Copper Alloying Improves the Microbiologically Influenced Corrosion Resistance of Pipeline Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Specimens
2.2. SRB Cultivation and Corrosion Tests
2.3. Weight Loss Test and Morphological Characterization
2.4. Electrochemical Test
3. Results
3.1. Uniform Corrosion Rates
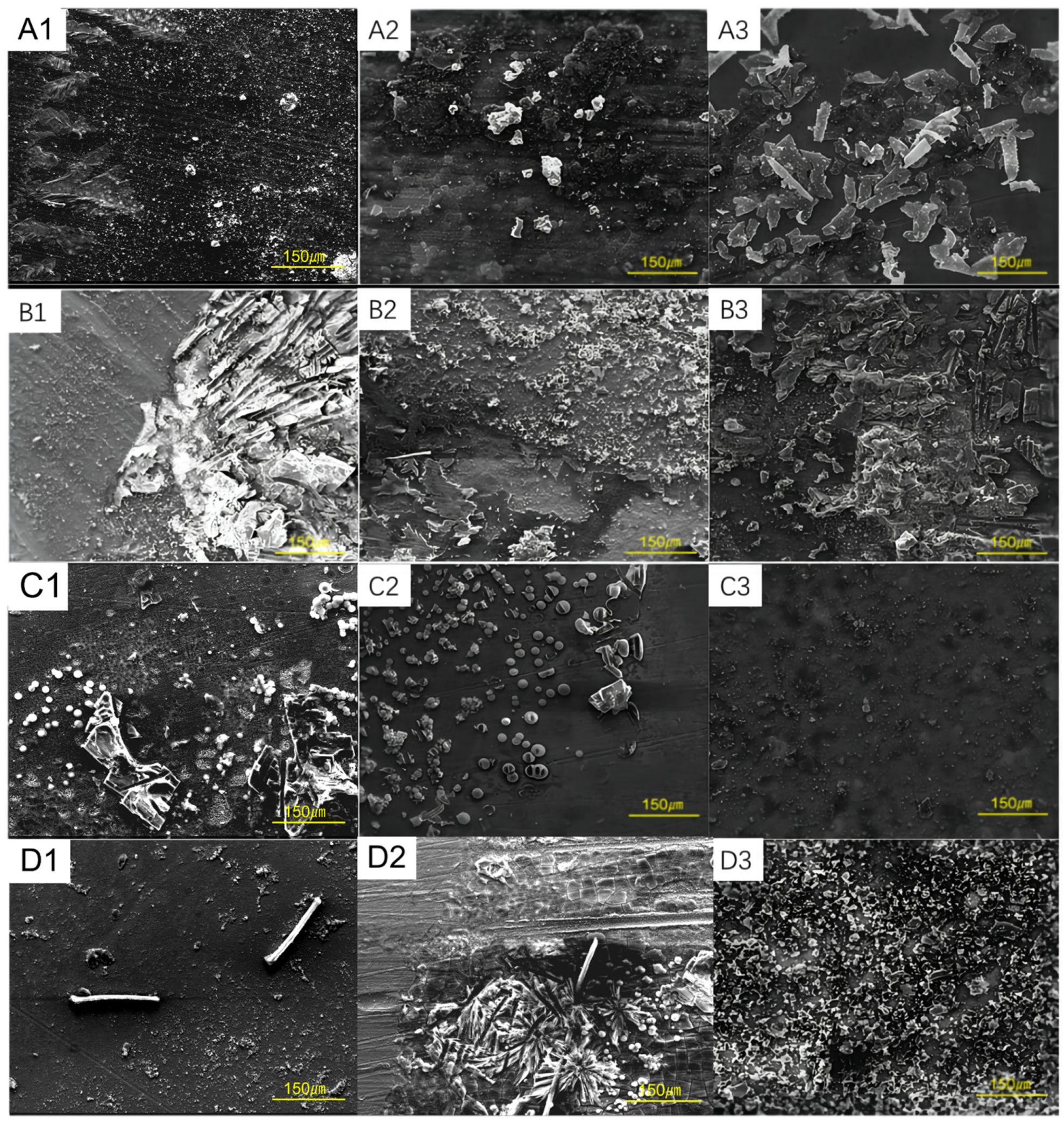
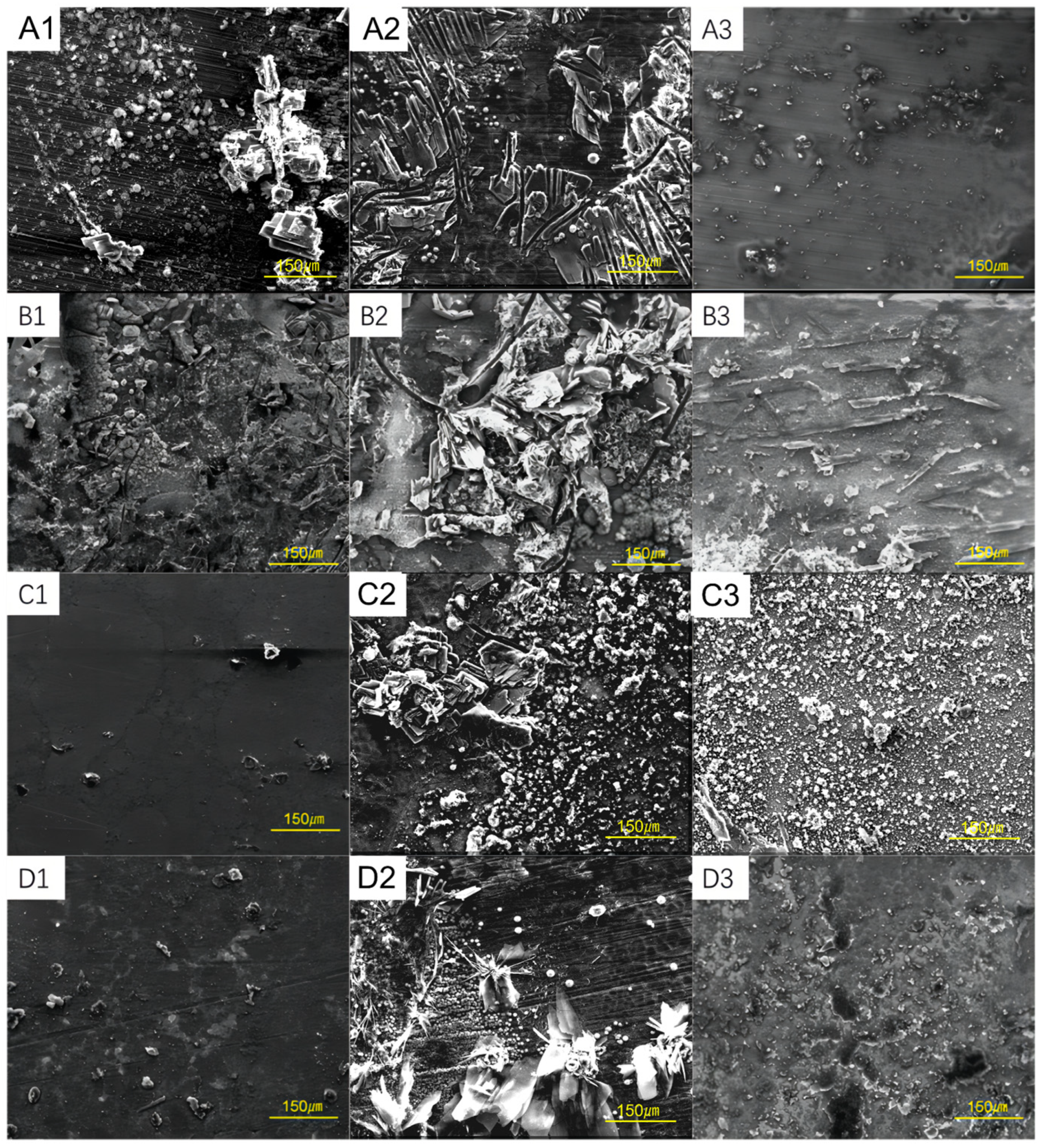
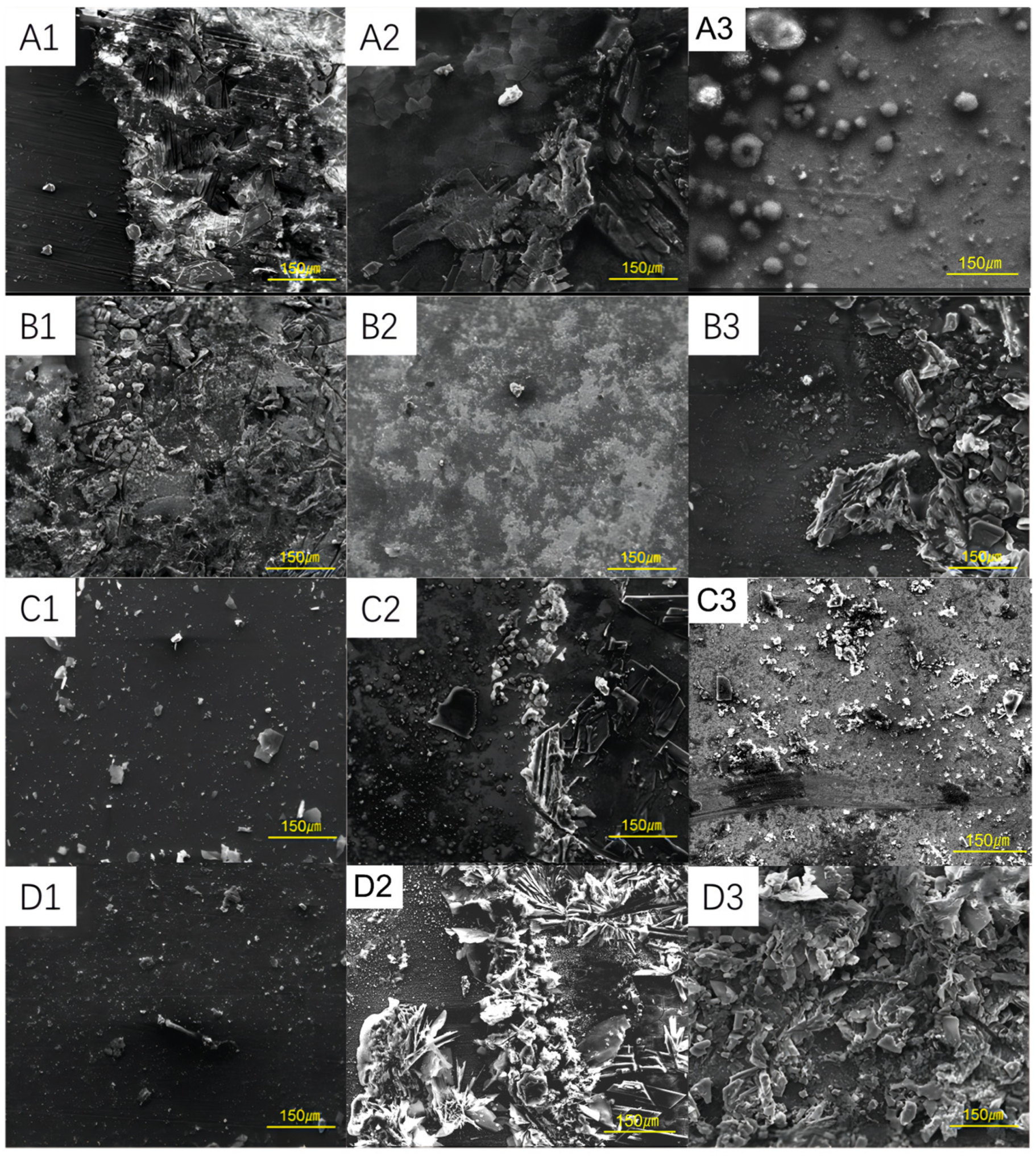
3.2. Corrosion Morphology of Steel Samples at Different Conditions
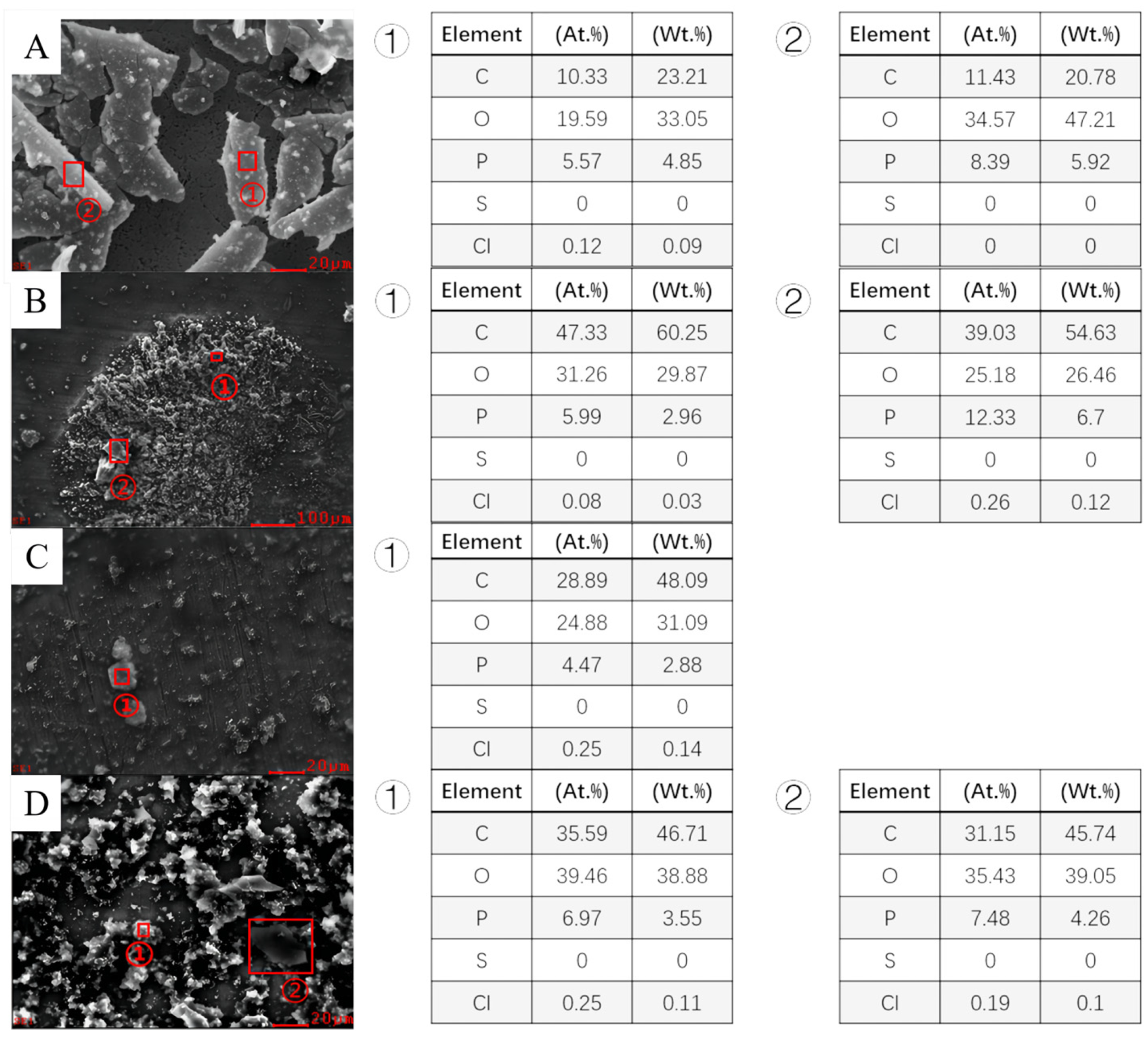
3.3. Analysis of Corrosion Product of Different Steels
3.4. Electrochemical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loto, C.A. Microbiological corrosion: Mechanism, control and impact—A review. Int. J. Adv. Manuf. Technol. 2017, 92, 4241–4252. [Google Scholar] [CrossRef]
- Lavanya, M. A Brief Insight into Microbial Corrosion and its Mitigation with Eco-friendly Inhibitors. J. Bio-Tribo-Corros. 2021, 7, 125. [Google Scholar] [CrossRef]
- Shrestha, R.; Černoušek, T.; Stoulil, J.; Kovářová, H.; Sihelská, K.; Špánek, R.; Ševců, A.; Steinová, J. Anaerobic microbial corrosion of carbon steel under conditions relevant for deep geological repository of nuclear waste. Sci. Total Environ. 2021, 800, 149539. [Google Scholar] [CrossRef] [PubMed]
