Expanding (Bio)Conjugation Strategies: Metal-Free Thiol-Yne Photo-Click Reaction for Immobilization onto PLLA Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
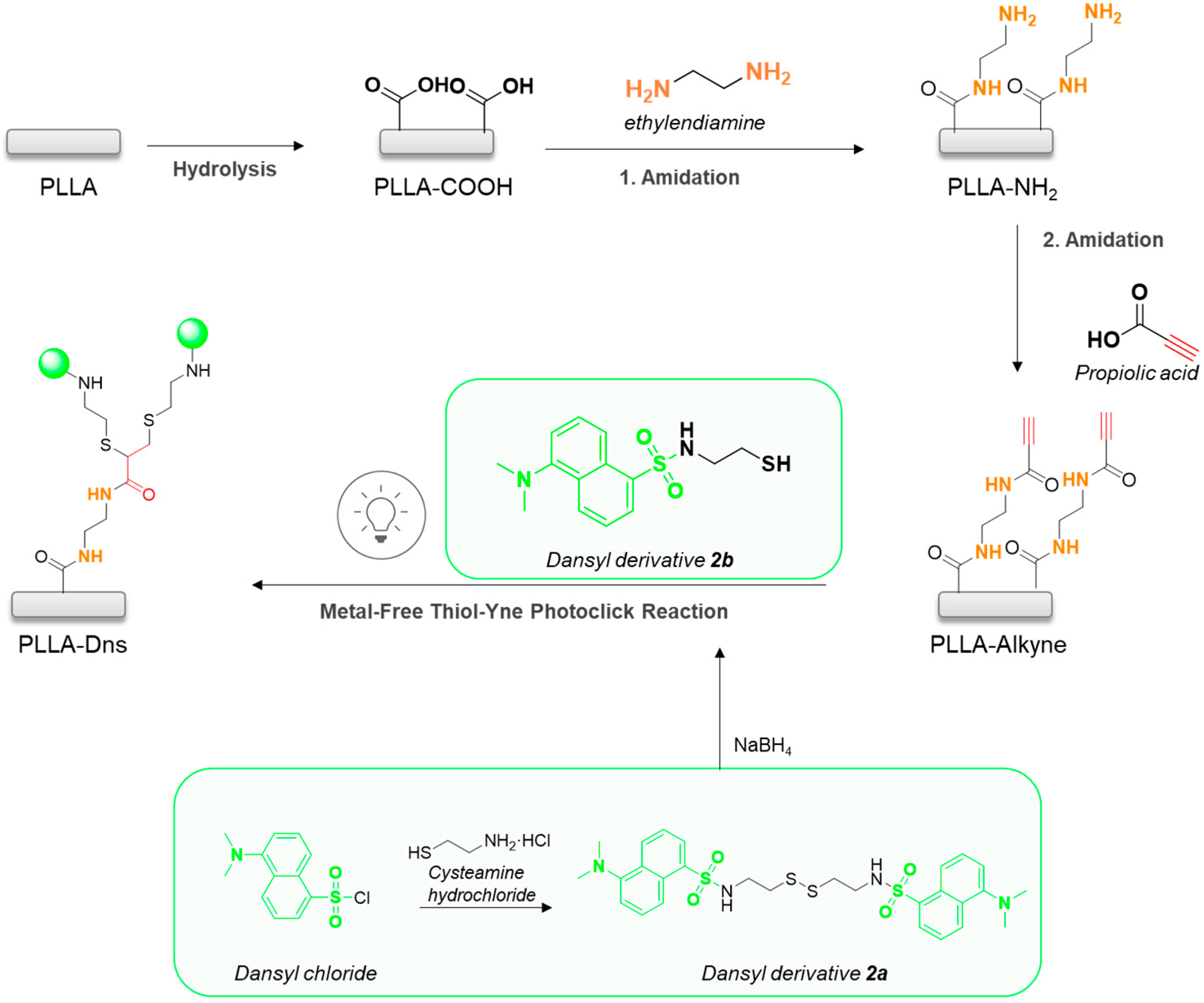
2.2.1. Synthesis of Dansyl Derivative 2a
2.2.2. Synthesis of Dansyl Derivative 2b
2.2.3. Preparation, Hydrolysis, and Amidation of PLLA Films
2.2.4. Immobilization of Dansyl Derivative 2b onto PLLA Films via Metal-Free Thiol-Yne Click Reaction
2.2.5. Nuclear Magnetic Resonance (NMR)
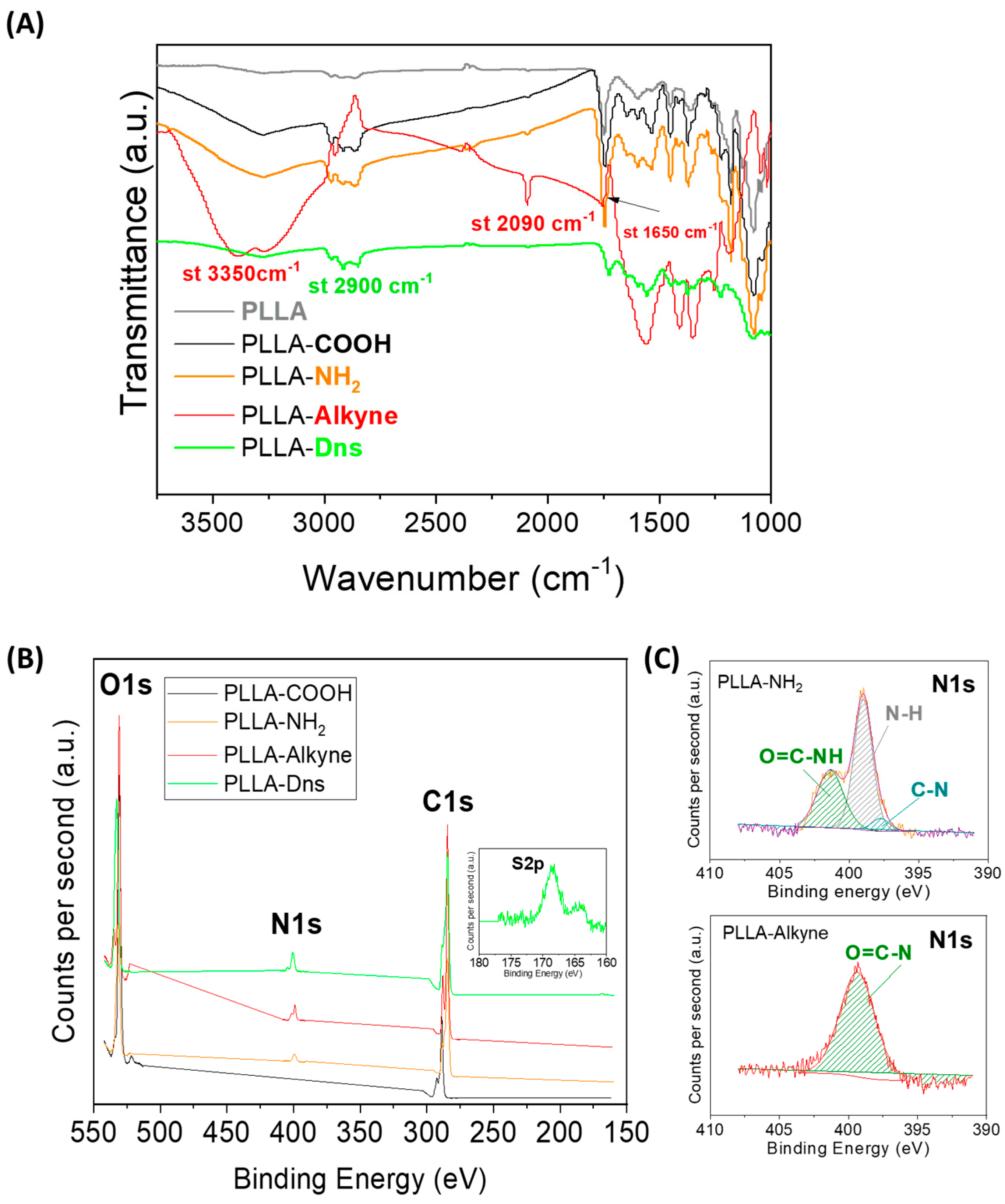
2.2.6. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR)
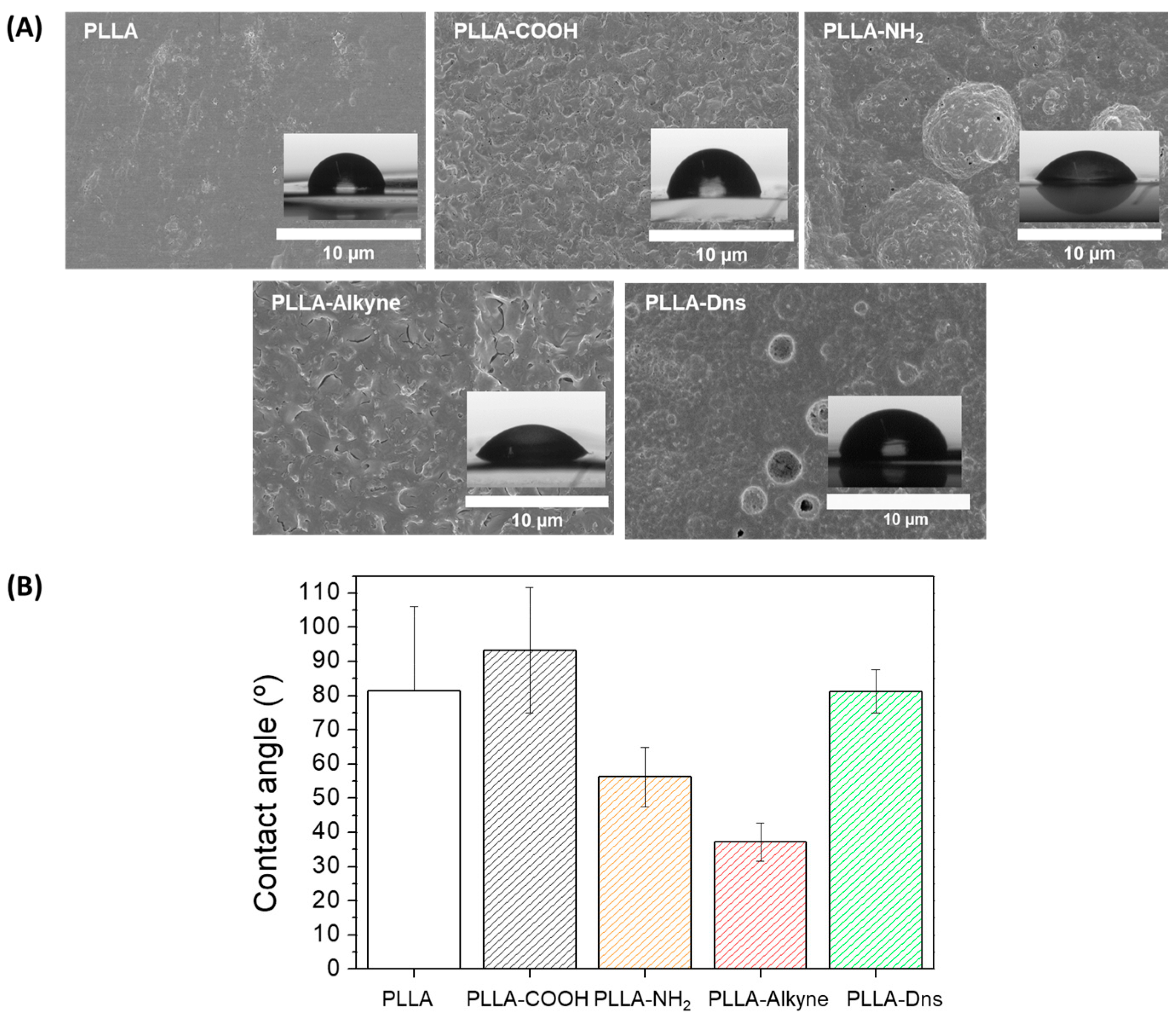
2.2.7. Water Contact Angle (WCA)
2.2.8. Scanning Electron Microscope (SEM)
2.2.9. X-ray Photoelectron Spectroscopy (XPS)
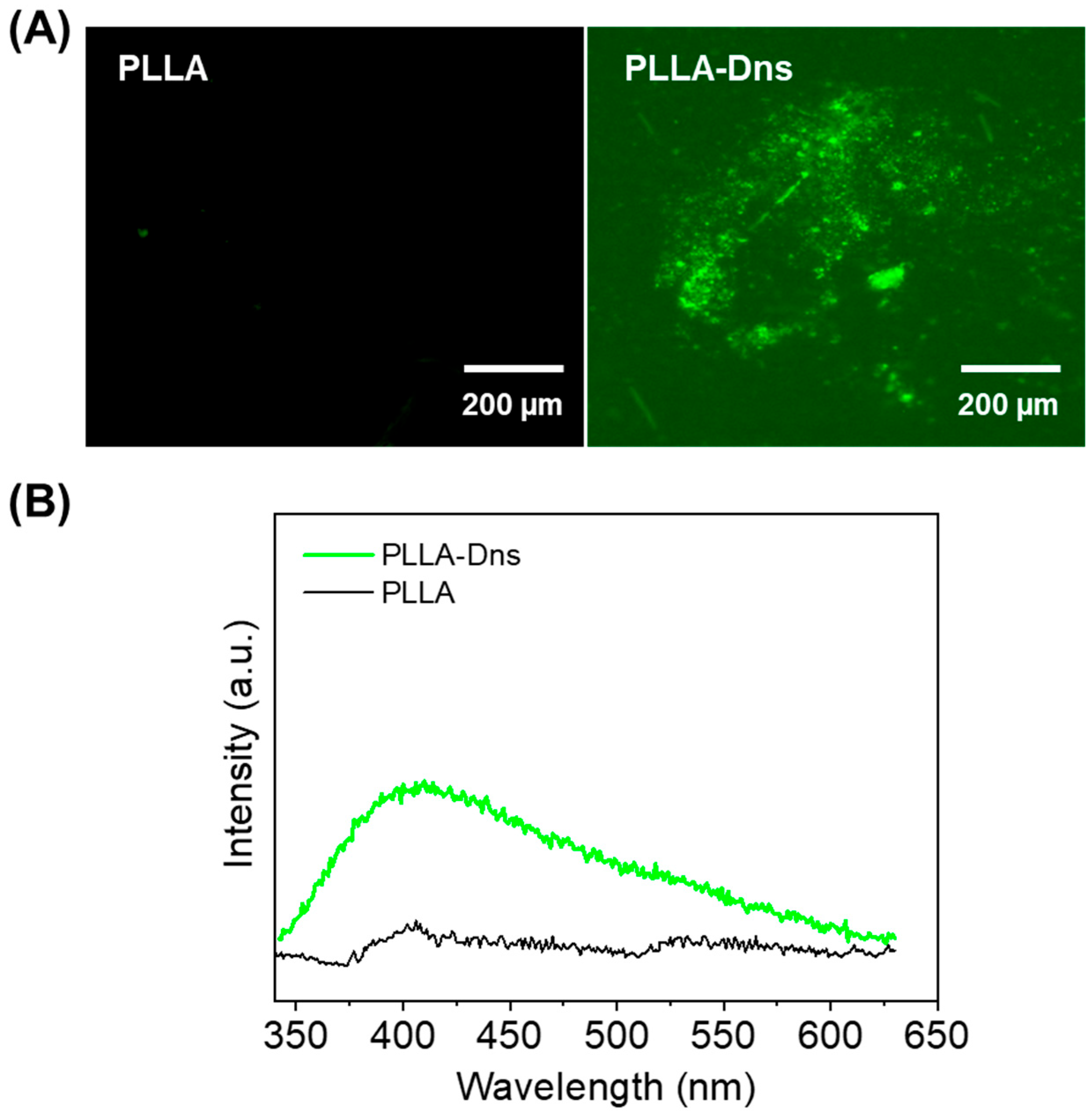
2.2.10. Fluorescence Microscopy
2.2.11. Fluorescence Spectroscopy
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bednarek, C.; Schepers, U.; Thomas, F.; Bräse, S. Bioconjugation in Materials Science. Adv. Funct. Mater. 2024, 34, 2303613. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Elsevier: London, UK, 2008; Volume 11, ISBN 0261-4189. [Google Scholar]
- Algar, W.R. A Brief Introduction to Traditional Bioconjugate Chemistry. In Chemoselective and Bioorthogonal Ligation Reactions; Wiley VCH: Weinheim, Germany, 2017; pp. 1–36. [Google Scholar]
- Debon, A.; Siirola, E.; Snajdrova, R. Enzymatic Bioconjugation: A Perspective from the Pharmaceutical Industry. JACS Au 2023, 3, 1267–1283. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, T.; Kondo, A. Enzyme-mediated methodologies for protein modification and bioconjugate synthesis. Biotechnol. J. 2012, 7, 1137–1146. [Google Scholar] [CrossRef]
