Effect of Structural and Material Modifications of Dye-Sensitized Solar Cells on Photovoltaic Performance
Abstract
1. Introduction
2. Experimental, Materials and Methods
2.1. Experimental
2.2. Materials and Methods
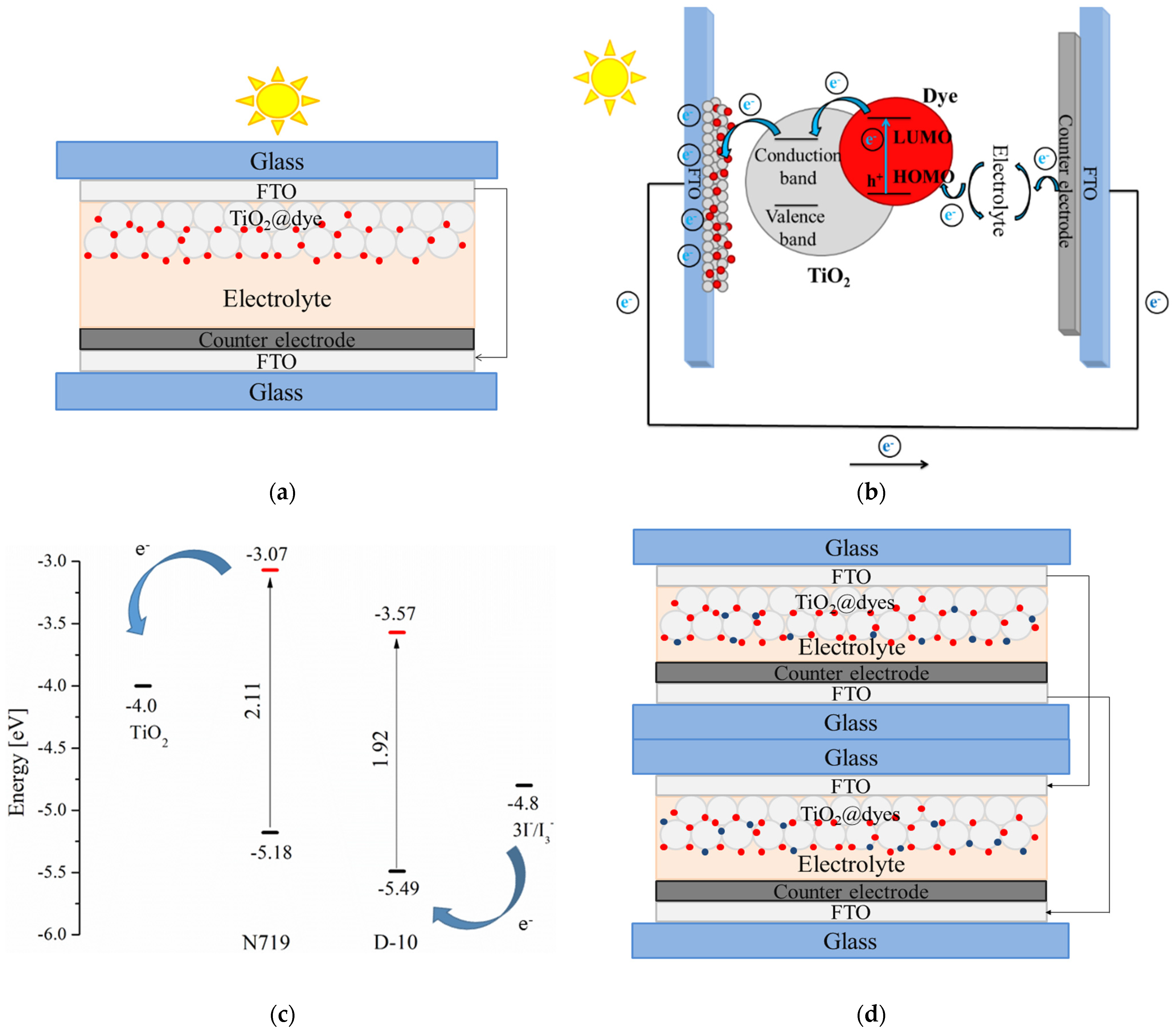
3. Results and Discussions
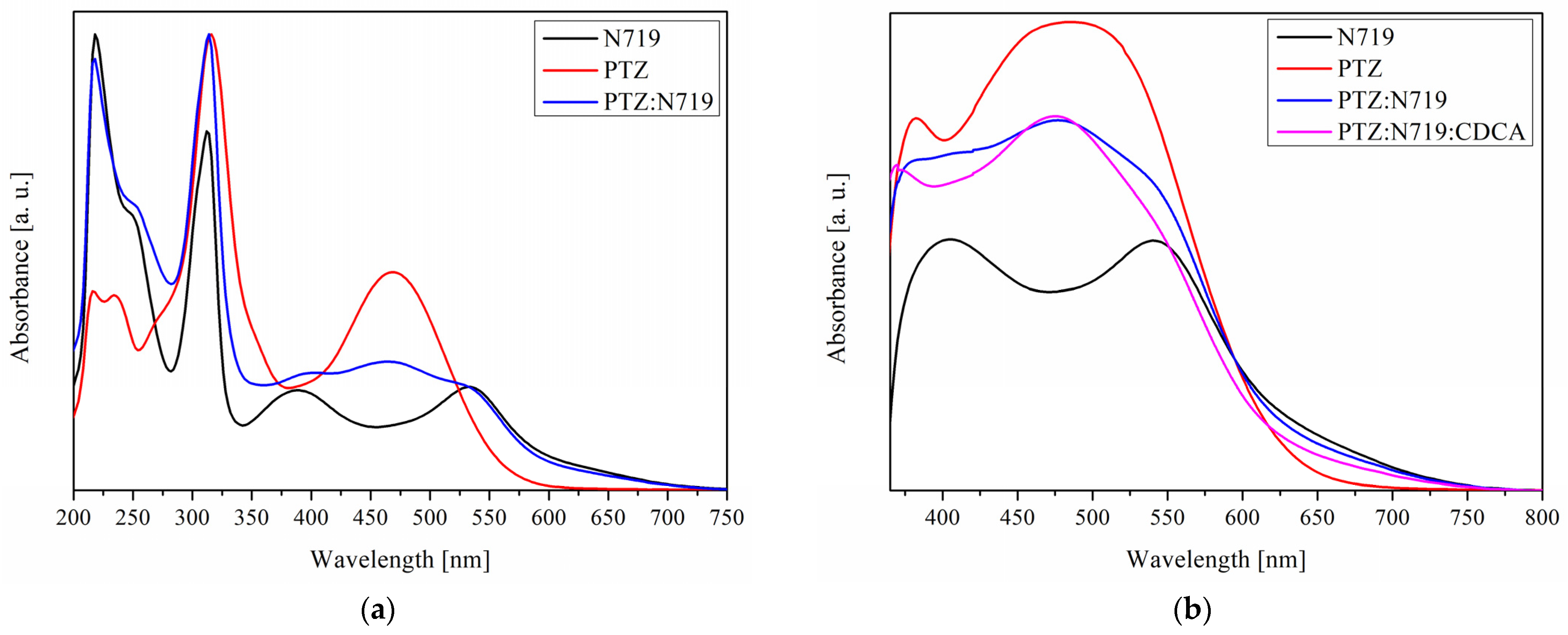
3.1. UV–Vis Properties of Photoanodes
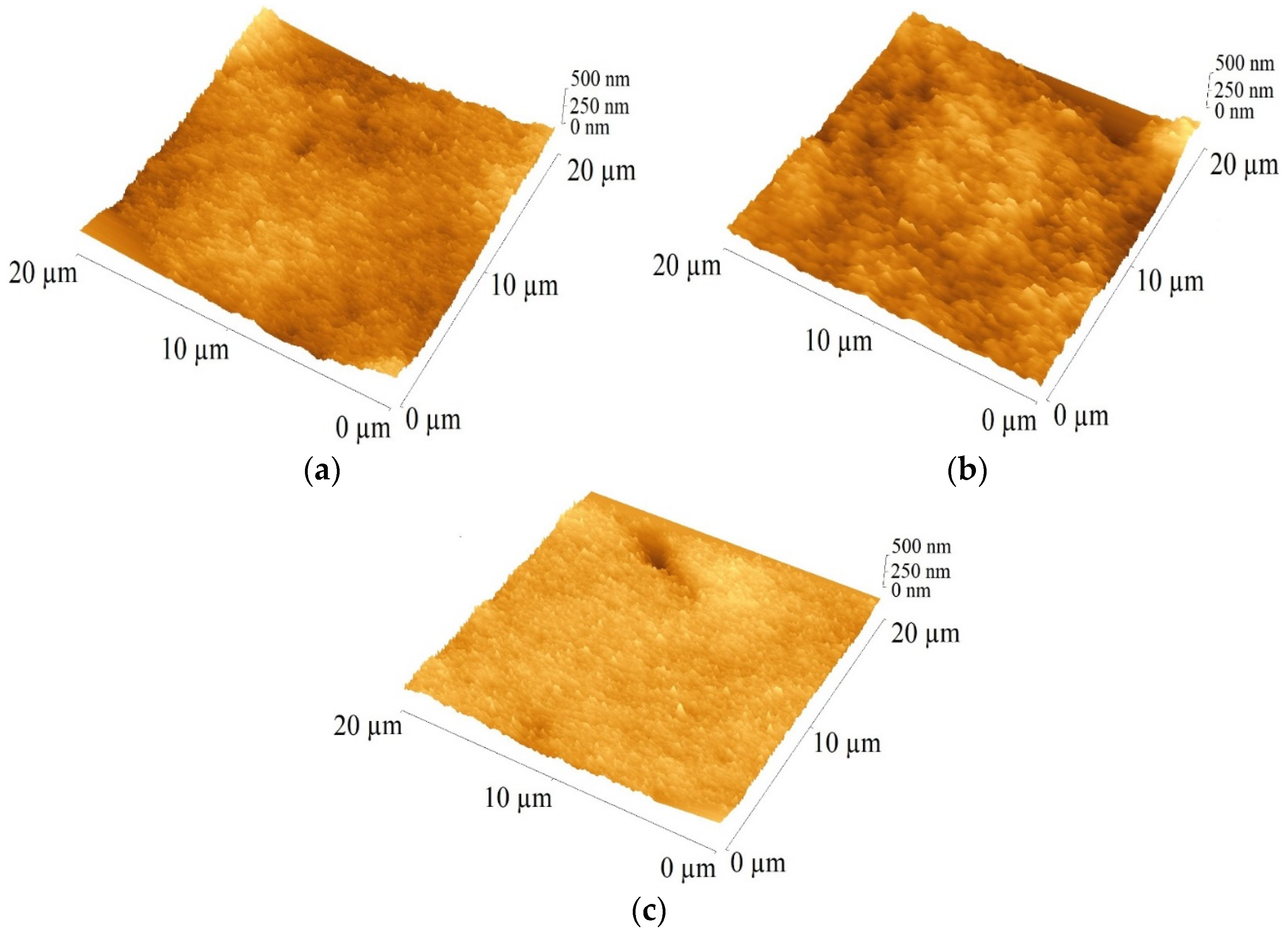
3.2. Photoanode Morphology
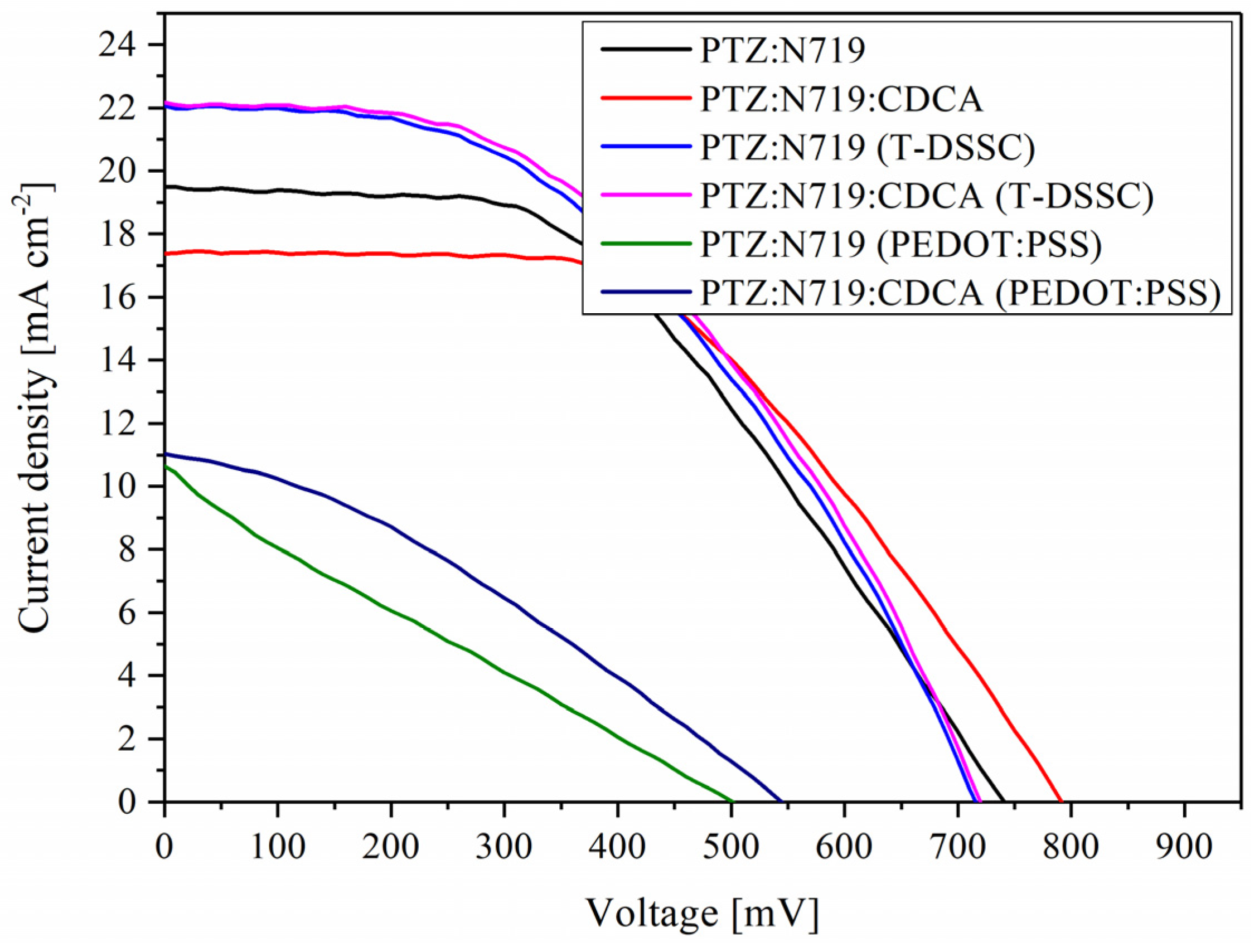
3.3. Photovoltaic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zdyb, A.; Krawczyk, S. Natural Flavonoids as Potential Photosensitizers for Dye-Sensitized Solar Cells. Ecol. Chem. Eng. S 2019, 26, 29–36. [Google Scholar] [CrossRef]
- Shakeel Ahmad, M.; Pandey, A.K.; Abd Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Goncalves, L.M.; De Zea Bermudez, V.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Song, L.; Du, P.; Xiong, J.; Ko, F.; Cui, C. Efficiency enhancement of dye-sensitized solar cells by optimization of electrospun ZnO nanowire/nanoparticle hybrid photoanode and combined modification. Electrochim. Acta 2015, 163, 330–337. [Google Scholar] [CrossRef]
- Barichello, J.; Mariani, P.; Vesce, L.; Spadaro, D.; Citro, I.; Matteocci, F.; Bartolotta, A.; Di Carlo, A.; Calogero, G. Bifacial dye-sensitized solar cells for indoor and outdoor renewable energy-based application. J. Mater. Chem. C 2023, 12, 2317–2349. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Sulaiman, Y. Fully flexible dye-sensitized solar cells photoanode modified with titanium dioxide-graphene quantum dot light scattering layer. Sol. Energy 2020, 212, 332–338. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Mustafa, A.N.; Azam, M.A.; Mahalingam, S.; Chelvanathan, P.; Amin, N. Current advancement of flexible dye sensitized solar cell: A review. Optik 2022, 254, 168089. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Kang, B.; Wang, P.; Qiu, Y. Review of recent progress in solid-state dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 549–573. [Google Scholar] [CrossRef]
