Abstract
Ni-based coating, as an excellent anti-corrosion material, is widely used in the petroleum field and has become a research hotspot in recent years. With the continuous progress of the petrochemical industry, especially in the carbon capture, utilization, and storage and enhanced oil recovery (CCUS-EOR) systems, Ni-based coatings face significant challenges. Based on the latest research status, this paper describes the influence factors of Ni-based coatings and the applicability of different Ni-based coatings. Research shows that CO2 and H2S are the key factors affecting corrosion. With the increase in CO2 and H2S content in the environment, the corrosion rate of carbon steel will be accelerated. When the CO2 content reaches a certain critical value, further increasing the CO2 content will reduce the corrosion rate. The corrosion mechanism of carbon steel under the combined action of CO2 and H2S was also analyzed. At a high CO2/H2S partial pressure ratio, the corrosion process is dominated by CO2 corrosion, and with the increase in the H2S/CO2 partial pressure ratio, the corrosion process shifts to H2S corrosion control. The application of Ni-P protective coatings in this corrosive environment has been investigated. A surface Ni-P coating is extremely hard, wear-resistant, and corrosion-resistant. It can withstand the corrosion of CO2, high salinity, and other media while preventing electrolyte penetration. It exhibits strong corrosion resistance in NaCl solution and can help prevent scaling. However, in the presence of CO2-H2s, it has been discovered that due to inherent faults in Ni-P, the corrosion phenomenon may be exacerbated by the action of H2S, and its protection ability has to be further investigated. The mechanical properties and corrosion resistance of the coating can be enhanced by adding particles to the coating, and four new coating systems, Ni-Fe-P, Ni-Cu-P, Ni-W-P, and Ni-W-P-nSiO2, were introduced. Finally, the future development focus and prospect of CO2 corrosion protection coatings under harsh conditions are prospected.
1. Introduction
Petroleum and petrochemical companies have recently linked themselves with the national significant strategic choice of “carbon peaking and carbon neutralization”. As a result, they combined carbon collection, utilization, and storage technology with CO2 oil displacement technology. Through autonomous experience, these businesses have gradually pioneered a revolutionary development mode based on the integration of CO2 capture, storage, and oil displacement. This approach is a successful strategy for achieving large-scale “carbon emission reduction” while actively supporting low-carbon and green development. Ultimately, these companies have made significant contributions to the transformation of national energy security and the achievement of the dual carbon goal [1,2].
The spontaneous combustion coal chemical sector provides the majority of the gas used in the CO2 flooding injection production system [3]. The high salinity of wellbore fluid, tubing steel fatigue stress, dissolved oxygen, acid impurities in the gas source, the uniqueness of the supercritical phase state, bacterial microorganisms, and other factors all combine to cause serious downhole tubing steel corrosion, scaling, failure, and fracture. This will endanger the safety of oil production in the field because CO2 will lower the pH of the solution and encourage local positive dissolution and cathodic hydrogen evolution process [4,5]. H2S not only causes electrochemical corrosion, but also it is particularly hazardous as a potent hydrogen-permeating medium that plays a toxic role. It can prevent hydrogen atoms from forming hydrogen molecules and speed the process of the diffusion and dissolution of hydrogen atoms in the metal matrix, leading to tubing steel fracture and failure. The corrosion mechanism and failure control of tubing steel columns under complex working conditions have always been a research hotspot and major challenge internationally, and they are also key issues for CO2 flooding to increase oil storage and production in the petroleum industry. This is a complex system engineering problem that urgently requires targeted research.
Coating protection is one of the most effective techniques for improving the mechanical and chemical characteristics of tubular steels among the numerous corrosion control strategies. Ni-based coatings have emerged as a hotspot for industrial research due to their exceptional resistance to wear, corrosion, and high temperatures. Sun et al. [6,7] investigated the corrosion resistance of metal Ni-P coatings in a supercritical CO2 environment contaminated with H2O-O2-NO2. The findings demonstrated that the protective property of the coating was persistent and that the corrosion inhibition rate may exceed 80% in a variety of gas conditions. Xu et al. [8] suggested that Ni-P coating had a dense cell structure, and its tiny porosity hindered the erosion of corrosive media. This improved the corrosion resistance of the matrix and had excellent corrosion resistance in a Cl−/H2S environment. Researchers have conducted a lot of in-depth research on Ni-based coatings and made significant progress [9]. They have developed into a functional chemical coating as the center of the surface treatment process, in order to improve its protection performance. For example, P can enhance the ability of a coating to shield against H2S/CO2 corrosion. Fe, W [10], WC [11], SiC [12], Al3O2 [13,14], and TiO2 [15], and other ceramic phases enhance the wear resistance, corrosion resistance, high-temperature resistance, and chemical stability of a coating. Cu and SiO2 can improve the hydrophobicity of a coating. The addition of trace elements, such as CeO2 or ZrO2 might promote the uniform distribution of hard particles and the second phase [16,17,18]. In this process, three main coatings, Ni-P, Ni-W, and Ni-W-P, are formed.
With the development of CO2 flooding in ultra-deep wells, higher requirements are put forward for the high temperature and acid resistance of Ni-based coatings. At present, there are few reports about the corrosion behavior of Ni-based coatings in oil and gas fields. Based on the relevant literature in recent years, the corrosion phenomena and influencing factors of downhole tubing steels in CO2 flooding and the corrosion behavior of Ni-based coatings are reviewed in this paper.
2. Corrosion of CO2 Flooding Tubing Steels
In the carbon capture utilization storage and oil displacement (CCUS-EOR) process, the collected CO2 is often compressed into a liquid or supercritical state before being transmitted via a tubular steel line. This avoids the two-phase flow condition and increases the density of CO2. There will unavoidably be H2S, O2, SOx, NOx, H2O, and other contaminants in the collected CO2 due to the limitations of the gas source, the capture procedure, and the financial cost. These contaminants will affect the steel tubing’s ability to corrode. In oil and gas fields, the high pressure and temperature environment can lead to corrosion, mechanical property deterioration, tubing steel failure, and unsafe production.
2.1. Influence of CO2 Partial Pressure
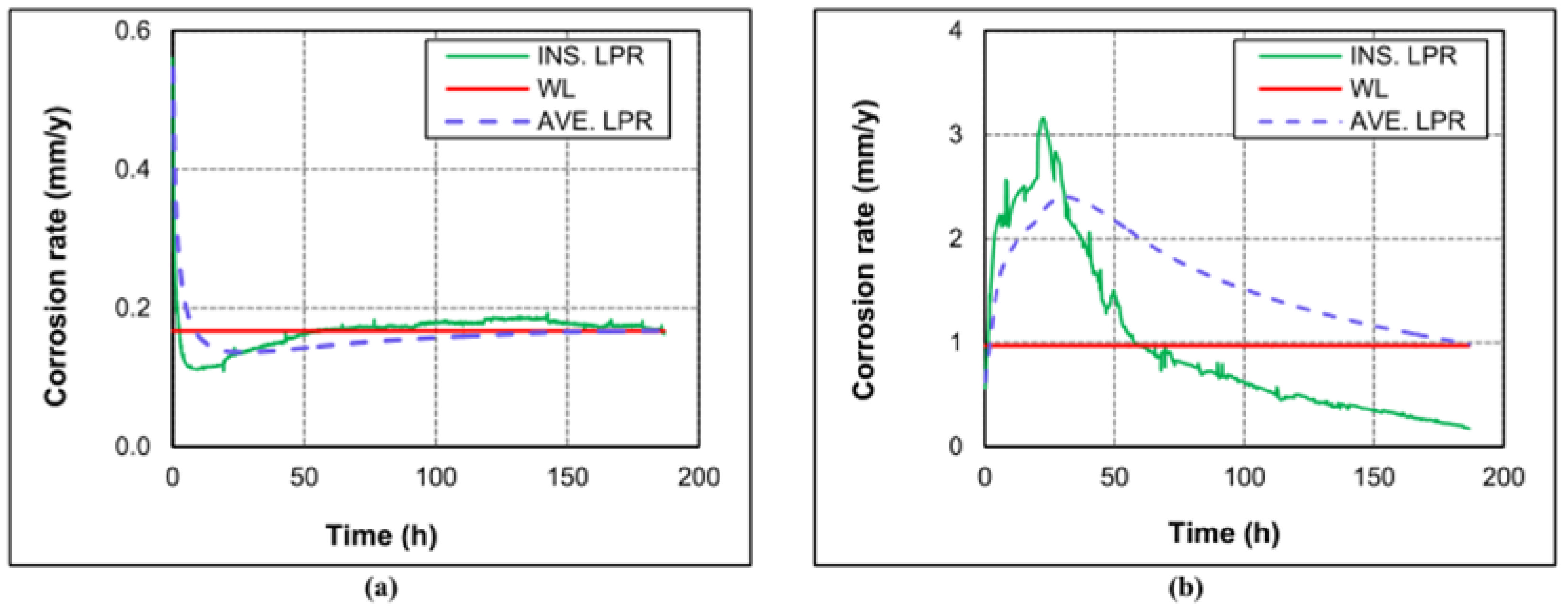
One of the critical factors that influence the corrosion of tubing steel in the CCUS-EOR system is the partial pressure of CO2. The impact of CO2 on the carbon steel corrosion rate in the environment was investigated by Rida Elgaddafi et al. [19]. The findings indicate that the corrosion rate rapidly decreases in a pure nitrogen environment (one without CO2) but that in a CO32−-containing environment, the anode–cathode reaction causes a swift instantaneous corrosion rate increase only at the initial contact stage, and the corrosion rate increases gradually as the reaction time increases. According to research by Dong et al. [20], the corrosion rate of N80 steel increased at various CO2 concentrations as CO2 content rose. According to the findings, low CO2 causes a limited number of particles to concentrate locally on the steel surface, exposing many metal substrates and facilitating simple interaction between the corrosion solution and the substrate. Even in sheets, the corrosion products on the steel surface progressively increased as the CO2 level rose. However, the flake corrosion products either show the matrix as a whole or have a lot of tiny holes and breaks in the corrosion product film, making it simple for the solution to come into contact with the matrix [21,22]. This encourages the corrosion rate of N80 steel to grow gradually (Figure 1).
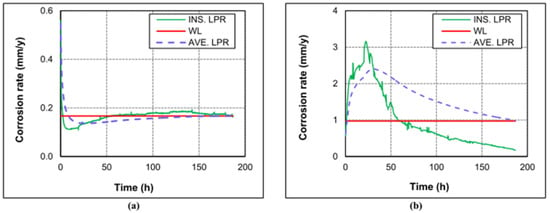
Figure 1.
Instantaneous (LPR) and average (WL) corrosion rates at 0.83 MPa and 43 °C, and different CPPs: (a) pure nitrogen (0% CO2); (b) pure carbon dioxide (100 CO2) [19] (copyright 2020, Elsevier).
