CMC-Ca(OH)2-TiO2 Nanocomposite for Paper Relics Multifunctional Restoration: Strengthening, Deacidification, UV Effect Resistance, and Antimicrobial Protection
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Synthesis of CMC-Ca(OH)2-TiO2 Nanohybrids
2.3. Paper Strengthening Methods
2.4. Tensile Strength Testing
2.5. UV Effect
2.6. Light Transmittance Testing
2.7. Antimicrobial Experiment
2.7.1. Preparation of Culture Medium
2.7.2. Fungal Preparation
2.7.3. Antimicrobial Effectiveness Testing Using the Oxford Cup Method [42,43]
2.8. Color Difference Testing
2.9. Instruments
3. Results and Discussion
3.1. Structural Characterization of CMC-Ca(OH)2-TiO2
3.2. Performance of CMC-Ca(OH)2-TiO2
Enhance Mechanical Strength Performance
3.3. Anti-UV Effect Performance
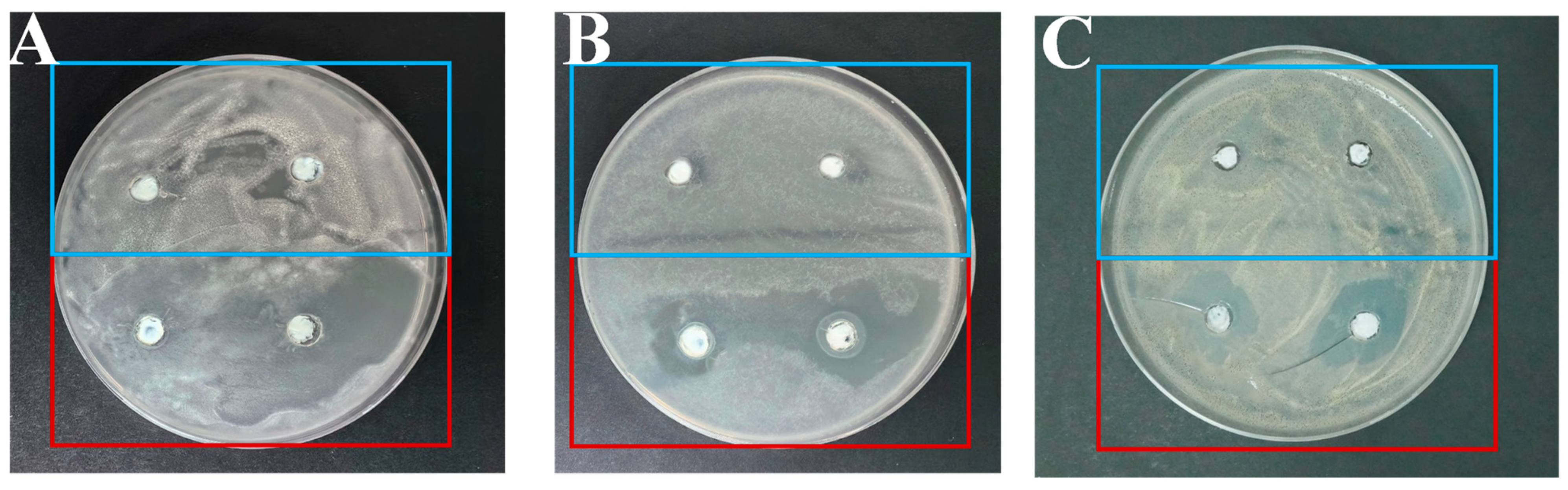
Antimicrobial Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vohrer, U.; Trick, I.; Bernhardt, J.; Oehr, C.; Brunner, H. Plasma treatment—An increasing technology for paper restoration? Surf. Coat. Technol. 2001, 142, 1069–1073. [Google Scholar] [CrossRef]
- Jin, S.S.; Wang, S.N. Recent research and prospect of deacidifying materials for paper and paper-based cultural relics. Acta Chim. Sin. 2023, 81, 309–318. [Google Scholar] [CrossRef]
- Yan, C.S.; Huang, C.; Han, S.T.; Han, X.L.; Ying, C.N.; Du, Y.D. Review on scientific detection technologies for ancient paper relics. Chin. Opt. 2020, 13, 936–964. [Google Scholar]
- Brandt, N.N.; Chikishev, A.Y.; Itoh, K.; Rebrikova, N.L. Atr-ftir and ft raman spectroscopy and laser cleaning of old paper samples with foxings. Laser Phys. 2009, 19, 483–492. [Google Scholar] [CrossRef]
- Balakhnina, I.A.; Brandt, N.N.; Chikishev, A.Y.; Pelivanov, I.M.; Rebrikova, N.L. Optoacoustic measurements of the porosity of paper samples with foxings. Appl. Phys. Lett. 2012, 101, 174101. [Google Scholar] [CrossRef]
- Balakhnina, I.A.; Brandt, N.N.; Chikishev, A.Y.; Rebrikova, N.L. Raman microspectroscopy of old paper samples with foxing. Appl. Spectrosc. 2014, 68, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Du, B.B.; Pan, L.W.; Zheng, M.Y.; Zhang, B.J.; Hu, Y.L. Preparation of agnps/oregano essential oil composite film and its antibacterial application in the conservation of paper relics. Inorg. Chem. Commun. 2024, 160, 112008. [Google Scholar] [CrossRef]
- Chen, Q.; Wen, W.Y.; Qiu, F.X.; Xu, J.C.; Yu, H.Q.; Chen, M.L.; Yang, D.Y. Preparation and application of modified carboxymethyl cellulose si/polyacrylate protective coating material for paper relics. Chem. Pap. 2016, 70, 946–959. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, P.; Huang, Y.Y.; Zhang, H.B. Nanocomposites composed of modified natural polymer and inorganic nanomaterial for safe, high-efficiency, multifunctional protection of paper-based relics. Sci. China-Technol. Sci. 2023, 66, 2225–2236. [Google Scholar] [CrossRef]