- Mahalingam, S.; Nugroho, A.; Floresyona, D.; Lau, K.S.; Manap, A.; Chia, C.H.; Afandi, N. Bio and non-bio materials-based quasi-solid state electrolytes in DSSC: A review. Int. J. Energy Res. 2022, 46, 5399–5422. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Yum, J.H.; Kessler, F.; Gómez García, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cobalt electrolyte/dye interactions in dye-sensitized solar cells: A combined computational and experimental study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Marchena, M.J.; De Miguel, G.; Cohen, B.; Organero, J.A.; Pandey, S.; Hayase, S.; Douhal, A. Real-time photodynamics of squaraine-based dye-sensitized solar cells with iodide and cobalt electrolytes. J. Phys. Chem. C 2013, 117, 11906–11919. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Zhang, Z.; Zhou, X.; Pang, S.; Cui, G. High-performance cobalt selenide and nickel selenide nanocomposite counter electrode for both iodide/triiodide and cobalt(II/III) redox couples in dye-sensitized solar cells. Chin. J. Chem. 2014, 32, 491–497. [Google Scholar] [CrossRef]
- Shaban, S.; Pradhan, S.; Pandey, S.S. Fabrication and Characterization of Bifacial Dye-Sensitized Solar Cells Utilizing Indoline Dye with Iodine- and Cobalt-Based Redox Electrolytes. Phys. Status Solidi Appl. Mater. Sci. 2023, 220, 2300241. [Google Scholar] [CrossRef]
- Yoosuf, M.; Pradhan, S.C.; Sruthi, M.M.; Soman, S.; Gopidas, K.R. Propellar shaped triple bond rigidified D-A-π-A triphenylamine dye as back electron interceptor in iodine and cobalt electrolyte DSSCs under full sun and indoor light. Sol. Energy 2021, 216, 151–163. [Google Scholar] [CrossRef]