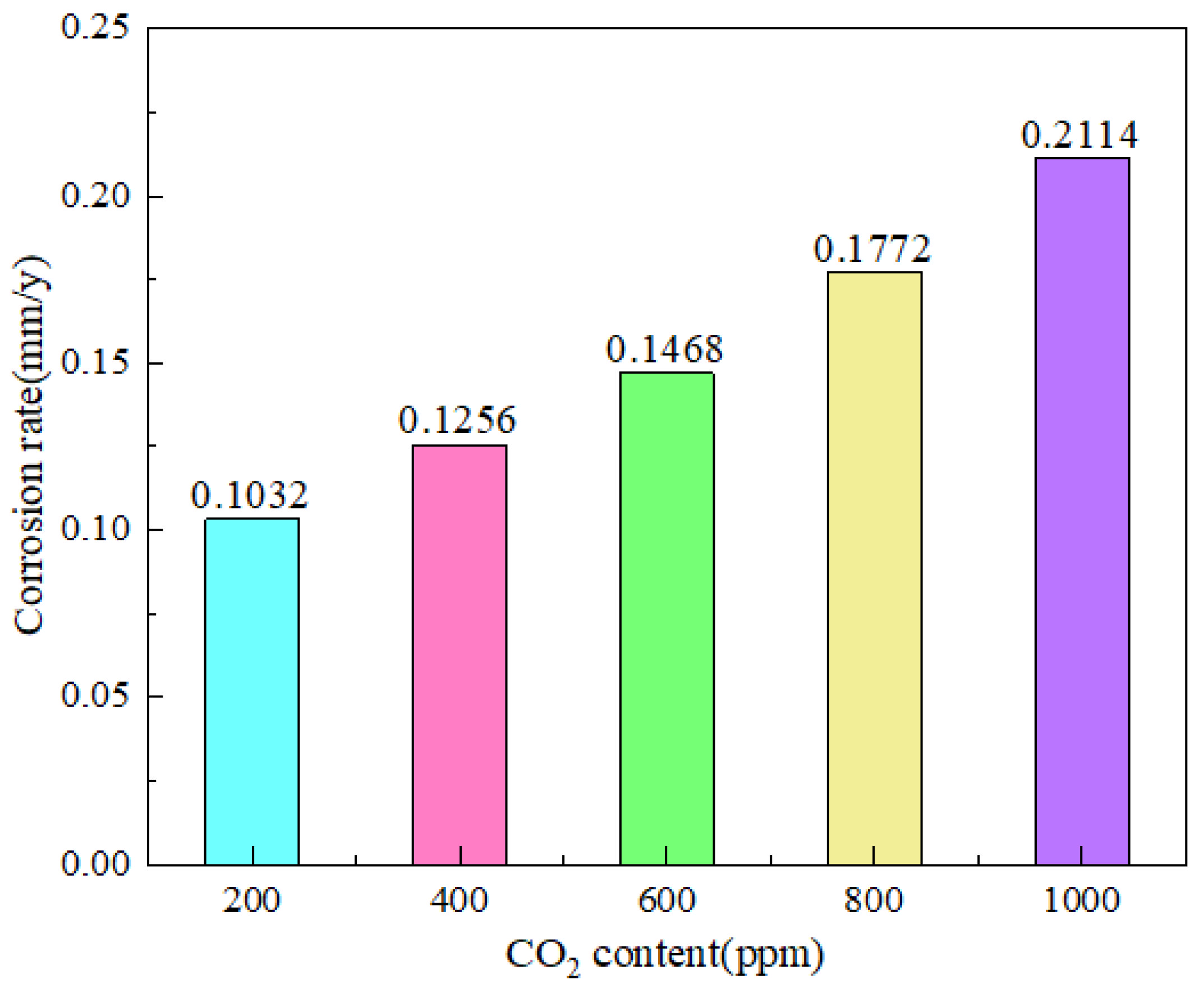
However, Lu et al. [23] found that, at a specific temperature, the corrosion cut-off value is 1 MPa CO2. Below this point, the corrosion rate would reduce while pressure remained constant and accelerate if CO2 pressure increased. The high-pressure CO2 accelerated the Fe breakdown and increased the CO32− concentration in the solution, which helped to produce a protective layer of FeCO3. The corrosion rate can be slowed down by allowing CO2 to produce a denser FeCO3 layer with Fe under high pressure. As CO2 partial pressure increases, a thick protective layer can develop fast. More investigation is required to determine how corrosion is impacted by sustained high CO2 partial pressure (Figure 2).
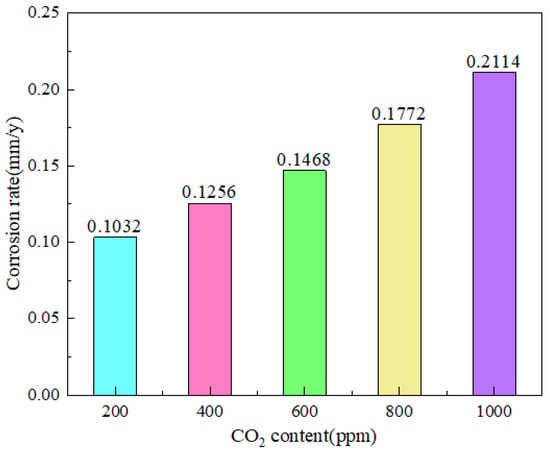
Figure 2.
Corrosion rate of N80 steel at different CO2 contents [20] (copyright 2023, John Wiley and Sons).
2.2. Influence of H2S Concentration
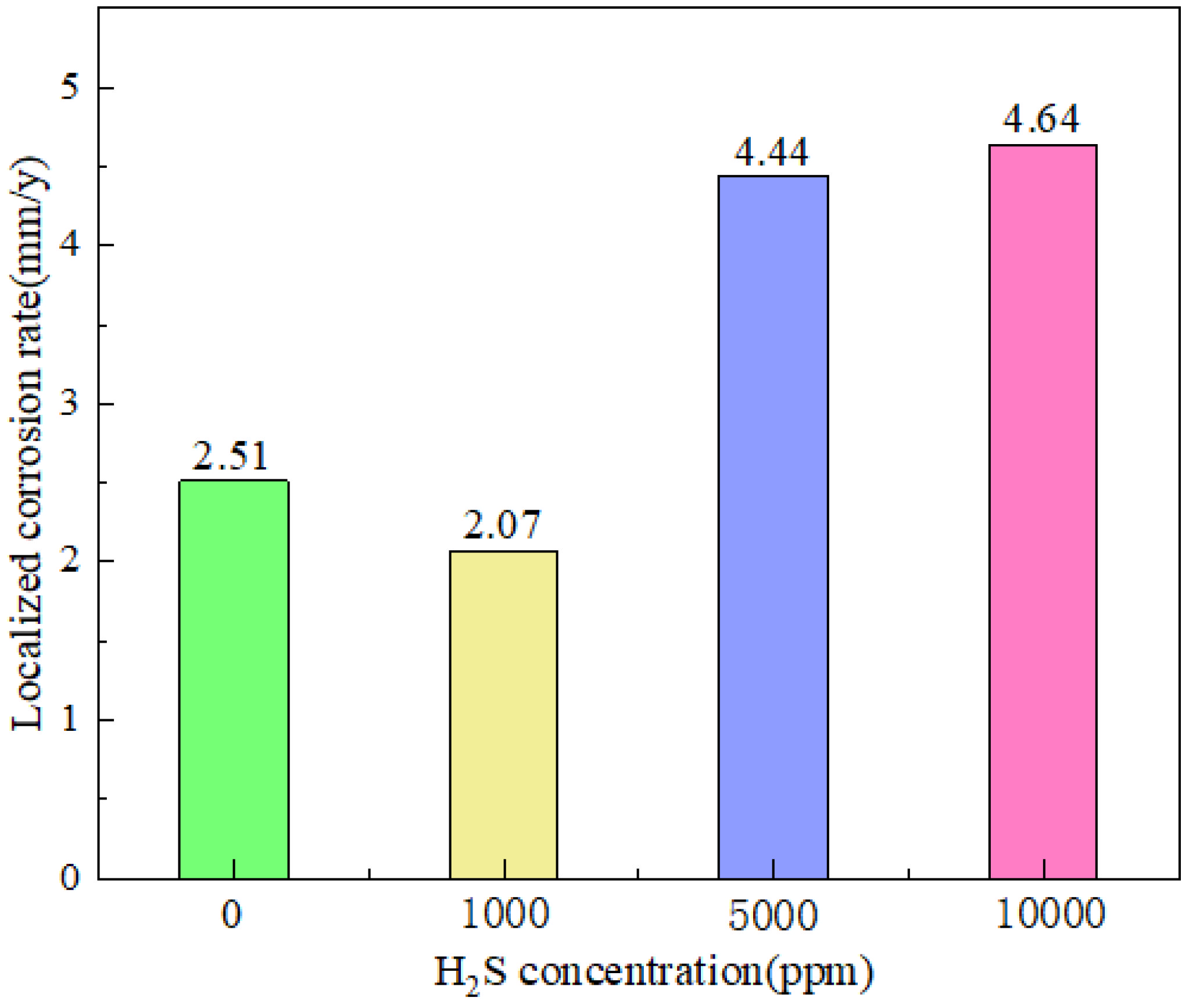
Another significant element influencing tubing steel corrosion is H2S. Determining the corrosion law of H2S is therefore essential, particularly in consideration of the corrosion impact of H2S coexisting with other contaminants on the tubing steel material in the CCUS-EOR system. In a CO2-O2-H2S environment, Liao et al. [24] investigated the effect of H2S concentration on the corrosion failure of L245NS steel. The findings indicate that local corrosion on the substrate is suppressed at concentrations of 1000 ppmv. Before a notable rise, the local corrosion rate reduces as the concentration approaches 5000 ppmv and 10,000 ppmv. Zhang et al. [25] discovered, however, that the corrosion rate rises as the concentration of H2S does, reaching its highest value (0.36 mm/a) at 0.04 vol% of PH2S. Following that, when the concentration of H2S increased, the rate of corrosion reduced. Increasing the H2S concentration aids in producing protective coatings made of ferric sulfide and ferric carbonate when the test conditions are favorable for their creation. Precipitation and film formation are accelerated at a given pH by increased CO2 partial pressure, which also causes a rise in CO32− concentration and supersaturation. Consequently, we may obtain a similar outcome for H2S concentration, meaning that an increase in H2S concentration causes an increase in S2− concentration, which speeds up the synthesis of sulfide. Similarly, nothing is known about how high H2S concentrations affect corrosion (Figure 3).
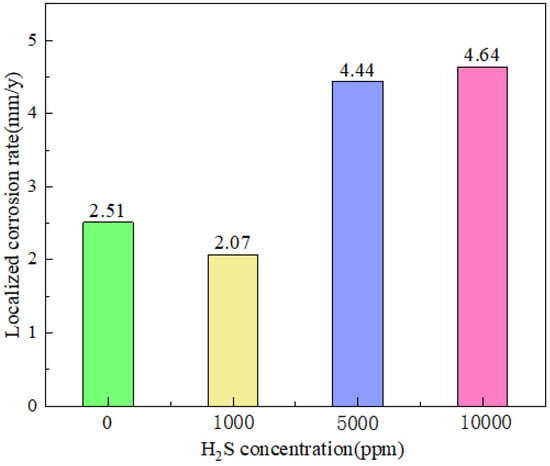
Figure 3.
The localized corrosion rate of L245NS steel substrate in different H2S concentrations [24] (copyright 2022, Elsevier).
2.3. Quantitative Analysis of PCO2/PH2S
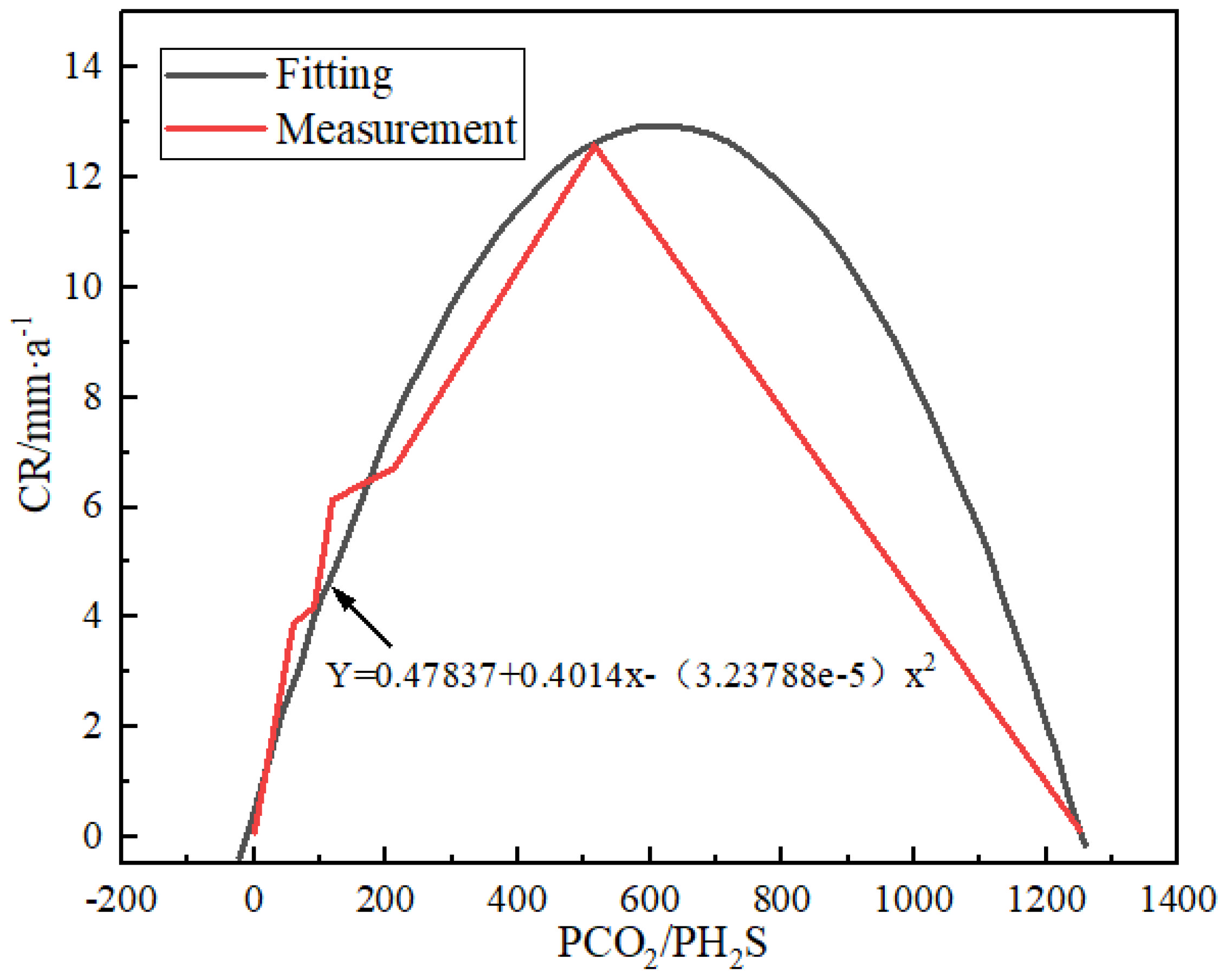
Yin et al. [26] provided a representative corrosion characteristic of sm80ss tubing steel under high temperature and pressure by NACE RP-0775-91 standard. This indicates a parabolic function link between the corrosion rate and PCO2/PH2S, and the following is the polynomial fitting equation:
where Y is the corrosion rate and X is PCO2/PH2S. A PCO2/PH2S of 31–520 indicates severe corrosion in the tubing steel. The corrosion is at the worst level when the PCO2/PH2S ratio reaches 600. The corrosion rate then falls when the PCO2/PH2S ratio rises (Figure 4).