- Kip, N.; Van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, Y.; Yu, D.; Lan, G.; Wang, Z.; Mou, X. Studies on the impact of fluid flow on the microbial corrosion behavior of product oil pipelines. J. Petrol. Sci. Eng. 2016, 146, 803–812. [Google Scholar] [CrossRef]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-Enhanced Microbial Corrosion of Concrete Sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef] [PubMed]
- Salgar-Chaparro, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Nutrient Level Determines Biofilm Characteristics and Subsequent Impact on Microbial Corrosion and Biocide Effectiveness. Appl. Environ. Microbiol. 2020, 86, e02885-19. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially influenced corrosion: Towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]
- Xu, D.; Gu, T.; Lovley, D.R. Microbially mediated metal corrosion. Nat. Rev. Microbiol. 2023, 21, 705–718. [Google Scholar] [CrossRef]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, W.; Cui, T.; Xu, D.; Zhang, D.; Lou, Y.; Qian, H.; Song, H.; Mol, A.; Cao, F.; et al. Adaptive bidirectional extracellular electron transfer during accelerated microbiologically influenced corrosion of stainless steel. Commun. Mater. 2021, 2, 67. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Liu, X.; Liu, P.; Li, X.; Zhang, M.; Zhou, E.; Zhao, Z.; Wang, X.; Zhang, Y.; et al. Accelerated corrosion of 316L stainless steel in a simulated oral environment via extracellular electron transfer and acid metabolites of subgingival microbiota. Bioact. Mater. 2024, 35, 56–66. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, S.; Liu, H.; Tian, X.; Xia, Y.; Li, M.; Xu, K.; Yu, H.; Liu, Q.; Chen, C. Bacterial distribution in SRB biofilm affects MIC pitting of carbon steel studied using FIB-SEM. Corros. Sci. 2020, 167, 108512. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Gu, T. Mechanistic modeling of biocorrosion caused by biofilms of sulfate reducing bacteria and acid producing bacteria. Bioelectrochemistry 2016, 110, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Jia, R.; Jin, P.; Liu, J.; Chen, S.; Gu, T. Investigation of the mechanism and characteristics of copper corrosion by sulfate reducing bacteria. Corros. Sci. 2018, 144, 237–248. [Google Scholar] [CrossRef]