- Sarkar, A.; Bera, S.; Chakraborty, A.K. CoNi2S4-reduced graphene oxide nanohybrid: An excellent counter electrode for Pt-free DSSC. Sol. Energy 2020, 208, 139–149. [Google Scholar] [CrossRef]
- Narudin, N.; Ekanayake, P.; Soon, Y.W.; Nakajima, H.; Lim, C.M. Enhanced properties of low-cost carbon black-graphite counter electrode in DSSC by incorporating binders. Sol. Energy 2021, 225, 237–244. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells—An overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Gnida, P.; Amin, M.F.; Pająk, A.K.; Jarząbek, B. Polymers in High-Efficiency Solar Cells: The Latest Reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef]
- Ding, S.; Yang, C.; Yuan, J.; Li, H.; Yuan, X.; Li, M. An overview of the preparation and application of counter electrodes for DSSCs. RSC Adv. 2023, 13, 12309–12319. [Google Scholar] [CrossRef] [PubMed]
- Richhariya, G.; Kumar, A.; Shukla, A.K.; Shukla, K.N.; Meikap, B.C. Effect of Different Counter Electrodes on Power Conversion Efficiency of DSSCs. J. Electron. Mater. 2023, 52, 60–71. [Google Scholar] [CrossRef]
- Nurazizah, E.S.; Aprilia, A.; Risdiana, R.; Safriani, L. Different Roles between PEDOT:PSS as Counter Electrode and PEDOT:Carrageenan as Electrolyte in Dye-Sensitized Solar Cell Applications: A Systematic Literature Review. Polymers 2023, 15, 2725. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Li, L.; Zhao, D.; Chen, S. Increased power conversion efficiency of dye-sensitized solar cells with counter electrodes based on porous polypyrrole. React. Funct. Polym. 2020, 148, 104483. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Sangiorgi, A.; Tarterini, F.; Sanson, A. Molecularly imprinted polypyrrole counter electrode for gel-state dye-sensitized solar cells. Electrochim. Acta 2019, 305, 322–328. [Google Scholar] [CrossRef]
