Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
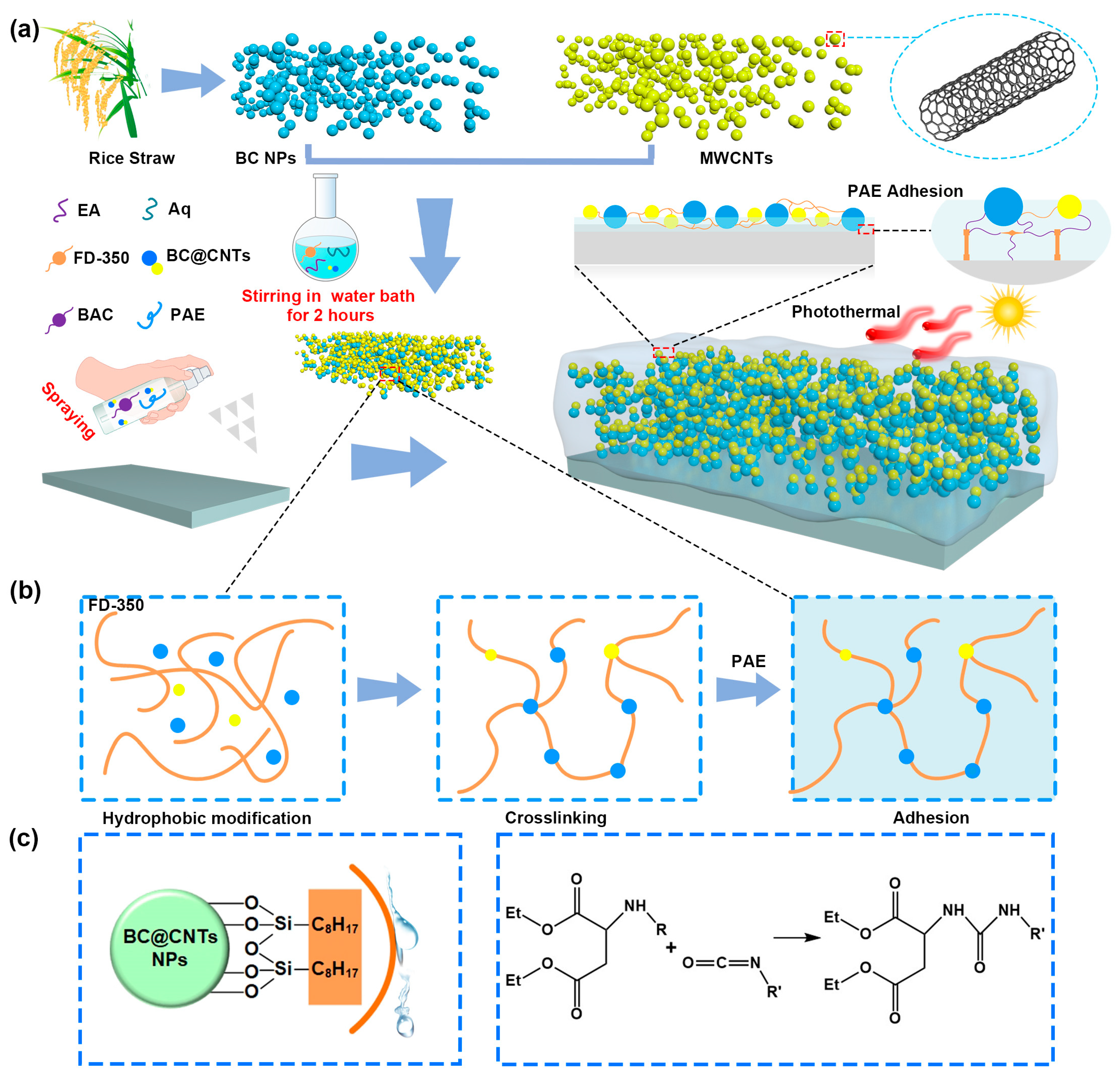
2.2. Preparation of BC NPs
2.3. Modification of BC@CNTs NPs
2.4. Fabrication of BCP Coatings
2.5. Characterization
2.6. Light Absorption and Photothermal Test
2.7. De-Icing and Defrosting Test
2.8. Antifouling and Self-Cleaning Test
2.9. Mechanical Durability Test
3. Results
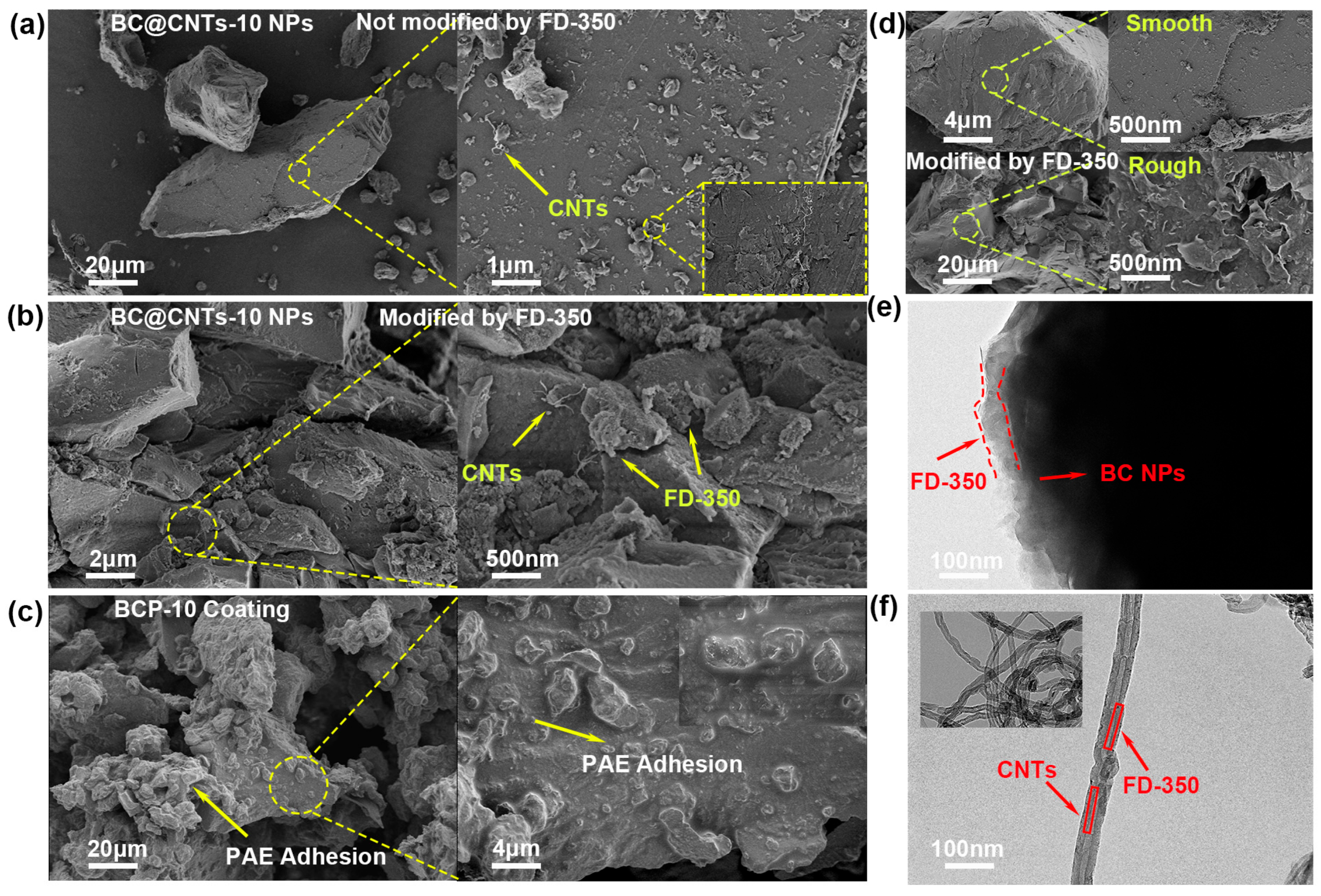
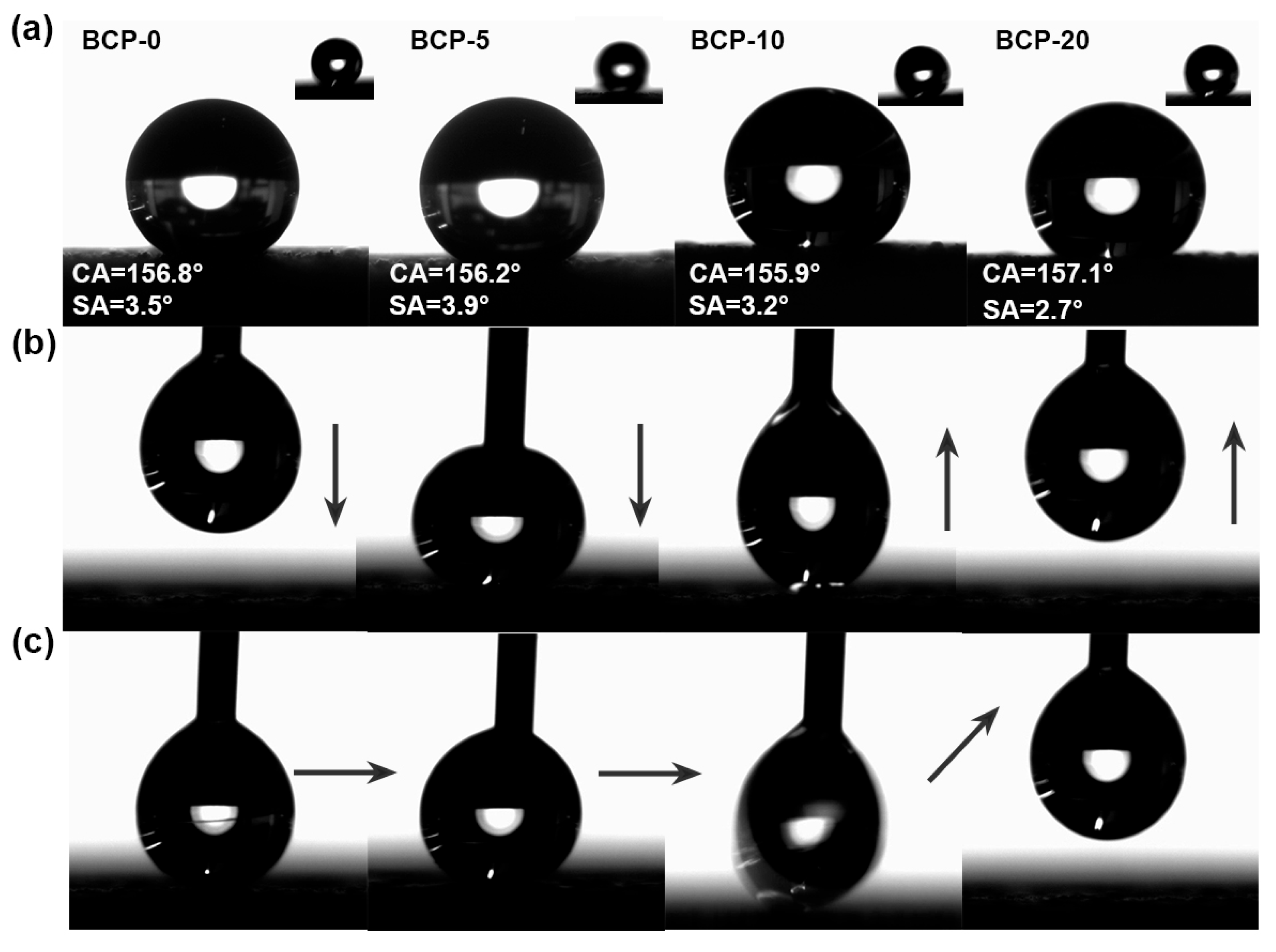
3.1. Characterization Analysis
3.2. Photothermal Performance of BCP Coating
3.3. De-Icing and Defrosting Performance
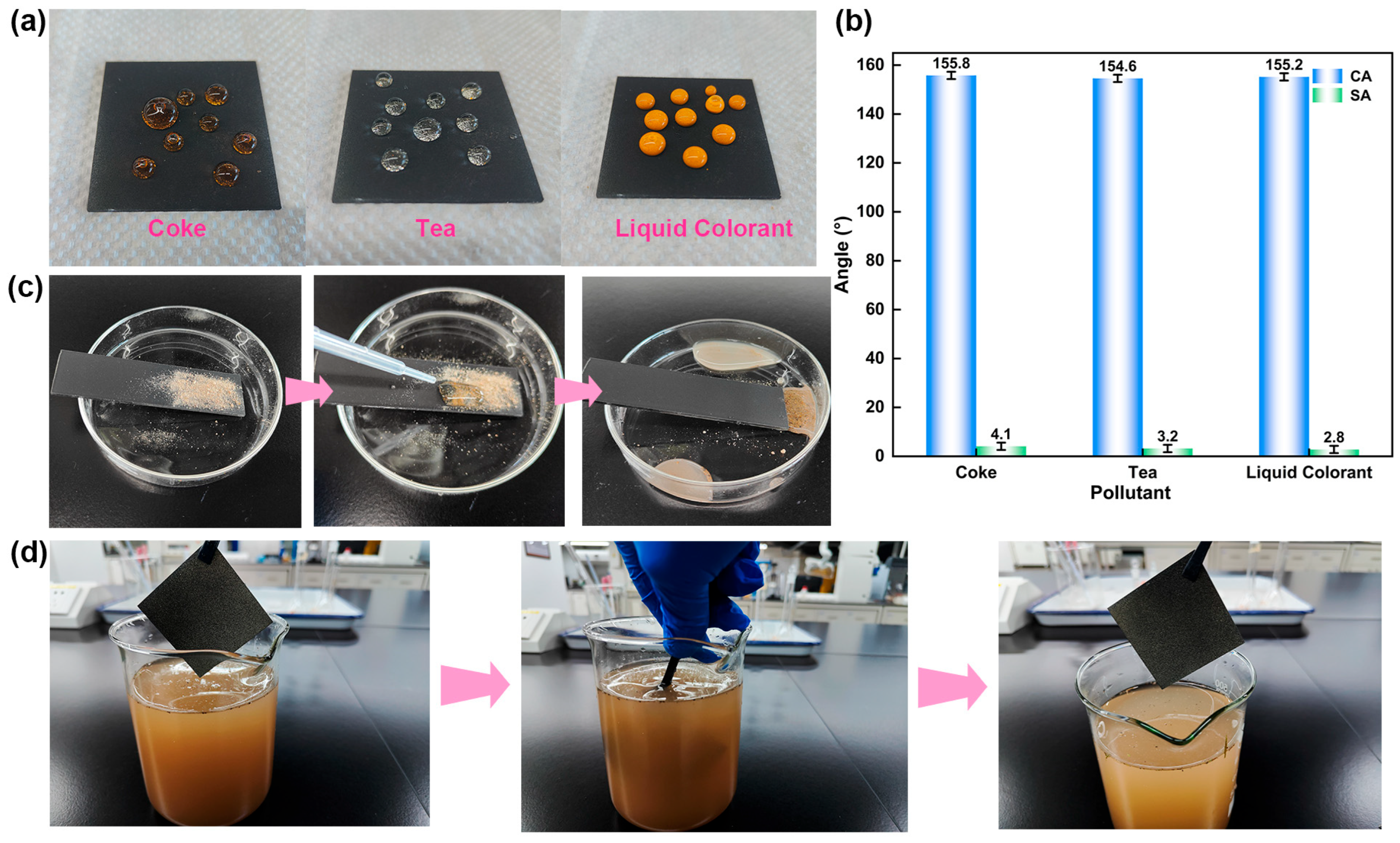
3.4. Antifouling and Self-Cleaning Performance of BCP Coatings
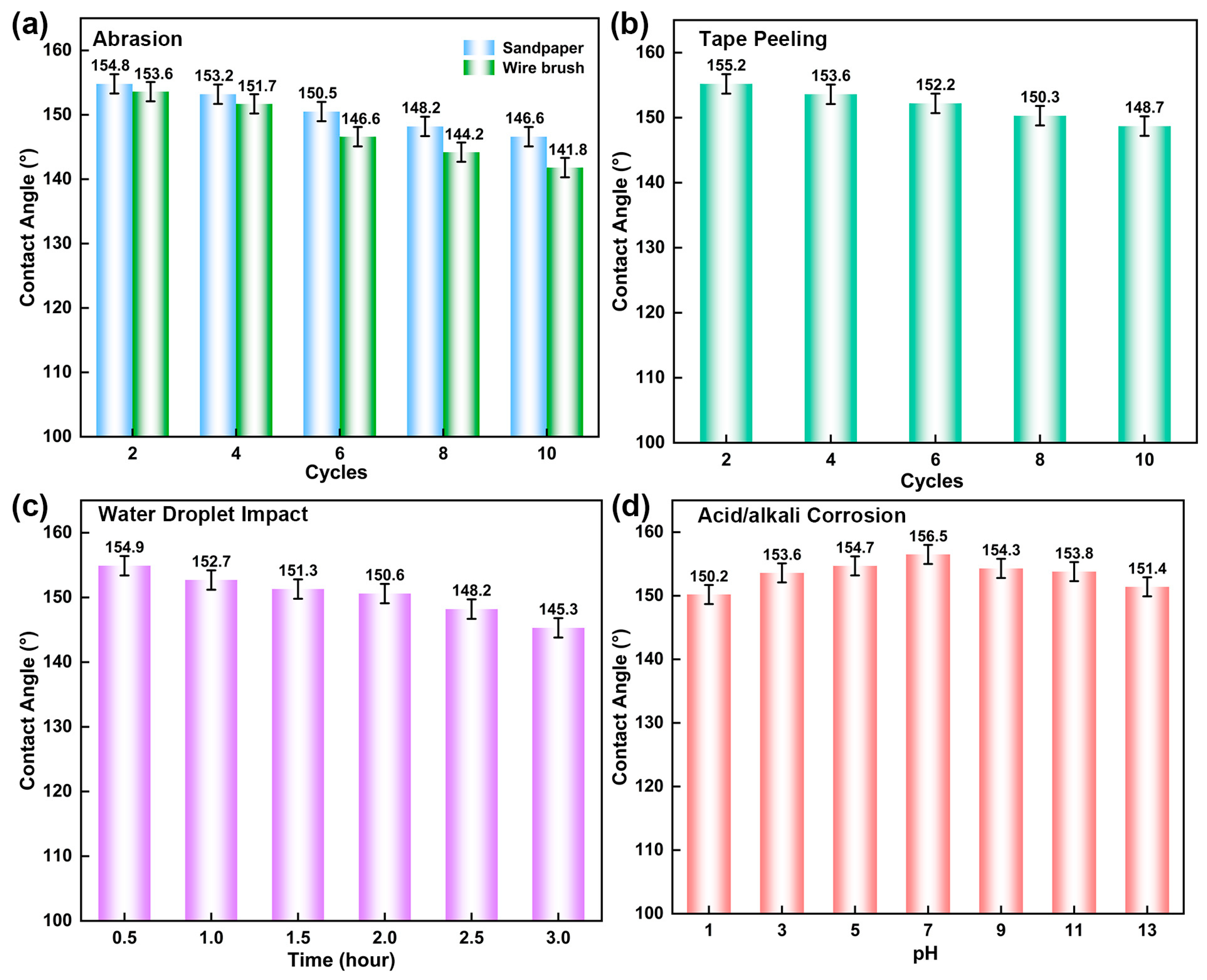
3.5. Mechanical Stability and Chemical Stability of BCP Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Xu, Y.; Zhang, F.; Jia, Y.; Du, Z.; Li, G.; Shi, B.; Li, P.; Ning, M.; Li, A. Preparation methods and research progress of super-hydrophobic anti-icing surface. Adv. Colloid Interface Sci. 2024, 323, 103069. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, H.J.; Chen, M.J. Multi-Scale Superhydrophobic Surface with Excellent Stability and Solar-Thermal Performance for Highly Efficient Anti-Icing and Deicing. Small 2024, 2312226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Yin, K.; Deng, Q.W.; Huang, Q.Q.; Arnusch, C.J. Multiscale hybrid-structured femtosecond laser-induced graphene with outstanding photo-electro-thermal effects for all-day anti-icing/deicing. Carbon 2024, 219, 118824. [Google Scholar] [CrossRef]