Y = 0.47873 + 0.04014X − (3.23788 × 10−5)X2
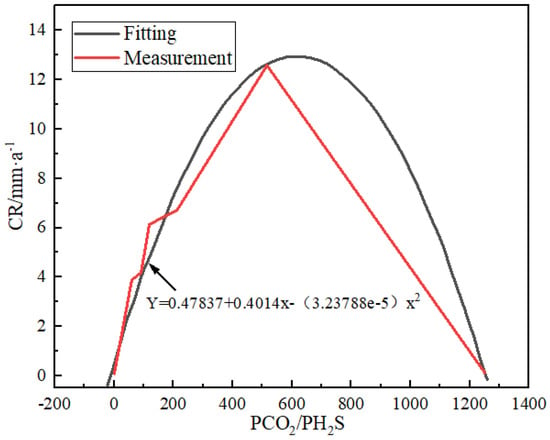
Figure 4.
The relation between corrosiveness and PCO2/PH2S for the representative SM 80SS tube steel using polynomial fitting method [26] (copyright 2008, Elsevier).
According to Masamura et al. [27] and Fierro et al. [28], when PCO2/PH2S > 200, CO2 is assumed to play a significant role in the system, resulting in the creation of a thick layer of FeS scale on the surface of the steel tube steel and a drop in corrosion rate. When PCO2/PH2S is less than 200, FeS corrosion products will first be formed, which hinders the formation of protective FeCO3 products and increases the corrosion rate. Zhang et al. [29] found through experiments that at higher PCO2/PH2S values, the corrosion process is dominated by CO2 corrosion, and there is a loose and unprotected corrosion product film on the specimen surface. At the same time, the corrosion rate of the material decreases with the increase in PCO2/PH2S, and the corrosion process gradually changes from CO2 control to H2S control.
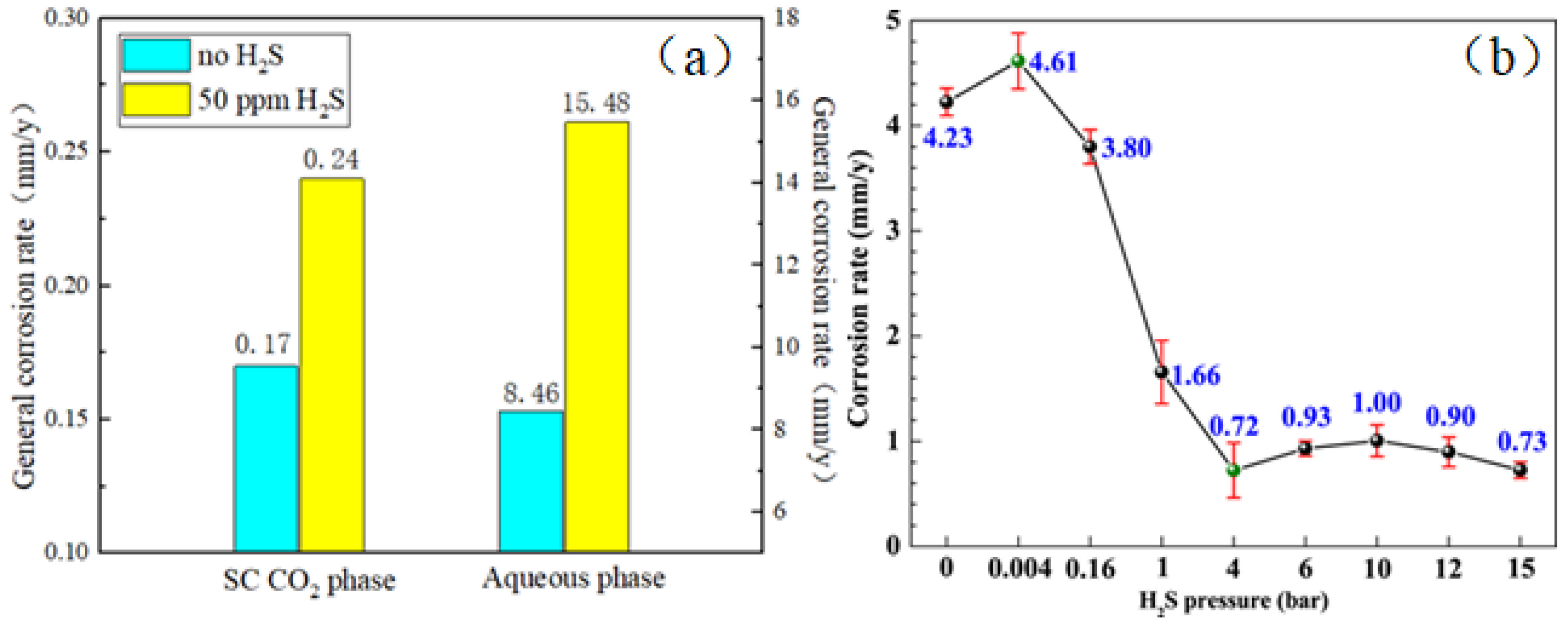
When H2S is added to CO2, the corrosiveness of FeS film will increase. The measured film thickness of the product conforms to the recognized critical PCO2/PH2S value. As mentioned above, general corrosion occurs only on the surface of the tubing steel. According to the above results, the trend of corrosion rate with PCO2/PH2S is consistent, but there is no recognized standard for the critical PCO2/PH2S value, and further exploration is needed (Figure 5).
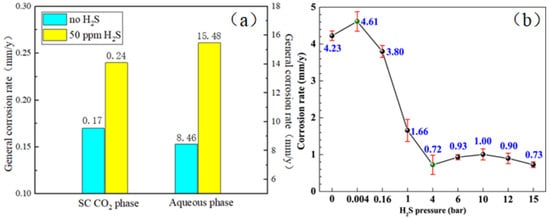
Figure 5.
(a) General corrosion rates of X65 steel exposed to both SC CO2 and aqueous phases with 50 ppm and without H2S at 10 MPa and 80 °C for 10 days [30] (copyright 2016, Elsevier). (b) Variation of corrosion rate of N80 steel with H2S pressure exposed to supercritical CO2–H2S environment at 80 bar CO2 and 80 °C for 72 h [31] (copyright 2023, Elsevier).
3. Ni-P Coating
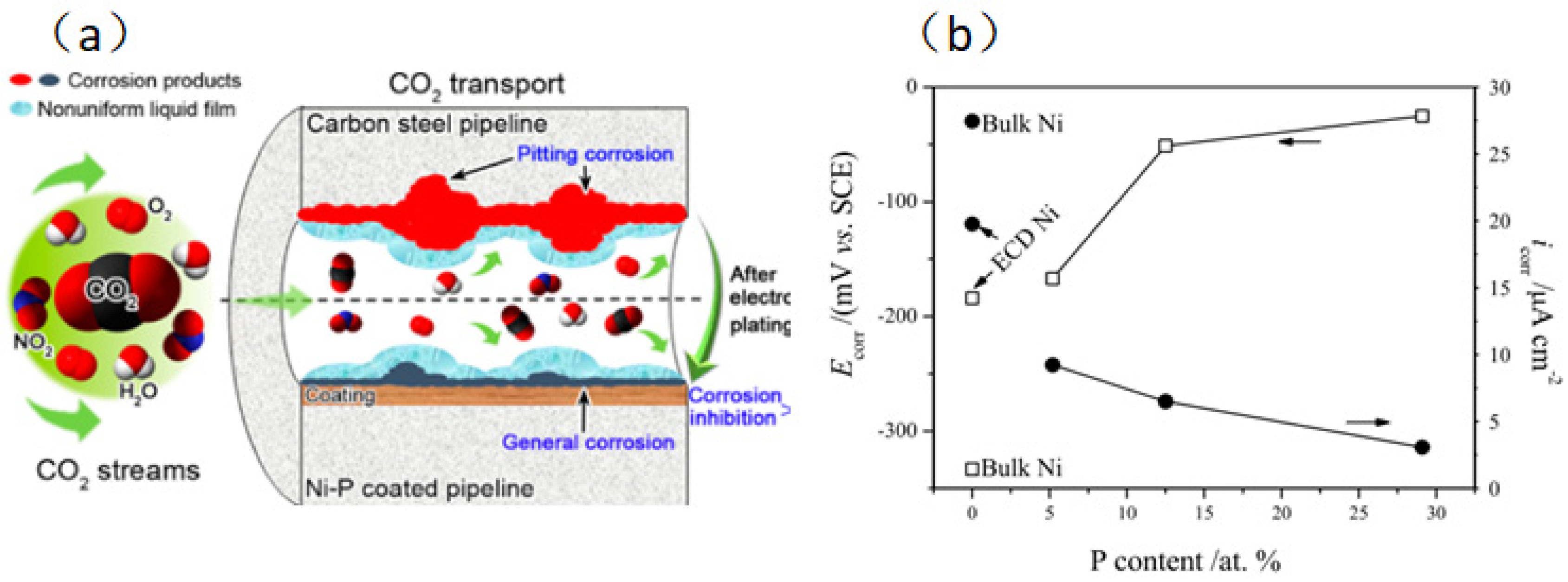
Since Ni-P coating has such good qualities—like high hardness, superior wear and corrosion resistance, and paramagnetism—it has become one of the most extensively used coatings in the industry. Moreover, a Ni-P coating resists corrosive media like CO2 and high salinity. Sun et al. [32] investigated the corrosion resistance of Ni-P coating in a supercritical CO2 environment with impurities of H2O, O2, and NO2. According to the findings, there is a decreased chance of perforation when a Ni-P coating forms on the metal surface, and extreme pitting changes to uniform corrosion. According to the co-factor calculation, Ni-P coating can impede electrolyte penetration and lessen the synergistic impact of O2 and NO2. The impact of P content on the Ni+ corrosion point Ecorr and corrosion current density score of Ni-P coating was investigated by Lu et al. [33]. In the active zone, the samples with more excellent surface P content demonstrated improved corrosion resistance due to their higher Ecorr and lower icorr. Because of their restricted active corrosion sites at nodulous borders and amorphous, chemically homogeneous microstructure, high-P-content Ni-P coatings show exceptional corrosion resistance in acidic conditions. NiO and Ni(OH)2 comprise most passivation films for Ni metal and Ni-based alloys in an acidic environment. NiO/Ni(OH)2 protective coating produced in CO32−-saturated solution can prevent corrosion in supercritical CO2 environments (Figure 6).
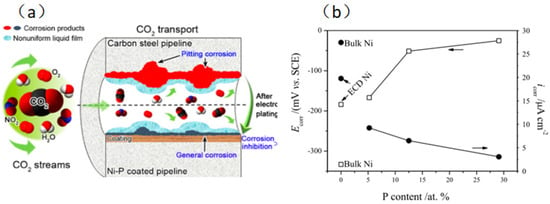
Figure 6.