- Karakuş, M.Ö.; Yakışıklıer, M.E.; Delibaş, A.; Ayyıldız, E.; Çetin, H. Anionic and cationic polymer-based quasi-solid-state dye-sensitized solar cell with poly(aniline) counter electrode. Sol. Energy 2020, 195, 565–572. [Google Scholar] [CrossRef]
- Jiao, S.; Wen, J.; Zhou, Y.; Sun, Z.; Liu, Y.; Liu, R. Preparation and Property Studies of Polyaniline Film for Flexible Counter Electrode of Dye-Sensitized Solar Cells by Cyclic Voltammetry. ChemistrySelect 2021, 6, 230–233. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Soman, S. Effect of thickness on charge transfer properties of conductive polymer based PEDOT counter electrodes in DSSC. Results Surf. Interfaces 2021, 5, 100030. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Di Cheng, J.; He, C.X.; Chen, D.; Gu, X.Y.; Wang, S.K.; Gao, X.P.; Sun, G.Z.; Zhang, Z.X.; Pan, X.J.; Pan, X.B.; et al. Effect of ruthenium(II)-bipyridine complex photosensitizer on the panchromatic light absorption and electron transfer in N719-dye sensitized photoanodes. Opt. Mater. 2022, 133, 112924. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, S.; Huang, D.; Zhou, X.; Zhu, X.; Zhao, J.; Chen, T.; Wong, W.Y.; Wong, W.K. Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes. Chem. Mater. 2013, 25, 2146–2153. [Google Scholar] [CrossRef]
- Marszalek, M.; Nagane, S.; Ichake, A.; Humphry-Baker, R.; Paul, V.; Zakeeruddin, S.M.; Grätzel, M. Tuning spectral properties of phenothiazine based donor-π-acceptor dyes for efficient dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 889–894. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, S.; He, J.; Zhang, C.; Zhao, J.; Chen, T.; Wong, W.Y.; Wong, W.K.; Zhu, X. Molecular engineering of simple phenothiazine-based dyes to modulate dye aggregation, charge recombination, and dye regeneration in highly efficient dye-sensitized solar cells. Chem. A Eur. J. 2014, 20, 6300–6308. [Google Scholar] [CrossRef]