- Amna, B.; Ozturk, T. Click chemistry: A fascinating method of connecting organic groups. Org. Commun. 2021, 78, 97–120. [Google Scholar] [CrossRef]
- Barbosa, M.; Martins, C.; Gomes, P. “Click” chemistry as a tool to create novel biomaterials: A short review. U. Porto J. Eng. 2015, 1, 22–34. [Google Scholar] [CrossRef]
- Binder, W.; Kluger, C. Azide/Alkyne-“Click” Reactions: Applications in Material Science and Organic Synthesis. Curr. Org. Chem. 2006, 10, 1791–1815. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Macdougall, L.J.; Mavila, S.; Sinha, J.; Kirkpatrick, B.E.; Anseth, K.S.; Bowman, C.N. Photoclick Chemistry: A Bright Idea. Chem. Rev. 2021, 121, 6915–6990. [Google Scholar] [CrossRef]
- Kumar, G.S.; Lin, Q. Light-Triggered Click Chemistry. Chem. Rev. 2021, 121, 6991–7031. [Google Scholar] [CrossRef]
- Ramil, C.P.; Lin, Q. Photoclick chemistry: A fluorogenic light-triggered in vivo ligation reaction. Curr. Opin. Chem. Biol. 2014, 21, 89–95. [Google Scholar] [CrossRef]
- Lutz, J.F. 1,3-Dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Pickens, C.J.; Johnson, S.N.; Pressnall, M.M.; Leon, M.A.; Berkland, C.J. Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide-Alkyne Cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper (I)—Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Stenzel, M.H. Bioconjugation using thiols: Old chemistry rediscovered to connect polymers with nature’s building blocks. ACS Macro Lett. 2013, 2, 14–18. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; He, D.; Ding, K.; Tian, Q.; Feng, G.; Qin, A.; Tang, B.Z. Bioconjugation and Reaction-Induced Tumor Therapy via Alkynamide-Based Thiol-Yne Click Reaction. Small 2023, 20, 2307309. [Google Scholar] [CrossRef]
- Worch, J.C.; Stubbs, C.J.; Price, M.J.; Dove, A.P. Click Nucleophilic Conjugate Additions to Activated Alkynes: Exploring Thiol-yne, Amino-yne, and Hydroxyl-yne Reactions from (Bio)Organic to Polymer Chemistry. Chem. Rev. 2021, 121, 6744–6776. [Google Scholar] [CrossRef]
- Daglar, O.; Luleburgaz, S.; Baysak, E.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. Nucleophilic Thiol-yne reaction in Macromolecular Engineering: From synthesis to applications. Eur. Polym. J. 2020, 137, 109926. [Google Scholar] [CrossRef]
- Lee, J.H.; Koo, Y.K.; Cho, H.W.; Cha, H.J.; Shin, D.U.; Oh, T.G.; Lee, S.J. Cysteine-loaded pH-responsive liposome/gold nanoparticles as a time-temperature indicator with instantaneous color change. Innov. Food Sci. Emerg. Technol. 2021, 73, 102794. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine as a molecular physiological and pathological biomarker. Biomolecules 2021, 11, 1689. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Massi, A.; Nanni, D. Thiol-yne coupling: Revisiting old concepts as a breakthrough for up-to-date applications. Org. Biomol. Chem. 2012, 10, 3791–3807. [Google Scholar] [CrossRef]