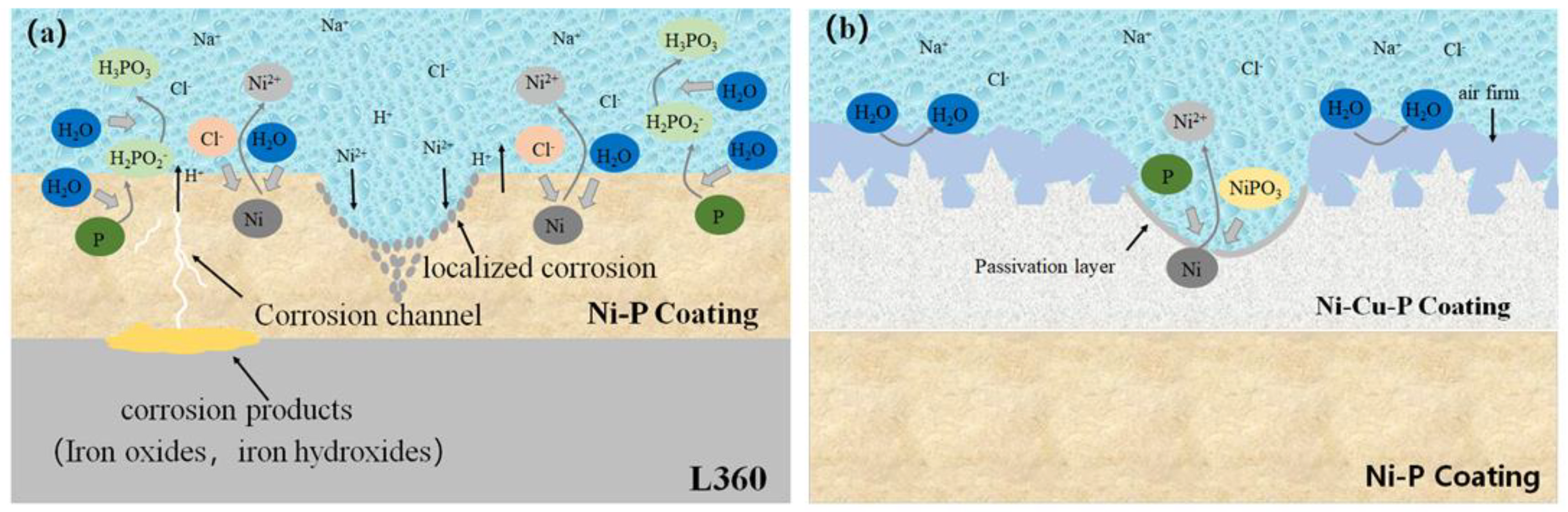
(a) Protective mechanism of Ni-P coating in supercritical CO2 environment [32] (copyright 2019, American Chemical Society). (b) Effect of P content on Ecorr and icorr for Ni-P films of various compositions [33] (copyright 2002, Elsevier).
A Ni-P coating resists high salinity well. The phenomena of Ni-P in a NaCl solution were investigated by Sudagar et al. [34]. They discovered that the Ni-P deposited layer in NaCl solution exhibited a significant passivation tendency, suggesting that a high-phosphorus electroless nickel plating coating has superior resistance to corrosion and chloride. The electrochemical corrosion behavior of Ni-P coatings in a NaCl solution was investigated by Wang et al. [35]. The findings demonstrated that a Ni-P coating remained stable over a 30-day soaking period in NaCl solution. Cl− tends to selectively absorb specific coating regions, including lattice flaws or high-nickel locations, and then erode the surrounding chemical Ni-P coating to create soluble NiCl2. An increasing amount of nickel was dissolved as the soaking period increased. The resistance of electroless Ni-P coating to corrosion is reduced upon long-term immersion, but the corrosive liquid does not penetrate the coating.
Potent scaling inhibition is also seen in the Ni-P coating. Due to the inherent properties of electroless plating, the surface of the Ni-P coating is smoother than that of the metal, which can conceal steel polishing marks. As a result, the rate of heterogeneous nucleation decreases, reducing the creation of scales. According to Li et al. [36], even at high Ca2+ concentrations, a little scale will form on the coated surface, considerably minimizing local corrosion caused by uneven carbon steel corrosion film deposition. Scale formation occurs when a solution containing a hydrophobic salt precipitates on the surface by ion coupling, nucleation, crystal growth, and, finally, scale agglomeration.
The deposition process is influenced by various environmental factors (such as pH, temperature, and hydrodynamic conditions) and surface characteristics like roughness. Surfaces with reduced surface energy, such as chemical coatings, have a lower adhesion strength to the crystals, thus extending the time of nucleation induction and resulting in a lower scaling rate. Wang et al. [37] found that the precipitation of CaxFe1−xCO3 may fill the pores of the corrosion product coating, isolate the steel from the corrosive medium, promote the “pseudo-passivation” of 3Cr steel, and thus improve the corrosion resistance of the steel. When the Ca2+ concentration attains and exceeds the critical point, leading to crystal disintegration, more Ca atoms participate in the corrosion film, increasing its density and densification by filling the void, while inhibiting further improvement of the uniform corrosion rate. Furthermore, a NiO/Ni(OH)2 passivation coating significantly lowers the active corrosion site, which is often the beginning point for nucleation. Surface roughness has a substantial impact on the development process of scale, as evidenced by the fact that when scale forms on the surface of existing defects (such as rough patches and scratches), it affects heterogeneous nucleation.
In general, the produced NiO/Ni(OH)2 corrosion layer has a low surface energy and little roughness, giving the Ni-P coating exceptional corrosion and scale resistance under the high-temperature and high-pressure environment of CO2 saturation with Ca2+. However, the blocking ability of Ni-P against H2S is not apparent, especially under CO2-H2S coexistence conditions. Sun et al. [38] studied the corrosion behavior of Ni-P coatings in CO2-Cl−, H2S-Cl−, and CO2-H2S-Cl− environments. The results show that the corrosion rate (0.0036 mm/y) is significantly lower than that of 0.025 mm/y in a CO2 environment, and the corrosion degree is relatively light. When CO2 and H2S exist simultaneously in the environment, the corrosion rate is the sum of the corrosion rates in CO2- and H2S-alone environments, suggesting that CO2 and H2S work together to accelerate the coating failure process. Li et al. [39] found that the corrosion rate of Ni-P coating in a CO2-H2S environment is greater than that in a CO2 environment because H2S corrodes the Ni-P coating by altering the cathode and anode processes. These researchers discovered that a high-phosphorus Ni-P coating eroded marginally in CO2 but moderately in H2S-dominating and CO2-H2S conditions. The inherent defects in a Ni-P coating create pathways for electrolyte penetration, leading to the continuous formation of corrosion paths inward, due to H2S. The electrolyte in the solution penetrates farther into the coating, hastening the pitting corrosion of the carbonized steel. The presence of CO2 and H2S has a synergistic impact on coating deterioration, with CO2 enhancing H2S corrosion. CO2 dominates changes in water chemistry. With rising CO2 and H2S concentrations and a high Ni affinity, the sulfide may easily migrate to the coating/solution interface and compete with the hydrate for adsorption, preventing the development of a protective oxide sheet. At the same time, the presence of CO2 enhances the effect of H2S on coating corrosion by acting as an extra cathode reaction and boosting the pace of the hydrogen evolution reaction. As a result, the electrolyte may enter the corrosion products and coating more easily, hastening the corrosion of the coating and substrate.
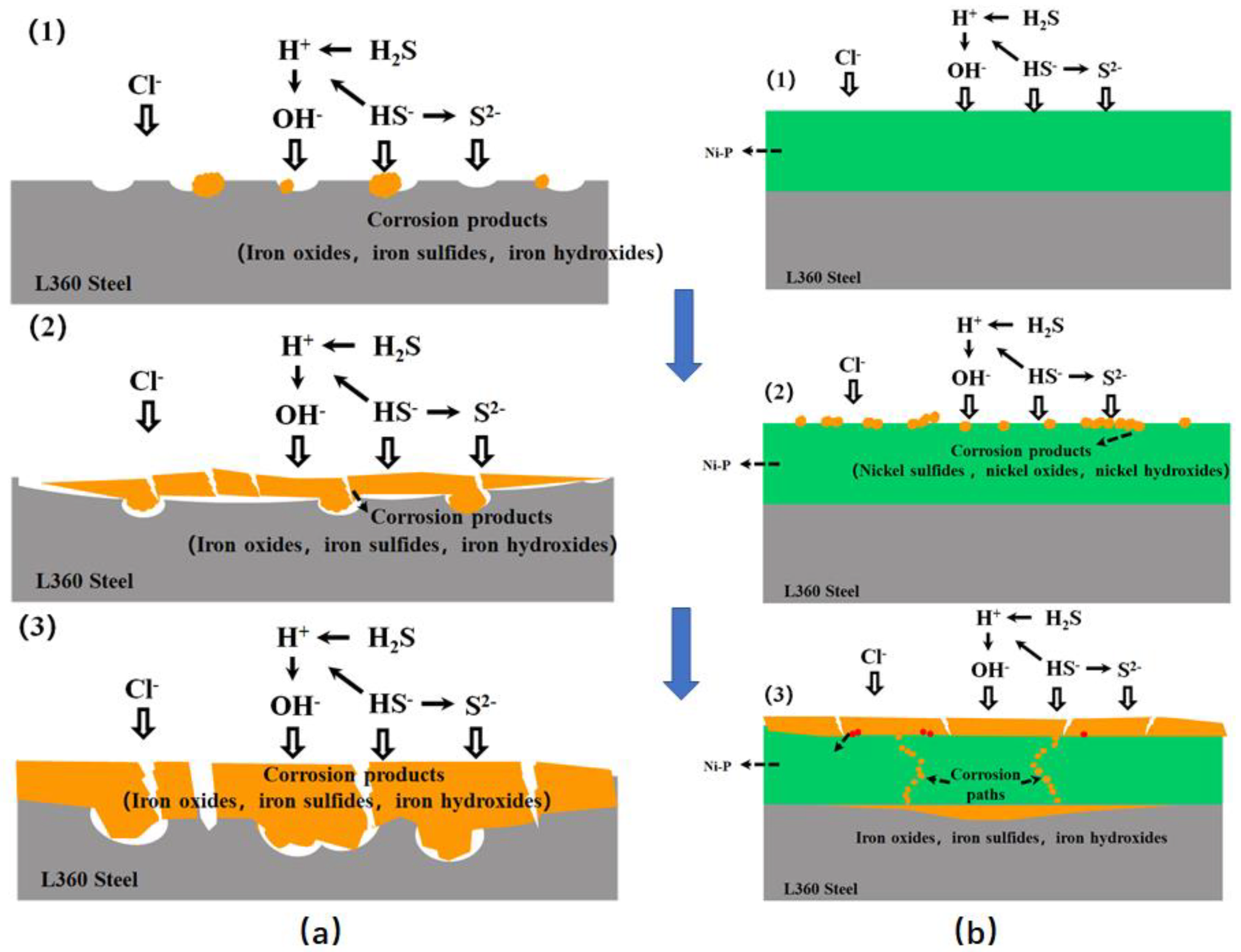
However, the competitive adsorption of CO2 and H2S results in the formation of a dense product, which decreases the corrosion rate. Li et al. [39] investigated the corrosion behavior of Ni-P coating on an L360 steel surface in saturated H2S media over time. The findings indicate that when uncoated L360 steel is subjected to a simulated acidic environment, H2S initially causes local corrosion of the material, followed by the development of corrosion products. H2S corrosion becomes more intense as soaking time increases, and the corrosion layer progressively thickens. Increased thickness of the corrosion product layer inhibits H2S corrosion and reduces the corrosion rate. The content of corrosion product NiS increases gradually during H2S corrosion. NiS features a cross-stacked structure that enhances the stability of the corrosion scale by tightly bonding it to the outer surface of the coating. This reduces direct contact between the chemical coating and the corrosive medium to a significant extent (Figure 7).
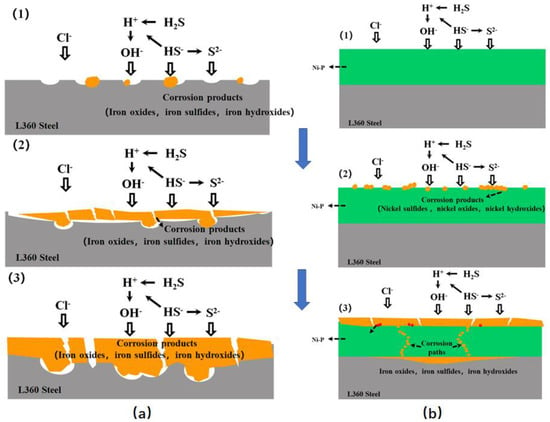
Figure 7.
Schematic illustrations of the degradation for (a) L360 steel and (b) Ni-P coatings in H2S-containing environments [39] (copyright 2021, Elsevier).
Many types of anti-corrosion coatings are employed in tube steel, including amorphous alloy coating, which has a high potential for usage in petrochemical and other industries due to its excellent corrosion resistance and mechanical characteristics. In the preparation of Ni-P amorphous alloy coating, the coating prepared by the electroless plating method has uniform thickness, is not limited by the shape of the part, has good bonding with the substrate, and has excellent corrosion resistance. The Ni-P amorphous coating prepared by electroplating has the advantages of a controllable deposition rate, convenient operation, and low operating temperature. Therefore, many scholars have begun to study new metal nanocomposite plating systems. However, the exact density of the coating cannot be maintained during the electroless plating process, and imperfections such as pinholes will eventually appear. If electrolyte collects in the flaw, it corrodes the porosity. The exposure of the substrate directly to the corrosive medium substantially diminishes the protective function of the coating, thereby reducing its service life and increasing maintenance costs [40,41,42,43,44,45,46,47].