- Chilkoor, G.; Karanam, S.P.; Star, S.; Shrestha, N.; Sani, R.K.; Upadhyayula, V.K.K.; Ghoshal, D.; Koratkar, N.A.; Meyyappan, M.; Gadhamshetty, V. Hexagonal Boron Nitride: The Thinnest Insulating Barrier to Microbial Corrosion. ACS Nano 2018, 12, 2242–2252. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Chinnaraj, S.B.; Jayathilake, P.G.; Dawson, J.; Ammar, Y.; Portoles, J.; Jakubovics, N.; Chen, J. Modelling the combined effect of surface roughness and topography on bacterial attachment. J. Mater. Sci. Technol. 2021, 81, 151–161. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, R.; Lou, L.; Jing, X.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Wang, J. Layer-by-Layer-Assembled antifouling films with surface microtopography inspired by Laminaria japonica. Appl. Surf. Sci. 2020, 511, 145564. [Google Scholar] [CrossRef]
- Bao, J.; Li, H.; Xu, Y.; Chen, S.; Wang, Z.; Jiang, C.; Li, H.; Wei, Z.; Sun, S.; Zhao, W.; et al. Multi-functional polyethersulfone nanofibrous membranes with ultra-high adsorption capacity and ultra-fast removal rates for dyes and bacteria. J. Mater. Sci. Technol. 2021, 78, 131–143. [Google Scholar] [CrossRef]
- Ogawa, A.; Takakura, K.; Hirai, N.; Kanematsu, H.; Kuroda, D.; Kougo, T.; Sano, K.; Terada, S. Biofilm Formation Plays a Crucial Rule in the Initial Step of Carbon Steel Corrosion in Air and Water Environments. Materials 