- Lin, R.Y.Y.; Wu, F.L.; Li, C.T.; Chen, P.Y.; Ho, K.C.; Lin, J.T. High-Performance Aqueous/Organic Dye-Sensitized Solar Cells Based on Sensitizers Containing Triethylene Oxide Methyl Ether. ChemSusChem 2015, 8, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Slodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of new phenothiazine-based compounds for dye-sensitized solar cells with theoretical insight. Materials 2020, 13, 2292. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Golba, S.; Zimosz, S.; Gnida, P.; Schab-Balcerzak, E. Dyes based on the D/A-acetylene linker-phenothiazine system for developing efficient dye-sensitized solar cells. J. Mater. Chem. C 2019, 7, 5830–5840. [Google Scholar] [CrossRef]
- Fabiańczyk, A.; Gnida, P.; Chulkin, P.; Kula, S.; Filapek, M.; Szlapa-Kula, A.; Janeczek, H.; Schab-Balcerzak, E. Effect of heterocycle donor in 2-cyanoacrylic acid conjugated derivatives for DSSC applications. Sol. Energy 2021, 220, 1109–1119. [Google Scholar] [CrossRef]
- Luo, J.S.; Wan, Z.Q.; Jia, C.Y. Recent advances in phenothiazine-based dyes for dye-sensitized solar cells. Chinese Chem. Lett. 2016, 27, 1304–1318. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Pan, Y.; Li, L.; Hagfeldt, A.; Sun, L. Phenothiazine derivatives for efficient organic dye-sensitized solar cells. Chem. Commun. 2007, 36, 3741–3743. [Google Scholar] [CrossRef]
- Gnida, P.; Libera, M.; Pająk, A.; Schab-Balcerzak, E. Examination of the Effect of Selected Factors on the Photovoltaic Response of Dye-Sensitized Solar Cells. Energy Fuels 2020, 34, 14344–14355. [Google Scholar] [CrossRef]