4. Research Status of Corrosion Resistance of Reinforced Ni-P Coating
Despite the fact that three-phase nanoparticles may significantly increase the corrosion resistance of Ni-P coatings, their high cost prevents their widespread application. Thus, researchers focused on ternary alloys, such as coating materials like Ni-Cu-P, Ni-Sn-P, Ni-W-P, and Ni-Fe-P, in order to add tertiary metals to a Ni-P coating and increase its corrosion resistance.
4.1. Ni-Fe-P Coating
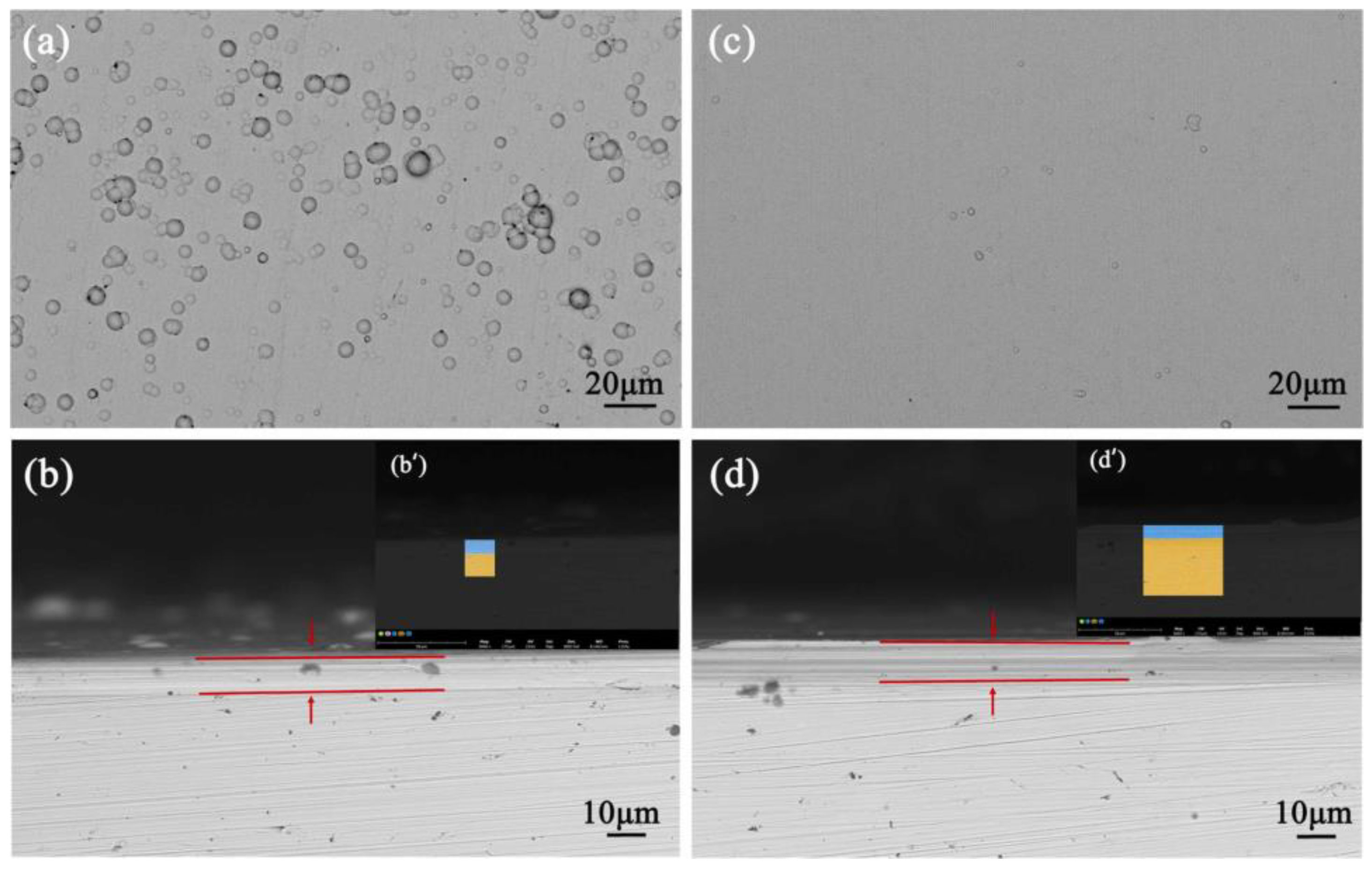
There have been reports in recent years suggesting that Ni-Fe-P coating exhibits superior corrosion resistance compared to Ni-P coating. Since iron is less expensive and easier to obtain than other ternary metals, Ni-Fe-P alloys have a wide range of potential applications. Ni-P and Ni-Fe-P coatings with the same P content were made by Zhang et al. [48]. They investigated the mechanism by which Fe affects the way that Ni-P coating corrodes in various corrosion situations. The outcomes demonstrate that the Ni-Fe-P coating has a smoother surface and many spherical formations. Fe improves the flatness of Ni-P coatings and reduces the effect of thickness fluctuations on corrosion resistance. The Ni-Fe-P coating exhibits superior corrosion resistance compared to the Ni-P coating because of its higher concentration of NiO and stronger resistance to Cl− (Figure 8).
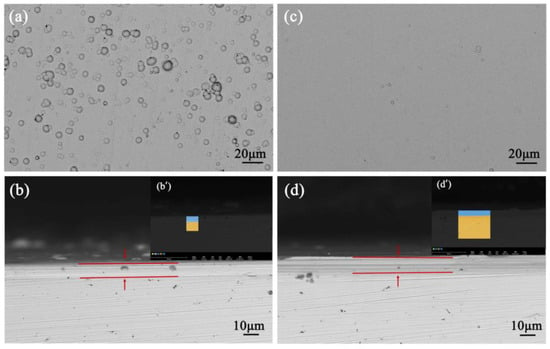
Figure 8.
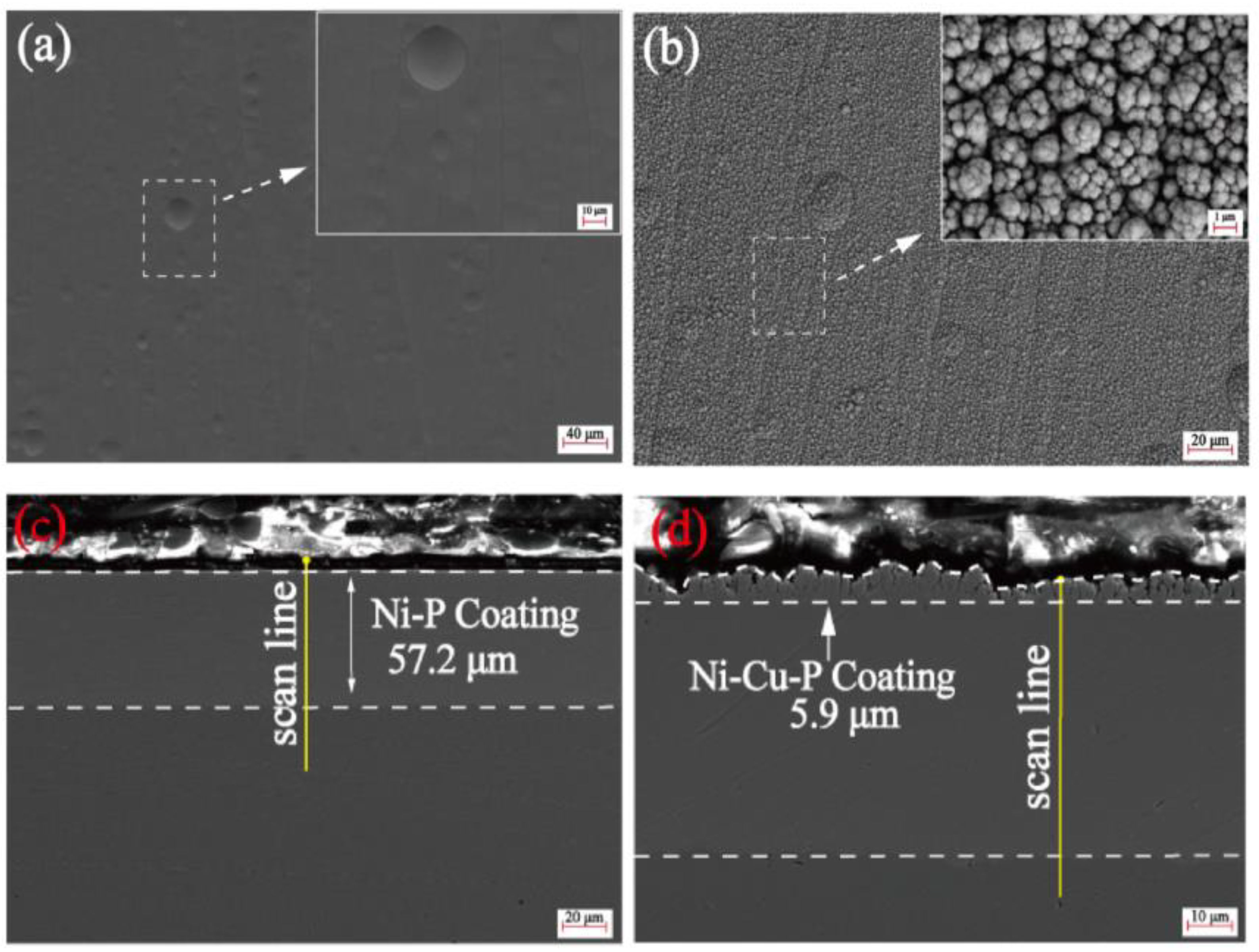
(a) Ni-P coatings, (b) cross-section of Ni-P coatings, (b′) corresponding EDS mapping, (c) Ni-Fe-P coatings, (d) cross-section of Ni-Fe-P coatings, (d′) corresponding EDS mapping [48] (copyright 2022, Elsevier).
Fe is added to a Ni-P coating to create a Ni-Fe-P ternary alloy coating. The Ni-Fe-P coating exhibits a smoother surface morphology, greater grain size significance, and visible crystal state as compared to the Ni-P coating [49]. As a result, the addition of iron to the Ni-P coating increases its flatness and encourages the formation of a crystalline structure. Furthermore, during deposition, the presence of iron encourages the conversion of Ni(OH)2 to NiO. The Ni-P and Ni-Fe-P coatings are both passivated. The Ni-Fe-P coating has superior corrosion resistance because NiO has greater corrosion resistance than Ni(OH)2.
Corrosive ions seep through the covering and affect the substrate directly in a saturated CO2 solution. The Ni-P coating has a smaller grain size than the Ni-Fe-P coating, which helps to better block the entry of corrosive ions. The Ni-P coating also exhibits superior corrosion resistance and a larger positive corrosion potential. The Ni-P coating exhibits deterioration in a manner akin to the CO2 environment. On the other hand, under the strong acid environment of the Ni-Fe-P coating, the iron rapidly combines with H+ ions and breaks down its structure.
4.2. Ni-Cu-P Coating
According to accounts, Cu promotes Ni breakdown within the coating, allowing for P enrichment. The formation of a passivation layer enhances the corrosion resistance of the coating, whilst the addition of Cu improves the mechanical properties of the Ni-P coating. Therefore, Ni-Cu-P also has broad application prospects.
The addition of Cu to Ni-Cu-P films restricts the formation of cellular structures on the surface, promotes an increase in the number of nucleation sites, and significantly improves the microstructure. Cu atoms precipitate in Ni-Cu-P amorphous coatings, resulting in a solid solution of Ni. The mechanical characteristics of the coating are improved, with considerable effects on hardness, elastic modulus, and wear resistance due to microstructure evolution and phase transitions. Zhang et al. [50] compared and analyzed the structural distinctions between Ni-P thin films and Ni-Cu-P composite thin films from the microstructure perspective. A big single cell has been observed to exist locally, and the Ni-P membrane has a rough surface. In comparison, the Ni-Cu-P coating had much higher microhardness, consistent thickness, great adherence to the substrate, and no pores, cracks, or obvious delamination. Liu et al. [51] investigated the influence of Cu on the microstructure and micromechanical properties of Ni-P coatings. Their findings indicate that the resulting Ni-Cu-P alloy is predominantly composed of solid solutions based on Ni and Cu, with a high Cu concentration. During heat treatment, phosphide precipitates such as NiP2 and Cu3P form, improving hardness via rapid precipitation (Figure 9).