- Miao, B.; Yuan, L.; Zhu, C. Piezoelectric-Actuator De-Icing Array Design Based on Structural Vibration Modes. J. Aircr. 2023, 61, 957–971. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Z.; Yang, H.; He, Z.; Wang, J. All-Day Anti-Icing/Deicing Film Based on Combined Photo-Electro-Thermal Conversion. ACS Appl. Mater. Interfaces 2021, 13, 44948–44955. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Li, B.; Xiang, H.; Dai, X.; Zhu, T.; Hua, X.; Yuan, Y. A Superhydrophobic Anti-Icing Surface with a Honeycomb Nanopore Structure. Coatings 2023, 13, 1971. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Chen, M.; Liu, Z.; Jiang, S.; Wang, L. Preparation of Durable Superhydrophobic Coatings Based on Discrete Adhesives. Coatings 2024, 14, 463. [Google Scholar] [CrossRef]
- Qian, C.; Wang, L.; Li, Q.; Chen, X. A Strategy of Candle Soot-Based Photothermal Icephobic Superhydrophobic Surface. Coatings 2024, 14, 612. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Sun, Y.; Feng, F.; Tagawa, K. Preparation of biochar-based photothermal superhydrophobic coating based on corn straw biogas residue and blade anti-icing performance by wind tunnel test. Renew. Energy 2023, 210, 618–626. [Google Scholar] [CrossRef]
