillo JP, Gómez Ortega A, González-Carmona JM, Franco Urquiza EA. Effect of Temperature on the Structure and Tribological Properties of Ti, TiN and Ti/TiN Coatings Deposited by Cathodic Arc PVD. Coatings. 2024; 14(7):823. https://doi.org/10.3390/coatings14070823
Chicago/Turabian StyleOrtega-Portilla, Carolina, Andrea Giraldo, Jorge Andrés Cardona, Alexander Ruden, Guillermo César Mondragón, Juan Pablo Trujillo, Arturo Gómez Ortega, Juan Manuel González-Carmona, and Edgar Adrián Franco Urquiza. 2024. "Effect of Temperature on the Structure and Tribological Properties of Ti, TiN and Ti/TiN Coatings Deposited by Cathodic Arc PVD" Coatings 14, no. 7: 823. https://doi.org/10.3390/coatings14070823