- Eom, Y.K.; Kang, S.H.; Choi, I.T.; Yoo, Y.; Kim, J.; Kim, H.K. Significant light absorption enhancement by a single heterocyclic unit change in the π-bridge moiety from thieno[3,2-b]benzothiophene to thieno[3,2-b]indole for high performance dye-sensitized and tandem solar cells. J. Mater. Chem. A 2017, 5, 2297–2308. [Google Scholar] [CrossRef]
- Yanagida, M.; Onozawa-Komatsuzaki, N.; Kurashige, M.; Sayama, K.; Sugihara, H. Optimization of tandem-structured dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2010, 94, 297–302. [Google Scholar] [CrossRef]
- Song, L.; Du, P.; Shao, X.; Cao, H.; Hui, Q.; Xiong, J. Effects of hydrochloric acid treatment of TiO2 nanoparticles/nanofibers bilayer film on the photovoltaic properties of dye-sensitized solar cells. Mater. Res. Bull. 2013, 48, 978–982. [Google Scholar] [CrossRef]
- Wang, X.; Bolag, A.; Yun, W.; Du, Y.; Eerdun, C.; Zhang, X.; Bao, T.; Ning, J.; Alata, H.; Ojiyed, T. Enhanced performance of dye-sensitized solar cells based on a dual anchored diphenylpyranylidene dye and N719 co-sensitization. J. Mol. Struct. 2020, 1206, 127694. [Google Scholar] [CrossRef]
- Atli, A.; Atilgan, A.; Yildiz, A. Multi-layered TiO2 photoanodes from different precursors of nanocrystals for dye-sensitized solar cells. Sol. Energy 2018, 173, 752–758. [Google Scholar] [CrossRef]
- Gierszewski, M.; Grądzka, I.; Glinka, A.; Ziółek, M. Insights into the limitations of solar cells sensitized with ruthenium dyes revealed in time-resolved spectroscopy studies. Phys. Chem. Chem. Phys. 2017, 19, 20463–20473. [Google Scholar] [CrossRef]
- Yun, D.J.; Ra, H.; Rhee, S.W. Concentration effect of multiwalled carbon nanotube and poly(3, 4-ethylenedioxythiophene) polymerized with poly(4-styrenesulfonate) conjugated film on the catalytic activity for counter electrode in dye sensitized solar cells. Renew. Energy 2013, 50, 692–700. [Google Scholar] [CrossRef]