- Lian, Z.; Zhou, J.; Liu, Z.; Wan, Y.; Liu, R.; Yang, J.; Xu, J.; Tian, Y.; Yu, H. Hierarchically structured superhydrophobic surfaces with photothermal conversion to avoid icing. Int. J. Mech. Sci. 2024, 275, 109341. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, R.; Jin, B.; Li, T.; Yao, L.; Feng, L.; He, J. Superhydrophobic VO2 Nanoparticle/PDMS Composite Films as Thermochromic, Anti-icing, and Self-Cleaning Coatings. ACS Appl. Nano Mater. 2022, 5, 5599–5608. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, H.; Geng, Y.; Li, M.; Deng, Q.; Tian, Y.; Chen, R.; Zhu, X.; Liao, Q. Carbon-Based Photothermal Superhydrophobic Materials with Hierarchical Structure Enhances the Anti-Icing and Photothermal Deicing Properties. ACS Appl. Mater. Interfaces 2021, 13, 48308–48321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, C.; Zhao, Y.; Zhang, S.; Wang, Y.; Ji, B.; Liu, R.; Wong, M.H.; Shan, S.; Zhang, J. An integrated constructed wetland-Microbial fuel cell system with sewage sludge-biochar to enhance treatment and energy recovery efficiencies. Chem. Eng. J. 2024, 486, 150431. [Google Scholar] [CrossRef]