- Wang, S.N.; Yang, X.; Li, Y.H.; Gao, B.X.; Jin, S.S.; Yu, R.; Zhang, Y.H.; Tang, Y. Colloidal magnesium hydroxide nanoflake: One-step surfactant-assisted preparation and paper-based relics protection with long-term anti-acidification and flame-retardancy. J. Colloid Interf. Sci. 2022, 607, 992–1004. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.E.; Yao, J.J.; Jin, S.T.; Tang, Y. Chemistry directs the conservation of paper cultural relics. Polym. Degrad. Stab. 2023, 207, 110228. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yang, W.; Zhang, K.; Chen, H.H.; Zhao, G.G.; Wang, Y.Y. Application of inorganic chemistry in restoration and protection of cultural relics. Chin. J. Inorg. Chem. 2018, 34, 2127–2134. [Google Scholar]
- Zhu, J.M.; Li, X.H.; Yan, J.; Zhao, X.C.; Cao, Y.J.; Camaiti, M.; Li, T.; Wei, B.Q. Tuning the dimensionality of nano CA(OH)2 with surfactants for wall painting consolidation. Chemnanomat 2019, 5, 1152–1158. [Google Scholar] [CrossRef]
- Xu, J.C.; Zhang, T.; Jiang, Y.; Chen, Q.; Yang, D.Y.; Qiu, F.X.; Yu, Z.P. Synthesis of microcrystalline cellulose/TiO2/fluorine/styrene-acrylate coatings and the application for simulated paper cultural relic protection. Cellulose 2020, 27, 6549–6562. [Google Scholar] [CrossRef]
- Mao, T.; Li, X.F.; Shi, X.T.; Hu, Y.; Zha, J.Y.; Luo, X.K.; Cheng, Y.L. Study on the performance of acrylic polyurethane for the protection of handwriting on paper relics. Coatings 2023, 13, 822. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.J.; Yan, Y.; Huang, X.Z.; Zhang, Y.H.; Tang, Y.; Yang, Y.L. Reversible deacidification and preventive conservation of paper-based cultural relics by mineralized bacterial cellulose. ACS Appl. Mater. Interfaces 2024, 16, 13091–13102. [Google Scholar] [CrossRef]
- Lu, D.M.; Diao, C.Y. Relic protection and digitization technology. In Proceedings of the IEEE International Conference on Systems, Man and Cybernetics, The Hague, The Netherlands, 10–13 October 2004. [Google Scholar]
- Balakhnina, I.A.; Brandt, N.N.; Dedova, A.E.; Zagladin, A.A.; Chikishev, A.Y. Laser ablation as a method for microsampling of paint layers. Laser Phys. 2022, 32, 066001. [Google Scholar] [CrossRef]
- Balakhnina, I.A.; Zagladin, A.A.; Chikishev, A.Y.; Brandt, N.N. Laser ablation microsampling of copper phthalocyanine blue by the second-harmonic radiation of nd:Yag laser. Laser Phys. 2023, 33, 105401. [Google Scholar] [CrossRef]
- Balakhnina, I.; Brandt, N.; Chikishev, A.; Rebrikova, N.; Yurchuk, Y. Laser ablation of paper: Raman identification of products. Appl. Phys. A-Mater. Sci. Process. 2014, 117, 1865–1871. [Google Scholar] [CrossRef]
- Brandt, N.N.; Brovko, O.O.; Chikishev, A.Y.; Itoh, K.; Lebedenko, S.I.; Polshakov, V.I.; Sakodynskaya, I.K. Laser control of the structure of a photosensitive substrate for enzymatic reaction. Laser Phys. 2007, 17, 1262–1265. [Google Scholar] [CrossRef]
- Brandt, N.N.; Chikishev, A.Y. Laser raman spectrometer for the studies of biomolecules with monitoring temperature of the samples. Laser Phys. 2002, 12, 647–652. [Google Scholar]
- Brandt, N.N.; Chikishev, A.Y.; Dolgovskii, V.I.; Lebedenko, S.I. Laser raman spectroscopy of the effect of solvent on the low-frequency oscillations of organic molecules. Laser Phys. 2007, 17, 1133–1137. [Google Scholar] [CrossRef]