- Ooyama, Y.; Harima, Y. Photophysical and electrochemical properties, and molecular structures of organic dyes for dye-sensitized solar cells. ChemPhysChem 2012, 13, 4032–4080. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, M.J.; Eom, Y.K.; Choi, I.T.; Kwon, S.M.; Yoo, Y.; Kim, J.; Kwon, J.; Park, J.H.; Kim, H.K. Porphyrin Sensitizers with Donor Structural Engineering for Superior Performance Dye-Sensitized Solar Cells and Tandem Solar Cells for Water Splitting Applications. Adv. Energy Mater. 2017, 7, 1602117. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, W. Recent progress on tandem structured dye-sensitized solar cells. Front. Optoelectron. 2012, 5, 371–389. [Google Scholar] [CrossRef]


| Dye | ACN:t-BuOH | TiO2 |
|---|---|---|
| λmax [nm] (ε [dm3 mol−1 cm−1]) | λmax [nm] | |
| N719 | 218 (26,333), 250 sh (16,000), 312 (20,667), 388 (6000), 532 (6333) | 405, 541 |
| PTZ | 216 (6667), 234 (6500), 316 (15,000), 468 (7333) | 382, 485 |
| PTZ:N719 | 218 (22,000), 250 sh (14,667), 314 (23,333), 404 (6000), 464 (6667), 534 (5333) | 477, 540 sh |
| PTZ:N719:CDCA | ̶ | 375, 475 |
| Dyes | RMS [nm] |
|---|---|
| N719 | 52 |
| PTZ | 23 |
| PTZ:N719 | 35 |
| PTZ:N719:CDCA | 37 |
| Dye | Electrolyte | Counter Electrode | Voc [mV] | Jsc [mA cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|---|
| N719 | I−/I3− | Pt | 723 ± 10 | 20.19 ± 0.21 | 0.42 ± 0.02 | 6.29 ± 0.05 |
| I−/I3− | PEDOT:PSS | 659 ± 5 | 4.91 ± 0.07 | 0.60 ± 0.01 | 2.05 ± 0.04 | |
| Co2+/3+ | Pt | 620 ± 13 | 1.52 ± 0.09 | 0.64 ± 0.02 | 0.62 ± 0.03 | |
| Co2+/3+ | PEDOT:PSS | 639 ± 12 | 1.05 ± 0.06 | 0.70 ± 0.01 | 0.45 ± 0.02 | |
| PTZ | I−/I3− | Pt | 701 ± 11 | 16.84 ± 0.12 | 0.50 ± 0.01 | 6.25 ± 0.04 |
| I−/I3− | PEDOT:PSS | 570 ± 8 | 9.10 ± 0.15 | 0.24 ± 0.02 | 1.55 ± 0.05 | |
| Co2+/3+ | Pt | 204 ± 12 | 1.91 ± 0.06 | 0.36 ± 0.01 | 0.14 ± 0.01 | |