- Zepeda, L.C.; Griffin, G.; Shah, K.; Al-Waili, I.; Parthasarathy, R. Energy potential, flow characteristics and stability of water and alcohol-based rice-straw biochar slurry fuel. Renew. Energy 2023, 207, 60–72. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Jha, J.M.; Srivastava, N.; Hashem, A.; Alakeel, M.A.; Abd_Allah, E.F.; Gupta, V.K. Low-cost biochar adsorbents prepared from date and delonix regia seeds for heavy metal sorption. Bioresour. Technol. 2021, 339, 125606. [Google Scholar] [CrossRef]
- Yao, B.; Luo, Z.; Du, S.; Yang, J.; Zhi, D.; Zhou, Y. Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms. Bioresour. Technol. 2021, 340, 125698. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Singh, J.; Verma, M. Waste derived modified biochar as promising functional material for enhanced water remediation potential. Environ. Res. 2024, 245, 117999. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Yang, Q.; Jing, Z.; Wang, W.; Li, P.; Tong, X.; Lin, F.; Wang, D.; Lio, G.E.; et al. Upcycling of biomass waste into photothermal superhydrophobic coating for efficient anti-icing and deicing. Mater. Today Phys. 2022, 24, 100683. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Zhang, J.; Hong, C.; Liu, M.; Wang, Y.; Zhang, J.; Zhai, R.; Zhou, C. Fluorine-free waterborne polyurethanes modified with bis-amino silane coupling agent: Synthesis and properties. Mater. Today Commun. 2024, 39, 109315. [Google Scholar] [CrossRef]