2020, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Galarce, C.; Fischer, D.; Díez, B.; Vargas, I.T.; Pizarro, G.E. Dynamics of Biocorrosion in Copper Pipes under Actual Drinking Water Conditions. Water 2020, 12, 1036. [Google Scholar] [CrossRef]
- Victoria, S.N.; Sharma, A.; Manivannan, R. Metal corrosion induced by microbial activity—Mechanism and control options. J. Indian Chem. Soc. 2021, 98, 100083. [Google Scholar] [CrossRef]
- Shi, X.; Yan, W.; Xu, D.; Yan, M.; Yang, C.; Shan, Y.; Yang, K. Microbial corrosion resistance of a novel Cu-bearing pipeline steel. J. Mater. Sci. Technol. 2018, 34, 2480–2491. [Google Scholar] [CrossRef]
- Mansour, R.; Elshafei, A. Role of Microorganisms in Corrosion Induction and Prevention. Br. Biotechnol. J. 2016, 14, 1–11. [Google Scholar] [CrossRef]
- Yu, H.; Li, Z.; Xia, Y.; Qi, Y.; Li, Y.; Liu, Q.; Chen, C. Effect of copper addition in carbon steel on biocorrosion by sulfate-reducing bacteria in solution. Anti-Corros. Methods Mater. 2021, 68, 302–309. [Google Scholar] [CrossRef]
- Udowo, V.; Yan, M.; Liu, F.; Ikeuba, A. Role of Fe oxide in the underdeposit corrosion of pipeline steel in oilfield produced water containing SRB. Mater. Corros. 2023, 1, 118–129. [Google Scholar] [CrossRef]