| Co2+/3+ | PEDOT:PSS | 190 ± 10 | 0.52 ± 0.03 | 0.40 ± 0.01 | 0.04 ± 0.01 |
| Thickness [nm] | Voc [mV] | Jsc [mA cm−2] | FF | PCE [%] |
|---|---|---|---|---|
| 250 | 657 ± 8 | 10.31 ± 0.15 | 0.25 ± 0.01 | 1.73 ± 0.01 |
| 500 | 659 ± 5 | 4.91 ± 0.07 | 0.60 ± 0.01 | 2.05 ± 0.04 |
| 1500 | 524 ± 3 | 5.01 ± 0.10 | 0.35 ± 0.02 | 0.92 ± 0.03 |
| Dye | Counter Electrode | Voc [mV] | Jsc [mA cm−2] | FF | PCE [%] |
|---|---|---|---|---|---|
| PTZ:N719 | Pt | 740 ± 7 | 19.49 ± 0.08 | 0.46 ± 0.01 | 6.87 ± 0.06 |
| PEDOT:PSS | 564 ± 9 | 10.66 ± 0.10 | 0.30 ± 0.01 | 1.67 ± 0.04 | |
| PTZ:N719:CDCA | Pt | 791 ± 6 | 17.44 ± 0.05 | 0.51 ± 0.02 | 7.10 ± 0.03 |
| PEDOT:PSS | 544 ± 10 | 11.04 ± 0.09 | 0.32 ± 0.02 | 1.99 ± 0.02 | |
| PTZ:N719 (T-DSSC) | Pt | 715 ± 20 | 22.05 ± 0.43 | 0.45 ± 0.03 | 7.27 ± 0.26 |
| PTZ:N719:CDCA (T-DSSC) | Pt | 720 ± 24 | 22.15 ± 0.68 | 0.46 ± 0.05 | 7.60 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnida, P.; Schab-Balcerzak, E. Effect of Structural and Material Modifications of Dye-Sensitized Solar Cells on Photovoltaic Performance. Coatings 2024, 14, 837. https://doi.org/10.3390/coatings14070837
Gnida P, Schab-Balcerzak E. Effect of Structural and Material Modifications of Dye-Sensitized Solar Cells on Photovoltaic Performance. Coatings. 2024; 14(7):837. https://doi.org/10.3390/coatings14070837
Chicago/Turabian StyleGnida, Paweł, and Ewa Schab-Balcerzak. 2024. "Effect of Structural and Material Modifications of Dye-Sensitized Solar Cells on Photovoltaic Performance" Coatings 14, no. 7: 837. https://doi.org/10.3390/coatings14070837
APA StyleGnida, P., & Schab-Balcerzak, E. (2024). Effect of Structural and Material Modifications of Dye-Sensitized Solar Cells on Photovoltaic Performance. Coatings, 14(7), 837. https://doi.org/10.3390/coatings14070837