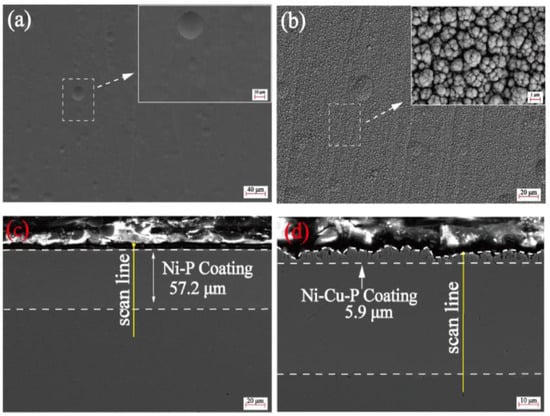
Figure 9.
Surface morphology of electroless (a) Ni-P coating and (b) electrodeposited Ni-Cu-P composite film; cross-section morphology of (c) electroless Ni-P coating and (d) electrodeposited Ni-Cu-P composite film [50] (copyright 2023, Elsevier).
The electrodeposited Ni-Cu-P coating contains more nucleation growth sites than a single electroless Ni-P sample. The electric current induces the co-deposition of Cu ions, which changes the Ni nucleation process [52]. The particles gradually expand to form a corrugated structure, which acts as an air barrier by creating a hollow beneath. The ability of this structure to prevent water droplets from further penetrating improves the hydrophobicity of the electrodeposited Ni-Cu-P coating.
Cu helps to passivate the composite coating and improves the mechanical properties of the Ni-P coating. This enhancement significantly contributes to the increased corrosion resistance of the composite coating. Cu has a larger self-corrosion potential than Ni, suggesting its poorer solubility in Ni. The quantities of Ni-P and Ni3(PO4)2 in Ni-Cu-P rise with the addition of Cu. The presence of P and copper Cu on the surface of Ni-Cu-P promotes the production of passivation films with dual semiconductor properties (P/N). This successfully prevents negative ions from entering the film and positive ions from leaving it. In addition, the passivation film also prevents Ni dissolution and Ni2+ diffusion so that the corrosion resistance of the film is improved. Zhang et al. [50] discovered that a Ni-Cu-P composite layer had the maximum corrosion potential, −0.4783 V, which was much greater than that of a Ni-P coating and L360 substrate. A chemically generated Ni-Cu-P composite coating has much higher resistance values, indicating that it is more corrosion-resistant than a single-layer Ni-P coating. An analysis of the superhydrophobic film picture in the low-frequency range exhibits Warburg characteristics, indicating restricted diffusion of corrosion products from the matrix to the solution or an impediment to the oxygen dissolution process. Superhydrophobic membranes are thought to have good barrier properties, thus leading to this phenomenon. The amorphous nature of Ni-P and its passivation characteristics contribute to high corrosion resistance, extending beyond the substrate to the film. In contrast, the outer surface of the Ni-Cu-P composite coating demonstrates excellent corrosion resistance by acting as a barrier film. This film effectively shields the substrate and the underlying layers from instability and prevents penetration in corrosive environments (Figure 10).
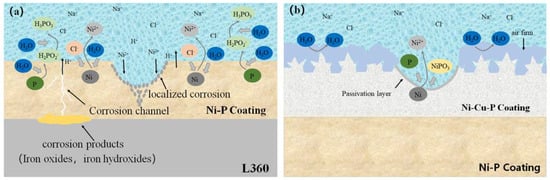
Figure 10.
Schematic illustration of corrosion of electroless Ni-P film/electrodeposited Ni-Cu-P coating in saturated NaCl solution: (a) Ni-P coating; (b) Ni-P/Ni-Cu-P composite film [50] (copyright 2023, Elsevier).
4.3. Ni-W-P Coating
To address the corrosion issues that arise in oil fields, Ni-W-P electrodeposition technology should be developed in time. It has excellent corrosion and wear resistance, especially in harsh environments with high temperatures and high pressure underground, and it has good anti-corrosion performance against H2S, CO2, and Cl−. It has been used in oil production equipment such as rods and casing. Many oil fields, such as Tarim Oilfield, Southwest Yuanba Oilfield, and Qinghai Oilfield, have been widely praised.
Much of the literature shows that the P and W elements co-deposited in Ni-W-P coatings improve the corrosion durability and tensile strength of Ni-W-P coatings, respectively. M. Asgari et al. [53] investigated the electrocatalytic properties of Ni-W-P. The results also show that the sample only changes color, the appearance is smooth, and no apparent corrosion occurs. At the same time, there is no visible rusting on the exterior of the coating. The corrosion rate of the Ni-W-P coating sample is approximately 0.0059 mm/y, suggesting that the Ni-W-P alloy coating is highly resistant to SSC.
The corrosion resistance and tensile resistance of Ni-W-P coating are improved by the P and W elements co-deposited in the coating. He et al. [54] proposed that corrosive wear (the material is prone to wear and corrosion concurrently) is a complex combined effect of these two events. In corrosive circumstances, the synergistic effect can have a dominant influence on material behavior. The combined effect of wear and corrosion is observed in a 3.5 wt% NaCl corrosion solution, indicating that the second phase precipitation increases the hardness of a Ni-W-P alloy, which is the primary reason for its improved wear resistance and has a significant impact on the synergistic effect (Figure 11).
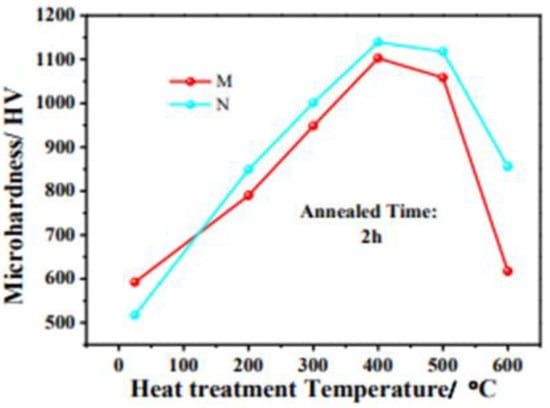
Figure 11.
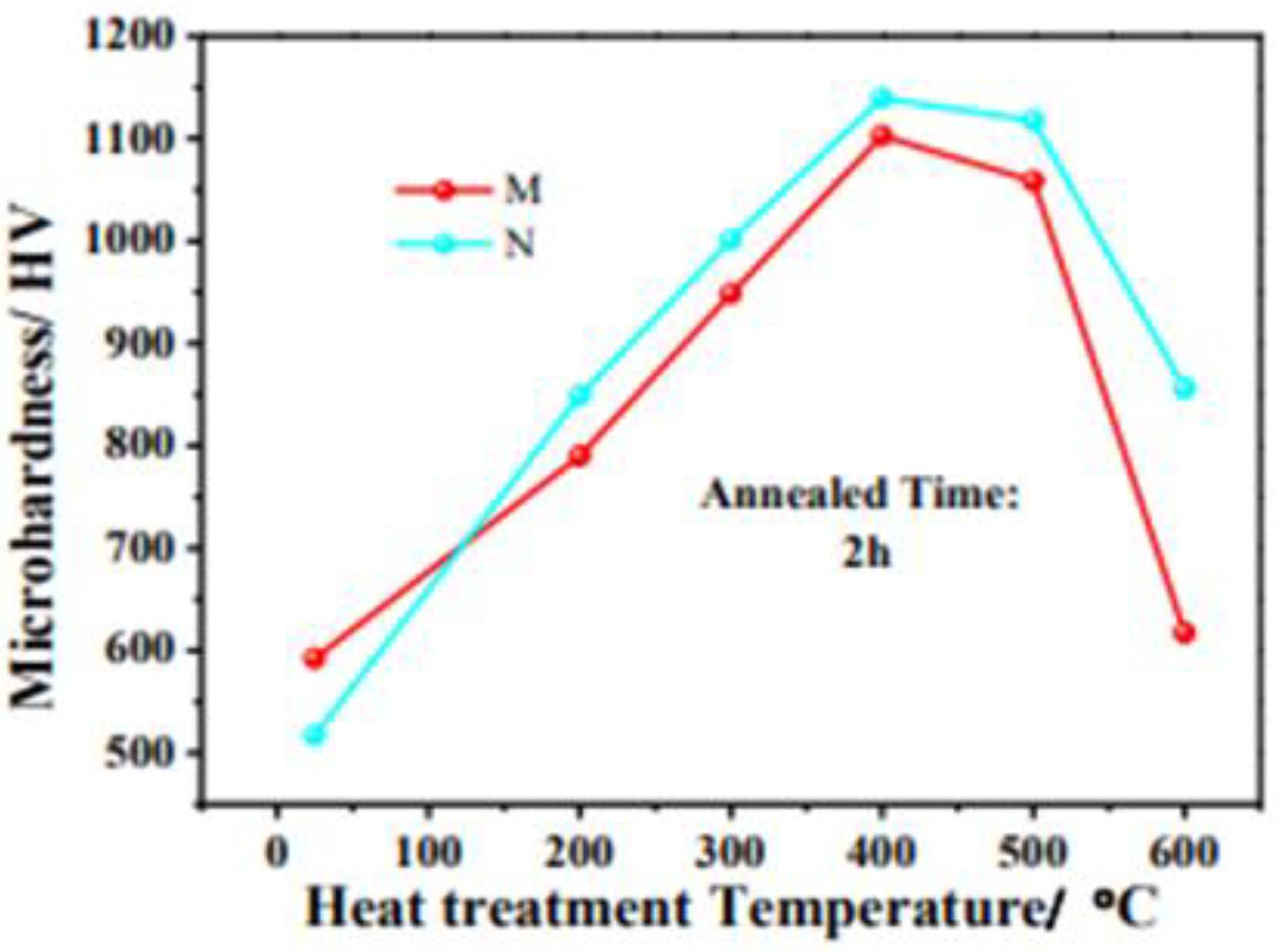
Microhardness curves of crystalline (M) and amorphous (N) Ni-W-P coatings annealed at different temperatures from 200 to 700 °C in air for 2 h [55] (copyright 2022, Elsevier).
Material loss is usually caused by a combination of wear and corrosion. The overall material removal rate, computed by summing the wear rate identified without corrosion and the corrosion rate seen without wear, differs from what would be expected when a passive metal or alloy experiences sliding wear in a corrosive environment. When exposed to corrosive environments, synergies are essential in attacking many industrial facilities. The same rule of corrosive wear behavior also applies to the sliding wear of Ni-W-P alloy in an atmosphere of corrosion. When the material slides and wears in a corrosive environment, the higher the hardness, the lower the synergistic effect, and the more excellent the resistance to mechanical plowing. When employed in a corrosive environment, it effectively increases the hardness of industrial materials and improves the performance of engineering materials. To improve the lifespan of nickel-based composite coatings, various researchers are looking at adding finely dispersed nanoparticles such as graphite, fiber, SiO2, ZrO2, SiC, TiC, TiO2, and diamond to a nickel-based coating matrix.
4.4. Ni-W-P-nSiO2 Coating
SiO2 nanoparticles are employed for enhancing the wear and corrosion resistance of a coating due to their cost-effectiveness. Through microhardness measurement experiments, Dong et al. [56] found that the hardness of a Ni-W-P coating significantly increased compared with the substrate, and the hardness of a Ni-W-P-NSiO2 coating was further increased (Figure 12). After the addition of SiO2 nanoparticles, the morphology of the composite coating appears to be small dispersed spherical particles, which is denser than that of the Ni-W-P coating. In the plating process, nano-SiO2 is nucleated as the core, and the ions of the plating solution are wrapped on the surface of the particles and grow and are deposited on the collective surface, which effectively refines the structure and makes the coating more hydrophobic. Wang et al. [57] also showed that incorporating SiO2 nanoparticles into a Ni-W-P matrix causes chaos in the atomic structure of Ni particles, resulting in the formation of amorphous nanoparticles over time. These minuscule, amorphous particles continue to develop until they cluster into small islands, which then merge into bigger islands and networks. Finally, these bigger islands and networks cover the metal matrix, resulting in continuous deposits. The composite coating comprises many amorphous small particles of SiO2 nanoparticles and presents an amorphous structure when deposited. Therefore, the Ni-W-P coating with SiO2 nanoparticles is more hydrophobic.
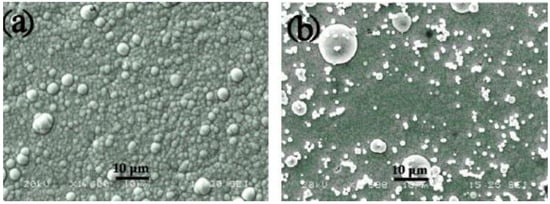
Figure 12.