- Xu, J.C.; Jiang, Y.; Zhang, T.; Chen, Q.; Yang, D.Y.; Qiu, F.X. Preparation of vinyl acetate/acrylate emulsion modified with carboxymethyl cellulose and fluorine for paper relic protection. J. Dispers. Sci. Technol. 2022, 43, 804–813. [Google Scholar] [CrossRef]
- Liu, J.J.; Xing, H.P.; Wang, J.L.; Cao, J.; Chao, X.L.; Jia, Z.H.; Li, Y.H. A new reinforcement method for the conservation of fragile, double-sided, printed paper cultural relics. Herit. Sci. 2021, 9, 123. [Google Scholar] [CrossRef]
- Kroftová, K.; Škoda, D.; Kuřitka, I.; Kubát, J. Technology of preparation of barium and magnesium hydroxide nanodispersion and possibilities of their use in monument care. Acta Polytech. CTU Proc. 2019, 21, 21–23. [Google Scholar] [CrossRef]
- Gu, W.T.; Wei, Y.F.; Liu, B.B.; Hu, L.Y.; Zhong, L.; Chen, G.K. Polyacrylic acid-functionalized graphene@Ca(OH)2 nanocomposites for mural protection. Acs Omega 2022, 7, 12424–12429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.M.; Zhang, P.Y.; Ding, J.H.; Dong, Y.; Cao, Y.J.; Dong, W.Q.; Zhao, X.C.; Li, X.H.; Camaiti, M. Nano Ca(OH)2: A review on synthesis, properties and applications. J. Cult. Herit. 2021, 50, 25–42. [Google Scholar] [CrossRef]
- Zhu, J.M.; Li, X.H.; Zhang, Y.Y.; Wang, J.; Wei, B.Q. Graphene-enhanced nanomaterials for wall painting protection. Adv. Funct. Mater. 2018, 28, 1803872. [Google Scholar] [CrossRef]
- Giorgi, R.; Dei, L.; Ceccato, M.; Schettino, C.; Baglioni, P. Nanotechnologies for conservation of cultural heritage: Paper and canvas deacidification. Langmuir 2002, 18, 8198–8203. [Google Scholar] [CrossRef]
- Xu, J.C.; Zhang, T.; Jiang, Y.; Yang, D.Y.; Qiu, F.X.; Chen, Q.; Yu, Z.P. Preparation of self-healing acrylic copolymer composite coatings for application in protection of paper cultural relics. Polym. Eng. Sci. 2020, 60, 288–296. [Google Scholar] [CrossRef]
- Xu, J.C.; Zhang, T.; Zhang, X.Y.; Jiang, Y.; Yang, D.Y.; Qiu, F.X.; Yu, Z.P. Preparation of polymeric material containing uv absorber for application in paper-based relics protection. Polym.-Plast. Technol. Mater. 2020, 59, 536–545. [Google Scholar] [CrossRef]
- Balakhnina, I.A.; Brandt, N.N.; Chikishev, A.Y.; Shpachenko, I.G. Single-pulse two-threshold laser ablation of historical paper. Laser Phys. Lett. 2018, 15, 065605. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzlito, F.; Scarano, A. Cytotoxic and bacteriostatic activity of nanostructured TiO2 coatings. Pol. J. Microbiol. 2016, 65, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Xing, H.P.; Zhou, Y.J.; Chao, X.L.; Li, Y.H.; Hu, D.D. An essential role of polymeric adhesives in the reinforcement of acidified paper relics. Polymers 2022, 14, 207. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhao, J.C.; Wang, S.N.; Dai, Z.Y.; Qin, S.T.; Mei, S.L.; Zhang, W.L.; Guo, R.Q. Carbon quantum dots for long-term protection against uv degradation and acidification in paper-based relics. ACS Appl. Mater. Interfaces 2024, 16, 5009–5018. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Ismagilov, Z.R.; Tsikoza, L.T.; Shikina, N.V.; Zarytova, V.F.; Zinoviev, V.V.; Zagrebelnyi, S.N. Synthesis and stabilization of nano-sized titanium dioxide. Russ. Chem. Rev. 2009, 78, 873–885. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Song, H.; Lin, D.; Wang, Y. Toxic effects of nano-TiO2 in bivalves-a synthesis of meta-analysis and bibliometric analysis. J. Environ. Sci. 2021, 104, 188–203. [Google Scholar] [CrossRef]
- GB/T 12914-2008; Paper and Board-Determination of Tensile Properties. Standards Press of China: Beijing, China, 2008.
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.X.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Xu, Y.R.; Niu, Y.F.; Wu, C.H.; Yan, J.; Rao, X.P.; Shi, Z.J.; Xu, K.M.; Algadi, H.; Guo, Z.H. Synthesis, characterization, antifungal properties of quaternary ammonium salts derived from natural rosin. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT-Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Hu, W.; Shi, S.; Shen, W.; Zhang, X.; Wang, H. In situ synthesis of cds nanoparticles on bacterial cellulose nanofibers. Carbohydr. Polym. 2009, 76, 509–512. [Google Scholar] [CrossRef]
- Miliani, C.; Monico, L.; Melo, M.J.; Fantacci, S.; Angelin, E.M.; Romani, A.; Janssens, K. Photochemistry of artists’ dyes and pigments: Towards better understanding and prevention of colour change in works of art. Angew. Chem. Int. Ed. Engl. 2018, 57, 7324–7334. [Google Scholar] [CrossRef]
- Cao, J.; Liu, X.; Wang, J.; Chen, H.; Liu, D.; Li, J.; Mai, B. Analysis of microbial diversity and its degradation function in wooden piles at shahe ancient bridge site in Xi’an and protection measures. Herit. Sci. 2024, 12, 33. [Google Scholar] [CrossRef]
- Mai, B.; Liu, N.; Liu, X.; Teri, G.; Liu, P.; Wang, J.; Li, Y.; Cao, J. Mould prevention of archive packaging based microenvironment intervention and regulation. J. Cult. Herit. 2022, 57, 16–25. [Google Scholar] [CrossRef]
- Ariafar, A.A.; Afsharpour, M.; Samanian, K. Use of TiO2/chitosan nanoparticles for enhancing the preservative effects of carboxymethyl cellulose in paper-art-works against biodeterioration. Int. Biodeterior. Biodegrad. 2018, 131, 67–77. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, R.; Wu, P.; Quan, M. CMC-Ca(OH)2-TiO2 Nanocomposite for Paper Relics Multifunctional Restoration: Strengthening, Deacidification, UV Effect Resistance, and Antimicrobial Protection. Coatings 2024, 14, 851. https://doi.org/10.3390/coatings14070851
Li J, Ma R, Wu P, Quan M. CMC-Ca(OH)2-TiO2 Nanocomposite for Paper Relics Multifunctional Restoration: Strengthening, Deacidification, UV Effect Resistance, and Antimicrobial Protection. Coatings. 2024; 14(7):851. https://doi.org/10.3390/coatings14070851
Chicago/Turabian StyleLi, Jing, Ruiwen Ma, Peng Wu, and Min Quan. 2024. "CMC-Ca(OH)2-TiO2 Nanocomposite for Paper Relics Multifunctional Restoration: Strengthening, Deacidification, UV Effect Resistance, and Antimicrobial Protection" Coatings 14, no. 7: 851. https://doi.org/10.3390/coatings14070851