- Nan, L.; Yang, W.; Liu, Y.; Xu, H.; Li, Y.; Lu, M.; Yang, K. Antibacterial Mechanism of Copper-bearing Antibacterial Stainless Steel against E.Coli. J. Mater. Sci. Technol. 2008, 24, 197–201. [Google Scholar]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; et al. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: In vitro and in vivo evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yang, C.G.; Xu, D.K.; Sun, D.; Nan, L.; Sun, Z.; Li, Q.; Gu, T.; Yang, K. Laboratory investigation of the microbiologically influenced corrosion (MIC) resistance of a novel Cu-bearing 2205 duplex stainless steel in the presence of an aerobic marine Pseudomonas aeruginosa biofilm. Biofouling 2015, 31, 481. [Google Scholar] [CrossRef] [PubMed]
- Sharifahmadian, O.; Salimijazi, H.R.; Fathi, M.H.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Ren, G.; Ren, L. Study on behaviour and mechanism of Cu2+ ion release from Cu bearing antibacterial stainless steel. Mater. Technol. 2015, 30, B126–B132. [Google Scholar] [CrossRef]
- Ercetin, A.; Özgün, Ö.; Aslantas, K.; Aykutoğlu, G. The microstructure, degradation behavior and cytotoxicity effect of Mg–Sn–Zn alloys in vitro tests. Discov. Appl. Sci. 2020, 2, 173. [Google Scholar] [CrossRef]