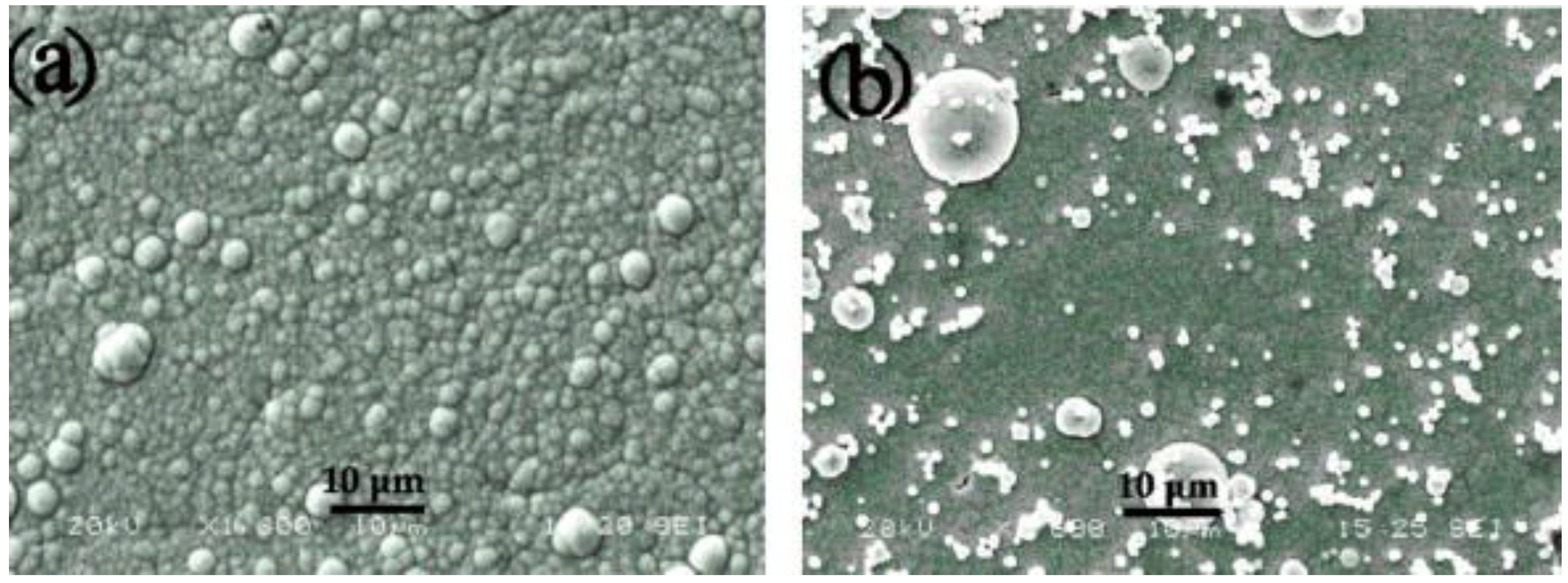
SEM images of electroless (a) Ni–P and (b) Ni–P/nano-SiO2 composite coatings [56] (copyright 2009, Elsevier).
Hu et al. [58] report that a Ni-W-P coating has a corrosion current density of 2.55 × 10−6 A·cm−2, whereas a Ni-W-P-nSiO2 coating has a corrosion current density of 1.31 × 10−6 A·cm−2. These values suggest that the former is about twice as high as the latter. A Ni-W-P-nSiO2 coating has better corrosion resistance than a Ni-W-P coating because of the homogeneous and gentle SiO2 nanoparticle deposition [59]. When the temperature rises over the melting point of S and the S is strongly oxidized, the coated surface becomes activated quickly, which speeds up the corrosion process. Ni and S make up the majority of the corrosion scale, with trace quantities of O and Ca. The three-dimensional microstructure of Ni-W-P-nSiO2-coated samples shows that the corrosion pit depth on their surface is around 3~4 μm. When the hardness approaches 480 HV, the internal porosity stabilizes in the Ni-W-P-nSiO2 coating, showing a robust connection with the matrix. A Ni3S2 corrosion scale layer forms in the corrosive environment with perchlorides and H2S/CO2. Severe high-temperature and high-pressure H2S-CO2-Cl− corrosion conditions are not able to harm a Ni-W-P-nSiO2 coating.
In summary, the co-deposition of SiO2 nanoparticles can improve the corrosion resistance of Ni-W-P coatings. The method of obtaining Ni-P nanocomposite coatings is limited to adding nanoparticles to a Ni-P plating solution. Although the properties of nanocomposite coatings are better than those of ordinary composite coatings or pure coatings in most cases, the large specific surface area and high surface energy of nanoparticles cause them to easily agglomerate in the plating solution, which has an important influence on the structure and properties of the coating. Currently, the primary methods for preventing the agglomeration of nanoparticles include (1) adding surfactants to improve the surface energy of nanoparticles and (2) using mechanical stirring or ultrasonic dispersion methods to reduce the agglomeration in the plating solution. However, the effect of these two methods is insignificant, and nanoparticles’ dispersion and action mechanisms in Ni-P plating solutions need to be further explored. In addition, compared with many studies on the process parameters of composite plating, the study on the mechanism of composite electrodeposition is not in-depth, and the relevant mechanism needs to be clarified.
5. Summary and Expectation
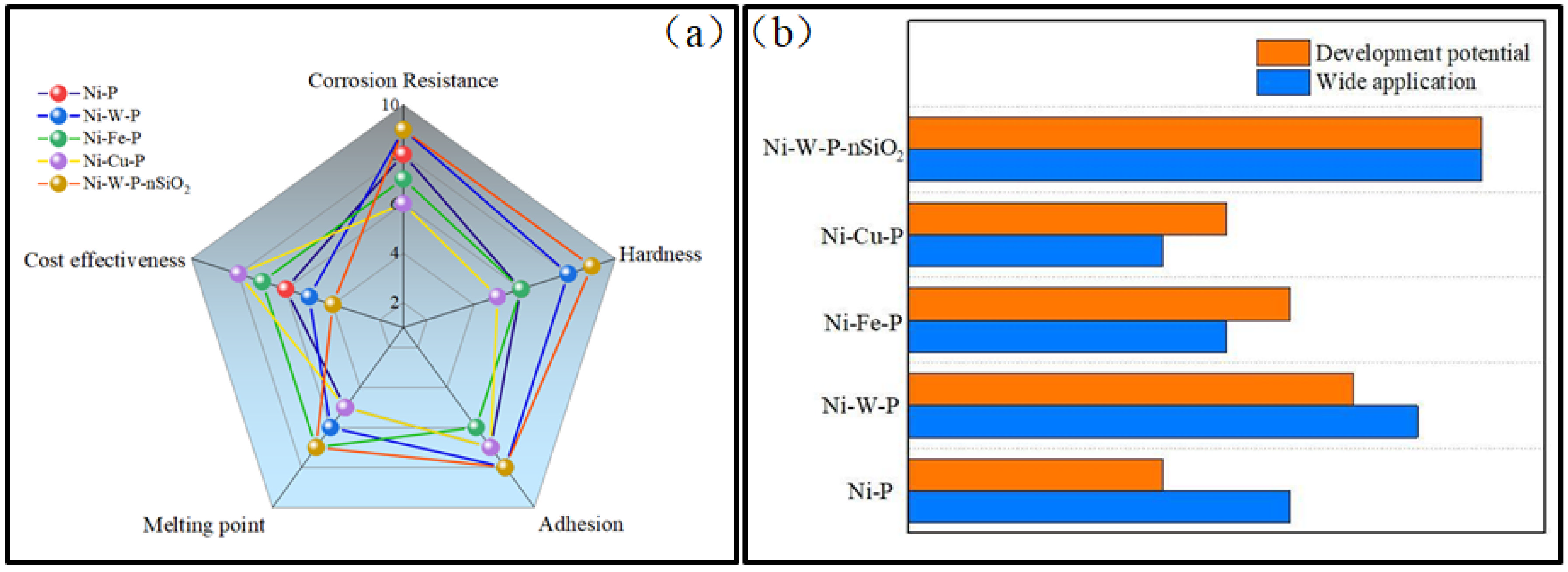
CCUS will be one of the essential measures in the carbon peak and neutrality process in the future, and the equipment corrosion problem it brings will be more prominent. In this paper, the CO2 corrosion process, corrosion mechanism, and corresponding protective measures under actual working conditions are reviewed, and the effects of CO2 and H2S on the corrosion of tubing steel are sorted out. In addition, the current tubing steel protection methods and existing problems in the CO2/H2S environment are summarized. The protection of Ni-P coating has a pronounced effect on the corrosion environment of the tubing steel, which has apparent corrosion resistance. However, the inherent defects of Ni-P coating may accelerate the corrosion of the tubing steel under the influence of H2S. Therefore, a new metal nanocomposite plating system is the development direction of future research. Improving the mechanical properties and corrosion resistance of Ni-P coatings by adding particles is a trending topic in the industry. There are still few related research reports on Ni-W-P and Ni-W-P-nSiO2 protective coatings, and new metal nanocomposite plating systems need further research and improvement (Table 1, Figure 13).

Table 1.
Summary.
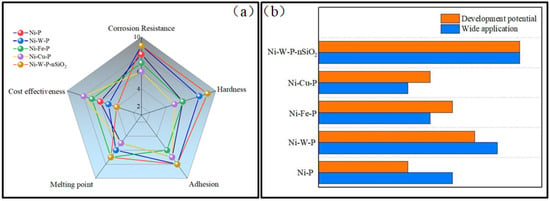
Figure 13.
Comparison of different coatings: (a) capability; (b) application and development.
In the future, research on CO2 corrosion protection coatings under harsh conditions should focus on the following points: (1) The instability of nanopowders in the plating solution is also the bottleneck of the development of nanocomposite plating technology; especially in higher-temperature electroless nickel plating solutions, nanopowders are more likely to agglomerate. Therefore, it is necessary to explore new dispersion methods further to solve the agglomeration phenomenon of nanoparticles so that the nanoparticles can be uniformly dispersed in the plating solution. (2) A new metal nanocomposite plating system should be studied to solve the problem of non-metallic nanopowder agglomeration in the plating solution. Although the nanocomposite nickel plating system of gold and silver has been studied, the application types of metal nanoparticles should be further expanded. (3) The co-deposition mechanism of nanoparticles and metal ions, the action mechanism of nanoparticles on the plating solution, and the effect on the properties of composite coatings still need to be further explored.