- Esfarjani, A.; Shokrieh, M.M. Effects of TiO2 nanoparticles and silane coupling agents on the adhesion strength and weathering properties of silicone rubber coatings. Int. J. Adhes. Adhes. 2024, 130, 103604. [Google Scholar] [CrossRef]
- Celik, N.; Sezen, B.; Sahin, F.; Ceylan, A.; Ruzi, M.; Onses, M.S. Mechanochemical Coupling of Alkylsilanes to Nanoparticles for Solvent-Free and Rapid Fabrication of Superhydrophobic Materials. ACS Appl. Nano Mater. 2023, 6, 14921–14930. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, S.; Ni, Y.; Gou, Y.; Huang, J.; Lai, Y. Transparent Liquid-like Slippery Coating with Excellent Photothermal Performance and Durability for Anti-/De-Icing Applications. Ind. Eng. Chem. Res. 2023, 62, 17701–17710. [Google Scholar] [CrossRef]
- Li, J.; Jiao, W.; Jin, H.; Lu, Q.; Sun, H.; Yin, Y.; He, X. Robust polyurea icephobic coatings with static large-scale de-icing and dynamic anti-icing performance. Chem. Eng. J. 2023, 478, 147339. [Google Scholar] [CrossRef]
- Wang, Z.; Du, M.; Fang, H.; Zhao, P.; Yao, X.; Chen, S. The development of high-strength, anticorrosive, and strongly adhesive polyaspartate polyurea coating suitable for pipeline spray repairs. J. Appl. Polym. Sci. 2024, 141, e55668. [Google Scholar] [CrossRef]
- Dural, S.; Camadanli, S.S.; Apohan, N.K. Improving the mechanical, thermal and surface properties of polyaspartic ester bio-based polyurea coatings by incorporating silica and titania. Mater. Today Commun. 2024, 38, 107654. [Google Scholar] [CrossRef]