- Sun, D.; Xu, D.; Yang, C.; Chen, J.; Shahzad, M.B.; Sun, Z.; Zhao, J.; Gu, T.; Yang, K.; Wang, G. Inhibition of Staphylococcus aureus biofilm by a copper-bearing 317L-Cu stainless steel and its corrosion resistance. Mater. Sci. Eng. C 2016, 69, 744–750. [Google Scholar] [CrossRef]
- Warnes, S.L.; Keevil, C.W. Mechanism of Copper Surface Toxicity in Vancomycin-Resistant Enterococci following Wet or Dry Surface Contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef]


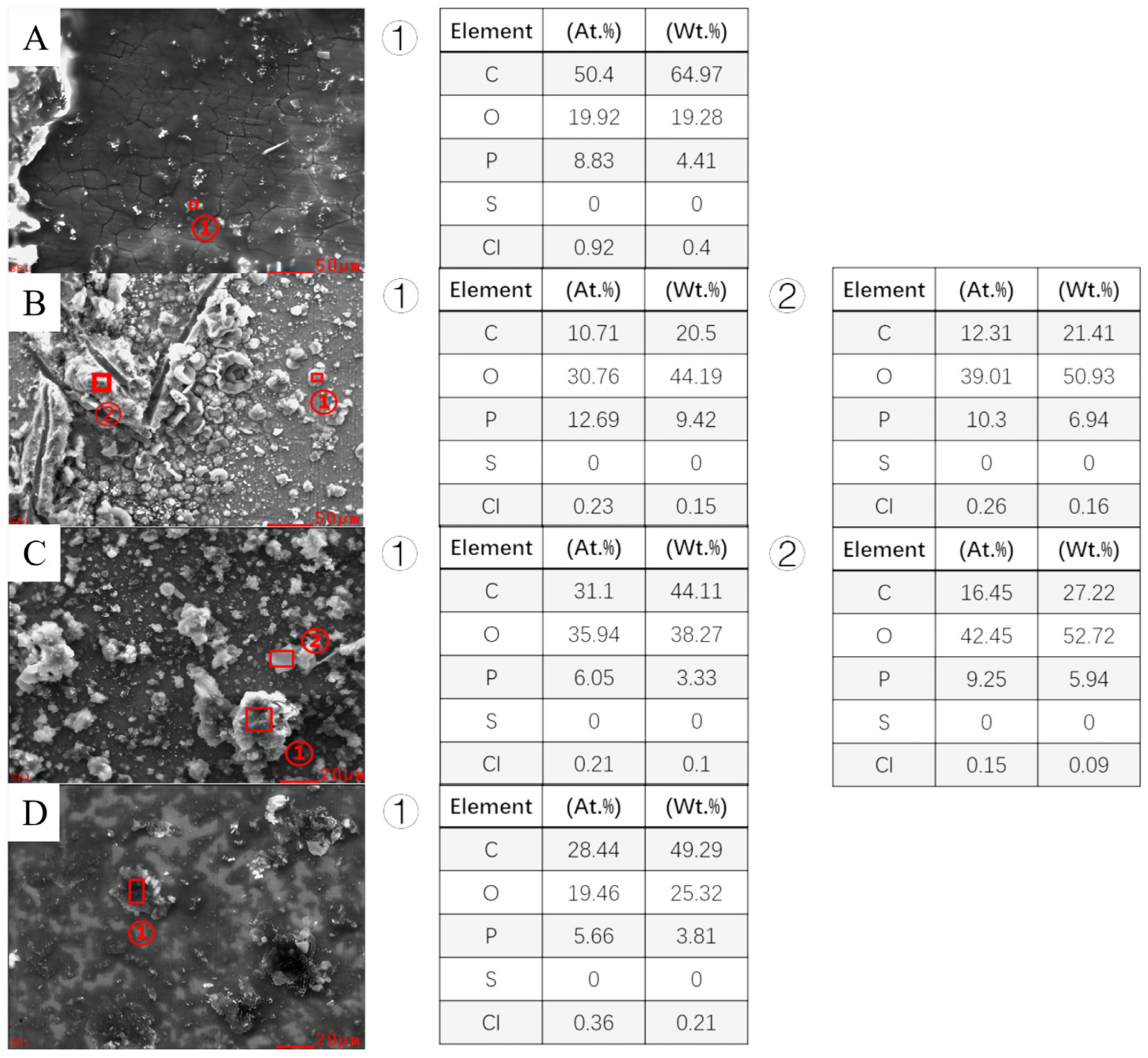
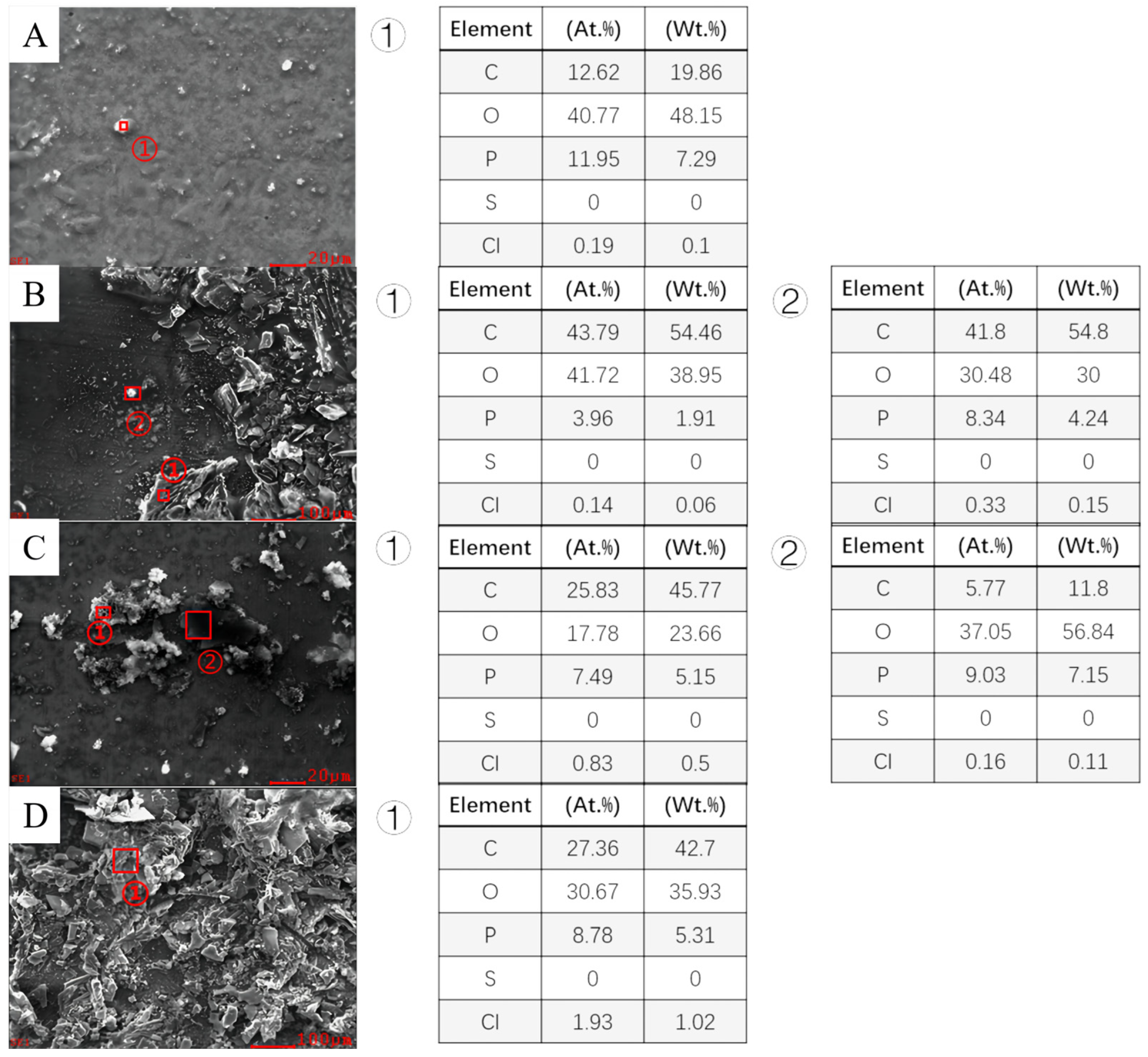

| C | Si | Mn | P | S | V | Ni | Ti | Cu | |
|---|---|---|---|---|---|---|---|---|---|
| Steel-1 | 0.16 | 0.45 | 1.65 | 0.02 | 0.01 | 0.09 | 0.05 | 0.06 | |
| Steel-2 | 0.18 | 0.45 | 1.7 | 0.025 | 0.015 | V + Ni + Ti < 0.15 | |||
| Steel-3 | 0.16 | 0.45 | 1.65 | 0.02 | 0.03 | 0.09 | 0.3 | 0.06 | |
| Steel-4 | 0.16 | 0.45 | 1.65 | 0.02 | 0.03 | 0.09 | 0.3 | 0.06 | 0.35 |
| Name | Sodium Lactate | Na2SO4 | NH4Cl | Yeast | KH2PO4 | Sodium Citrate | CaCl2·6H2O | MgSO4·7H2O | FeSO4·7H2O | Deionized Water |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (g/L) and Volume | 6.0 | 4.5 | 1.0 | 1.0 | 0.5 | 0.3 | 0.06 | 0.06 | Few | 1 L |