Funding
This research was funded by the National Natural Science Foundation of China (No. 52201064), the Science and Technology Project of Changzhou City (No. CJ20220157), and the Science and Technology Program of Changzhou University (No. ZMF22020065).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
Conflicts of Interest
Author Pengcheng Cao and Guangqin Wang were employed by the the Oil Production Plant 10th of PetroChina Changqing Oilfield Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lv, G.; Li, Z.; Li, X.; Zhang, J.; Wang, S. Technology and Application of CO2 Capture, Utilization and Storage for Coal fired Power Plant. Sci. Technol. Rev. 2014, 32, 40–45. [Google Scholar]
- Hu, Y.; Hao, M.; Chen, G.; Sun, R.; Li, S. Technologies and practice of CO2 flooding and sequestration in China. Pet. Explor. Dev. 2019, 12, 716–727. [Google Scholar] [CrossRef]
- Marbun, B.T.H.; Santoso, D.; Kadir, W.G.A.; Wibowo, A.; Sule, R. Improvement of borehole and casing assessment of CO2-EOR/CCUS injection and production well candidates in Sukowati Field, Indonesia in a well-based scale. Energy Rep. 2021, 7, 1598–1615. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, X.; Sun, C.; Xu, X.; Sun, J. Corrosion behavior of X65 steel in gaseous and liquid CO2 transport environments containing multiple impurities and different H2O content. IOP Conf. Ser. Mater. Sci. Eng. 2024, 2686, 12–18. [Google Scholar] [CrossRef]
- Wu, B.; Song, Z.; Chen, P. Corrosion Behavior of N80/J55 Tubing Steel in Supercritical CO2/H2O System. Drill. Prod. Technol. 2021, 44, 66–70. [Google Scholar]
- Sun, C.; Sun, J.; Liu, S.; Wang, Y. Effect of water content on the corrosion behavior of X65 pipeline steel in supercritical CO2-H2O-O2-H2S-SO2 environment as relevant to CCS application. Corros. Sci. 2018, 137, 151–162. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Sun, J. Probing the Initial Corrosion Behavior of X65 Steel in CCUSEOR Environments with Impure Supercritical CO2 Fluids. Corros. Sci. 2021, 189, 109585. [Google Scholar] [CrossRef]
- Xu, X.; Miao, J.; Bai, Z.; Feng, Y.; Ma, Q.; Zhao, W. The corrosion behavior of electroless Ni–P coating in Cl−/H2S environment. Appl. Surf. Sci. 2012, 258, 8802–8806. [Google Scholar]
- Ashassi-Sorkhabi, H.; Es’Haghi, M. Corrosion resistance enhancement of electroless Ni–P coating by incorporation of ultrasonically dispersed diamond nanoparticles. Corros. Sci. 2013, 77, 185–193. [Google Scholar] [CrossRef]
- Liu, H.; Guo, R.X.; Zong, Y.; Bian, J.S.; Li, S. Annealing crystallization and wear behavior of electroless deposited Ni-P and Ni-W-P coatings. Trans. Mater. Heat Treat. 2011, 32, 139–145. [Google Scholar]
- Luo, H.; Leitch, M.; Behnamian, Y.; Ma, Y.; Zeng, H.; Luo, J. Development of electroless Ni–P/nano-WC composite coatings and investigation on its properties. Surf. Coat. Technol. 2015, 277, 99–106. [Google Scholar] [CrossRef]
- Ghavidel, N.; Allahkaram, S.R.; Naderi, R.; Barzegar, M.; Bakhshandeh, H. Corrosion and wear behavior of an electroless Ni-P/nano-SiC coating on AZ31 Mg alloy obtained through environmentally-friendly conversion coating. Surf. Coat. Technol. 2019, 382, 125156. [Google Scholar] [CrossRef]
- Kousar, H.; Umer, M.A.; Shehzad, K.; Ferdous, R.; Mehmood, K.; Basit, A.; Shahbaz, T.; Yasir, M.; Shakoor, A. Exceptional mechanical properties and wear behavior of Al2O3 nanoparticle reinforced Ni-W-P coatings. Tribol. Int. 2024, 194, 109533. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Li, H.; Liu, Y. Study of the corrosion resistance of a superhydrophobic Ni-P-Al2O3 composite coating based on electrochemical machining. Int. J. Electrochem. Sci. 2019, 14, 6032–6044. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Wang, Y.; Guo, P.; Jiang, W.; Yao, C.; Shu, X. Preparation and properties of Ni-W-P-TiO2 nanocomposite coatings developed by a sol-enhanced electroplating method. Chin. J. Chem. Eng. 2021, 44, 369–376. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Liu, J.; Wang, S.; Li, Z. New method for CO2 corrosion resistance Ni-W-Y2O3-ZrO2 nanocomposite coatings. Ceram. Int. 2019, 45, 6163–6174. [Google Scholar] [CrossRef]
- Chinchu, K.S.; Riyas, A.H.; Sha, M.A.; Geethanjali, C.V.; Saji, V.S.; Shibli, S.M. ZrO2–CeO2 assimilated electroless Ni–P anti-corrosion coatings. Surf. Interface 2020, 21, 100704. [Google Scholar] [CrossRef]
- Cheng, W.; Wen, P.; Wang, T.; Yu, H.; Jiang, M.; Wen, H.; Wang, B. A study of Ni–P electroless coating modified by rare earth oxide (La2O3, Y2O3 and Ce2O3) on pre-phosphorized zirconium alloy. Ferroelectrics 2023, 606, 161–174. [Google Scholar] [CrossRef]
- Elgaddafi, R.; Ahmed, R.; Shah, S. Corrosion of carbon steel in CO2 saturated brine at elevated temperatures. J. Pet. Sci. Eng. 2020, 196, 107638. [Google Scholar] [CrossRef]
- Dong, B.; Zeng, D.; Favero, C.; Divers, T.; Boisse, N.; Xu, Q.; Ling, J.; Xie, K.; Wang, Q. Insight into polymer concentration, temperature, CO2 content, and Cl− content on corrosion of N80 steel tubing in polymer flooding. Mater. Corros. 2023, 74, 943–961. [Google Scholar] [CrossRef]
- Cui, Z.; Wu, S.; Zhu, S.; Yang, X. Study on corrosion properties of pipelines in simulated produced water saturated with supercritical CO2. Appl. Surf. Sci. 2006, 252, 2368–2374. [Google Scholar] [CrossRef]
- Li, D.; Han, D.; Zhang, L.; Lu, M.; Wang, L.; Ma, W. Effects of temperature on CO2 corrosion of tubing and casing steel. NACE-Int. Corros. Conf. Ser. 2013, 17, 2426. [Google Scholar]
- Lu, S.; Liu, W.; Zhang, S.; Qi, X.; Li, X.; Wang, X. Corrosion Performance of Carbon Steel in CO2 Aqueous Environment Containing Silty Sand with Different Sizes. Acta Metall. Sin. 2017, 30, 1055–1066. [Google Scholar] [CrossRef]
- Liao, K.; Leng, J.; Cheng, Y.F.; He, T.; He, G.; Zhao, S.; Liu, X.; Huang, Q. Effect of H2S concentrations on corrosion failure of L245NS steel in CO2-O2-H2S system. Proc. Safety Environ. Prot. 2022, 168, 224–238. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, D.; Zhang, Z.; Zhao, W.; Yao, G. Effect of flow velocity on pipeline steel corrosion behaviour in H2S/CO2 environment with sulphur deposition. Corros. Eng. Sci. Technol. 2018, 53, 370–377. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, W.; Bai, Z.; Feng, Y.; Zhou, W. Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2. Electrochim. Acta 2008, 53, 3690–3700. [Google Scholar] [CrossRef]
- Masamura, K.; Hashizume, S.; Sakai, J.; Matsushima, I. Polarization Behavior of High-Alloy OCTG in CO2 Environment as Affected by Chlorides and Sulfides. Corros. Sci. 1987, 43, 359–365. [Google Scholar] [CrossRef]
- Fierro, G.; Ingo, G.M.; Mancia, F. XPS Investigation on the Corrosion Behavior of 13Cr-Martensitic Stainless Steel in CO2-H2S-Cl Environments. Corros. Sci. 1989, 45, 814–823. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, Y.; Guo, X.; Jiang, F.; Shi, D.; Chen, Z. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of small amount of H2S on the corrosion behavior of carbon steel in the dynamic supercritical CO2 environments. Corros. Sci. 2023, 103, 132–144. [Google Scholar] [CrossRef]
- Sun, C.; Ding, T.; Sun, J.; Lin, X.; Zhao, W.; Chen, H. Insights into the effect of H2S on the corrosion behavior of N80 steel in supercritical CO2 environment. J. Mater. Res. Technol. 2023, 26, 5462–5477. [Google Scholar] [CrossRef]
- Sun, C.; Liu, S.; Li, J.; Zeng, H.; Luo, J. Insights into the Interfacial Process in Electroless Ni-P Coating on Supercritical CO2 Transport Pipeline as Relevant to Carbon Capture and Storage. ACS Appl. Mater. Interfaces 2019, 11, 16243–16251. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zangari, G. Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions. Electrochim. Acta 2002, 47, 2969–2979. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Chen, X.; Lang, P.; Liang, Y. High corrosion resistance of electroless Ni-P with chromium-free conversion pre-treatments on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 921–928. [Google Scholar]
- Wang, J.; Wu, Q. The effects of anodic interlayer on the morphology and mechanical performances of electroless Ni–P coating on Al alloy. Appl. Phys. A 2017, 123, 435. [Google Scholar] [CrossRef]
- Li, J.; Sun, C.; Roostaei, M.; Mahmoudi, M.; Luo, J. Role of Ca2+ in the CO2 corrosion behavior and film characteristics of N80 steel and electroless Ni-P coating at high temperature and high pressure. Mater. Chem. Phys. 2021, 267, 124618. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.; Lu, M. Corrosion behavior and mechanism of 3Cr steel in CO2 environment with various Ca2+ concentration. Corros. Sci. 2018, 136, 210–220. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, H.; Luo, J. Unraveling the effects of CO2 and H2S on the corrosion behavior of electroless Ni-P coating in CO2/H2S/Cl– environments at high temperature and high pressure. Corros. Sci. 2019, 148, 317–330. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xiao, J.; Yan, J.; Tang, Z. Time-dependent corrosion behavior of electroless Ni–P coating in H2S/Cl environment. Int. J. Hydrogen Energy 2021, 46, 11849–11864. [Google Scholar] [CrossRef]
- Yu, J.; Jing, T.; Yang, J.; Li, Q. Determination of activation energy for crystallizations in Ni–Sn–P amorphous alloys. J. Mater. Process. Technol. 2012, 85, 761–767. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Li, R. Characterization of Ni and Ni-Co Alloy Coatings Prepared by Pulse Current Electroplating. Adv. Mater. Res. 2011, 295–297, 1590–1593. [Google Scholar] [CrossRef]
- Atta, N.F.; Fekry, A.M.; Hassaneen, H.M. Corrosion inhibition, hydrogen evolution and antibacterial properties of newly synthesized organic inhibitors on 316L stainless steel alloy in acid medium. Int. J. Hydrogen Energy 2011, 36, 6462–6471. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Electrochemical studies on electroless ternary and quaternary Ni–P based alloys. Electrochim. Acta 2006, 52, 1064–1074. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Nazari, M.H.; Mamaghani, S.; Zarebidaki, A. Characterization and corrosion behavior of electroless Ni–P/nano-SiC coating inside the CO2 containing media in the presence of acetic acid. Mater. Des. 2011, 32, 750–755. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fekry, A.M. Inhibition effect of newly synthesized heterocyclic organic molecules on corrosion of steel in alkaline medium containing chloride. Int. J. Hydrogen Energy 2010, 35, 11387–11396. [Google Scholar] [CrossRef]
- He, W.; Knudsen, O.Ø.; Diplas, S. Corrosion of stainless steel 316L in simulated formation water environment with CO2–H2S–Cl. Corros. Sci. 2009, 51, 2811–2819. [Google Scholar] [CrossRef]
- Xu, C.; Chen, L.; Yu, L.; Zhang, J.; Zhang, Z.; Wang, J. Effect of pickling processes on the microstructure and properties of electroless Ni–P coating on Mg–7. 5Li–2Zn–1Y alloy. Prog. Nat. Sci. 2014, 24, 655–662. [Google Scholar] [CrossRef]
- Zhang, Z.; He, Y.; Bai, Y.; Song, R.; He, Y.; Liu, B.; Li, H.; Shangguan, J. Influence of iron element on the structure and corrosion resistance of Ni-P coatings in different corrosive environments. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130100. [Google Scholar] [CrossRef]
- Liu, L.; Peng, J.; Du, X.; Zheng, H.; Cao, X. Synthesis, composition, morphology, and wettability of electroless Ni-Fe-P coatings with varying microstructures. Thin Solid Films 2020, 706, 138080. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, D.; Tang, D.; Han, E.; Wang, J. Study on corrosion behavior of Ni–P/Ni–Cu–P superhydrophobic composite coatings preparation on L360 steel by two-step method. J. Mater. Res. Technol. 2023, 23, 3035–3047. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Wang, L.; Wang, S.; Liu, C.; Wang, J. Corrosion behavior of electroless deposited Ni–Cu–P coating in flue gas condensate. Surf. Coat. Technol. 2010, 204, 3382–3386. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Duan, Y.; Mstsuda, S.; Yong, K. Metastable phase evolution and nanoindentation behavior of amorphous Ni–Cu–P coating during heat treatment process. J. Alloys Compd. 2019, 805, 597–608. [Google Scholar] [CrossRef]
- Asgari, M.; Abedi, B.; Ashrafi, A.; Darband, G.B.; Monirvaghefi, M. Electroless Deposition of Ni-W-P films as binder-free, efficient and durable electrode for electrochemical hydrogen evolution. Mater. Res. Bull. 2023, 166, 112318. [Google Scholar] [CrossRef]
- He, F.; Fang, Y.; Jin, S. The study of corrosion-wear mechanism of Ni-W-P alloy. Wear 2014, 311, 14–20. [Google Scholar] [CrossRef]
- Sa, Z. Study on Electrodeposition of Ni-W-P Alloy Coating on Q235 carbon steel from Pyrophosphate Bath and Its Corrosion Resistance. Int. J. Electrochem. Sci. 2022, 10, 20964. [Google Scholar] [CrossRef]
- Dong, D.; Chen, X.; Xiao, W.; Yang, G.; Zhang, P. Preparation and properties of electroless Ni–P–SiO2 composite coatings. Appl. Surf. Sci. 2009, 255, 7051–7055. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Xu, R.; Zhan, P. Probe into deposition mechanism of double pulse electrodepositing Ni-W-P matrix composite coatings containing CeO2 and SiO2 nano-particles(Article). J. Rare Earths 2010, 28, 437–441. [Google Scholar] [CrossRef]
- Hu, J.; Wang, B.; Xu, Y.; Zhou, L. The Anticorrosive and Antifouling Properties of Ni-W-P-nSiO2 Composite Coating in A Simulated Oilfield Environment. JOM 2018, 70, 2619–2625. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Zhang, Y.; Xu, X. The effects of SiO2 coating on diamond abrasives in sol-gel tool for SiC substrate polishing. Diam. Relat. Mater. 2017, 76, 123–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).