- Feng, W.; Li, L.; Ueda, E.; Li, J.; Heißler, S.; Welle, A.; Trapp, O.; Levkin, P.A. Surface patterning via thiol-yne click chemistry: An extremely fast and versatile approach to superhydrophilic-superhydrophobic micropatterns. Adv. Mater. Interfaces 2014, 1, 1400269. [Google Scholar] [CrossRef]
- Liang, H.; Yin, D.; Shi, L.; Liu, Y.; Hu, X.; Zhu, N.; Guo, K. Surface modification of cellulose via photo-induced click reaction. Carbohydr. Polym. 2023, 301, 120321. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Scott, T.F.; Kloxin, C.J.; Anseth, K.S.; Bowman, C.N. Thiol-Yne Photopolymerizations: Novel Mechanism, Kinetics, and Step-Growth Formation of Highly Cross-Linked Networks. Macromolecules 2009, 42, 211–217. [Google Scholar] [CrossRef]
- Sradha, S.; Sariga, A.; George, L.; Varghese, A. Advancements in thiol-yne click chemistry: Recent trends and applications in polymer synthesis and functionalization. Mater. Today Chem. 2024, 38, 102112. [Google Scholar] [CrossRef]
- Lowe, A.B.; Hoyle, C.E.; Bowman, C.N. Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 20, 4745–4750. [Google Scholar] [CrossRef]
- Fu, X.; Qin, A.; Tang, B.Z. Dynamic covalent polymers generated from X-yne click polymerization. J. Polym. Sci. 2023, 62, 787–798. [Google Scholar] [CrossRef]
- Seyednejad, H.; Ghassemi, A.H.; Van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Functional aliphatic polyesters for biomedical and pharmaceutical applications. J. Control. Release 2011, 152, 168–176. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; Carrubba, V. La Poly-L-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153–1182. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Taib, N.A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A Review on Poly Lactic Acid (PLA) as a Biodegradable Polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2017, 107, 247–276. [Google Scholar] [CrossRef]
- Agrawal, R.; Kumar, A.; Mohammed, M.K.A.; Singh, S. Biomaterial types, properties, medical applications, and other factors: A recent review. J. Zhejiang Univ. Sci. A 2023, 24, 1027–1042. [Google Scholar] [CrossRef]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Guo, C.; Xiang, M.; Dong, Y. Surface modification of poly (lactic acid) with an improved alkali-acid hydrolysis method. Mater. Lett. 2015, 140, 144–147. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Diaz-Galbarriatu, M.; Pérez-Álvarez, L.; Moreno-Benítez, I.; Vilas-Vilela, J.L. Strategies to Enhance Biomedical Device Performance and Safety: A Comprehensive Review. Coatings 2023, 13, 1981–2005. [Google Scholar] [CrossRef]
- Jeznach, O.; Kołbuk, D.; Marzec, M.; Bernasik, A.; Sajkiewicz, P. Aminolysis as a surface functionalization method of aliphatic polyester nonwovens: Impact on material properties and biological response. RSC Adv. 2022, 12, 11303–11317. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; García-García, A.; Diaz-Galbarriatu, M.; Vilas-Vilela, J.L.; Moreno-Benítez, I. An easy and simple method for the immobilization of dyes through click reactions: Activated alkyne, copper not needed. RSC Adv. 2024, 14, 14289–14295. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Diaz-Galbarriatu, M.; Sola-Llano, R.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Moreno-Benitez, I. Catalyst-Free Amino-Yne Click Reaction: An Efficient Way for Immobilizing Amoxicillin onto Polymeric Surfaces. Polymers 2024, 16, 246. [Google Scholar] [CrossRef]
- Fu, X.; Qin, A.; Tang, B.Z. X-yne click polymerization. Aggregate 2023, 4, e350. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Ruiz-Rubio, L.; Hernáez-Laviña, E.; Vilas-Vilela, J.L.; Moreno-Benítez, M.I. Poly(L-lactide)-based anti-inflammatory responsive surfaces for surgical implants. Polymers 2021, 13, 34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bodón, J.; Diaz-Galbarriatu, M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Moreno-Benítez, I. Expanding (Bio)Conjugation Strategies: Metal-Free Thiol-Yne Photo-Click Reaction for Immobilization onto PLLA Surfaces. Coatings 2024, 14, 839. https://doi.org/10.3390/coatings14070839
Sánchez-Bodón J, Diaz-Galbarriatu M, Pérez-Álvarez L, Vilas-Vilela JL, Moreno-Benítez I. Expanding (Bio)Conjugation Strategies: Metal-Free Thiol-Yne Photo-Click Reaction for Immobilization onto PLLA Surfaces. Coatings. 2024; 14(7):839. https://doi.org/10.3390/coatings14070839
Chicago/Turabian StyleSánchez-Bodón, Julia, Maria Diaz-Galbarriatu, Leyre Pérez-Álvarez, José Luis Vilas-Vilela, and Isabel Moreno-Benítez. 2024. "Expanding (Bio)Conjugation Strategies: Metal-Free Thiol-Yne Photo-Click Reaction for Immobilization onto PLLA Surfaces" Coatings 14, no. 7: 839. https://doi.org/10.3390/coatings14070839