| 25 °C | 40 °C | 60 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecorr/(V) | Icorr/(μA/cm2) | Eb(V) | Ecorr/(V) | Icorr/(μA/cm2) | Eb (V) | Ecorr/(V) | Icorr/(μA/cm2) | Eb (V) | |
| Steel-1 | −0.656 | 5.85 | −0.294 | −0.678 | 6.21 | −0.352 | −0.579 | 5.67 | −0.240 |
| Steel-2 | −0.503 | 6.03 | −0.239 | −0.625 | 6.38 | −0.205 | −0.578 | 6.65 | −0.140 |
| Steel-3 | −0.582 | 6.41 | −0.241 | −0.629 | 6.46 | −0.301 | −0.388 | 6.31 | −0.134 |
| Steel-4 | −0.620 | 6.23 | −0.327 | −0.634 | 6.36 | −0.308 | −0.607 | 6.12 | −0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Li, P.; Wu, B.; Wei, Y.; Jiang, H.; Shen, J.; Liang, Q. Copper Alloying Improves the Microbiologically Influenced Corrosion Resistance of Pipeline Steel. Coatings 2024, 14, 834. https://doi.org/10.3390/coatings14070834
Liu Q, Li P, Wu B, Wei Y, Jiang H, Shen J, Liang Q. Copper Alloying Improves the Microbiologically Influenced Corrosion Resistance of Pipeline Steel. Coatings. 2024; 14(7):834. https://doi.org/10.3390/coatings14070834
Chicago/Turabian StyleLiu, Qingjian, Pei Li, Baihong Wu, Yulong Wei, Huifang Jiang, Junjie Shen, and Qingwen Liang. 2024. "Copper Alloying Improves the Microbiologically Influenced Corrosion Resistance of Pipeline Steel" Coatings 14, no. 7: 834. https://doi.org/10.3390/coatings14070834