- Du, Y.; Hu, L.; Dong, L.; Du, S.; Xu, D. Experimental Study on Anti-Icing of Robust TiO2/Polyurea Superhydrophobic Coating. Coatings 2023, 13, 1162. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Yin, F.; Shi, J.; Jing, D. The mechanism of enhanced photothermal conversion of low-dimensional plasmonic nanofluids with LFPs resonance. Int. J. Heat Mass Transf. 2023, 208, 124056. [Google Scholar] [CrossRef]
- Wei, W.; He, J.; He, L.; Qi, X.; Zhang, X.; Wan, W.; Gao, Z. Study of direct drinking water production and sea salt recovery from photothermal conversion based on MWCNTs. Sep. Purif. Technol. 2024, 335, 126068. [Google Scholar] [CrossRef]
- Dong, L.; Ding, S.; Wu, Z.; Xie, H.; Wang, J.; Wang, T.; Wang, Y.; Huang, Y. Photothermal Conversion Characteristics of ZnO/MWCNTs Binary Nanofluids and ZnO/MWCNTs/Au Ternary Hybrid Nanofluids in Direct Absorption Solar Collectors. Energy Technol. 2022, 10, 2200661. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Li, Y.; Teng, F.; Liang, C. Superhydrophobic surface of hybrid nanocomposites made of TiO2 and multi-walled carbon nanotubes: Photothermal ice removal performance and wear resistance. Appl. Surf. Sci. 2023, 640, 158318. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wu, J.; Jia, X.; Zhang, Z.; Yang, J.; Miao, X.; Feng, L.; Song, H. Facile Recycling of Waste Fabrics for Preparing Multifunctional Photothermal Protective Materials. ACS Sustain. Chem. Eng. 2023, 11, 10566–10577. [Google Scholar] [CrossRef]
- Wei, J.; Wei, X.; Hou, M.; Wang, J. Fluorine-Free Photothermal Superhydrophobic Copper Oxide Micro-/Nanostructured Coatings for Anti-icing/De-icing Applications. ACS Appl. Nano Mater. 2023, 6, 9928–9938. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, H.; Zhong, M. Triple-Scale Superhydrophobic Surface with Excellent Anti-Icing and Icephobic Performance via Ultrafast Laser Hybrid Fabrication. ACS Appl. Mater. Interfaces 2021, 13, 1743–1753. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Hu, L.; Du, S.; Xu, D.; Yang, J. Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing. Coatings 2024, 14, 838. https://doi.org/10.3390/coatings14070838
Lei Y, Hu L, Du S, Xu D, Yang J. Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing. Coatings. 2024; 14(7):838. https://doi.org/10.3390/coatings14070838
Chicago/Turabian StyleLei, Yuhang, Lina Hu, Shuming Du, Dong Xu, and Jingxiao Yang. 2024. "Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing" Coatings 14, no. 7: 838. https://doi.org/10.3390/coatings14070838
APA StyleLei, Y., Hu, L., Du, S., Xu, D., & Yang, J. (2024). Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing. Coatings, 14(7), 838. https://doi.org/10.3390/coatings14070838




