Composite Paints with High Content of Metallic Microparticles for Electromagnetic Shielding Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Composite Paints with Metallic Microparticles
2.2. Substrate Materials for Paint Deposition
2.3. Deposition of Paints on Plastic Substrate Samples
2.4. Testing Equipment and Methods
3. Results and Discussion
3.1. Dielectric Tests on Conductive Paints with Additives
3.2. DSC Analysis
3.3. Thickness of the Paint Layers
3.4. Surface Topography (Roughness)
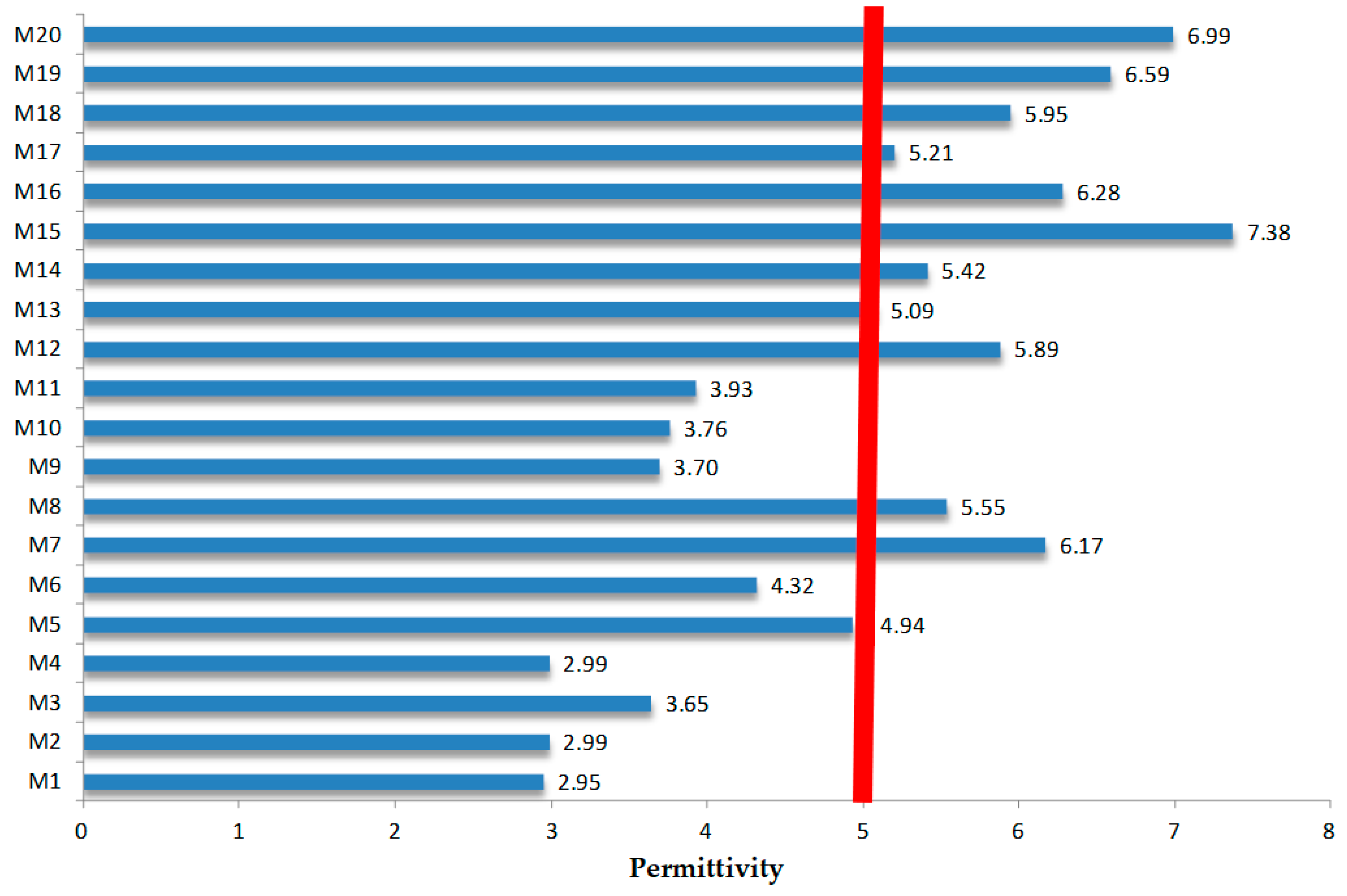
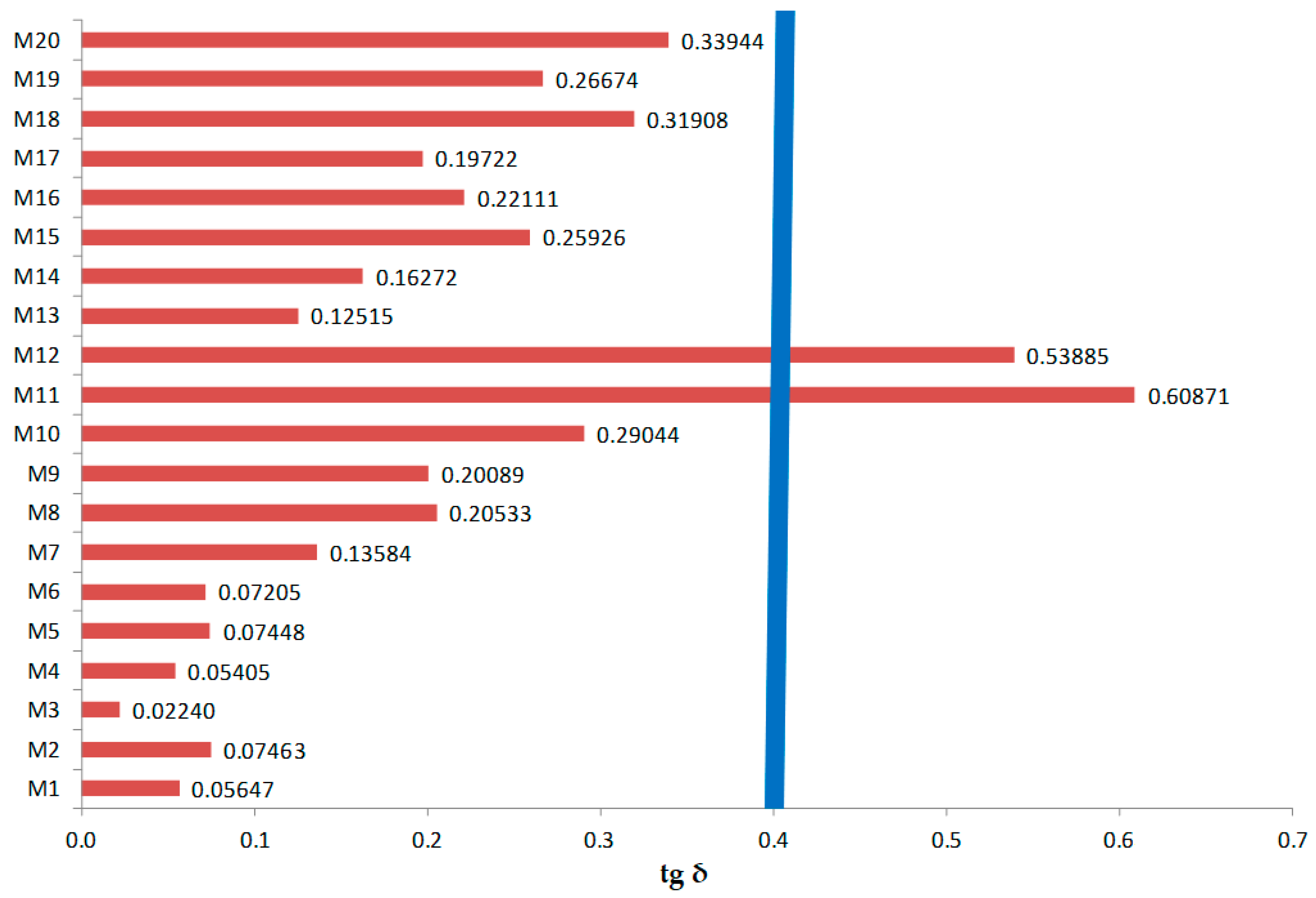
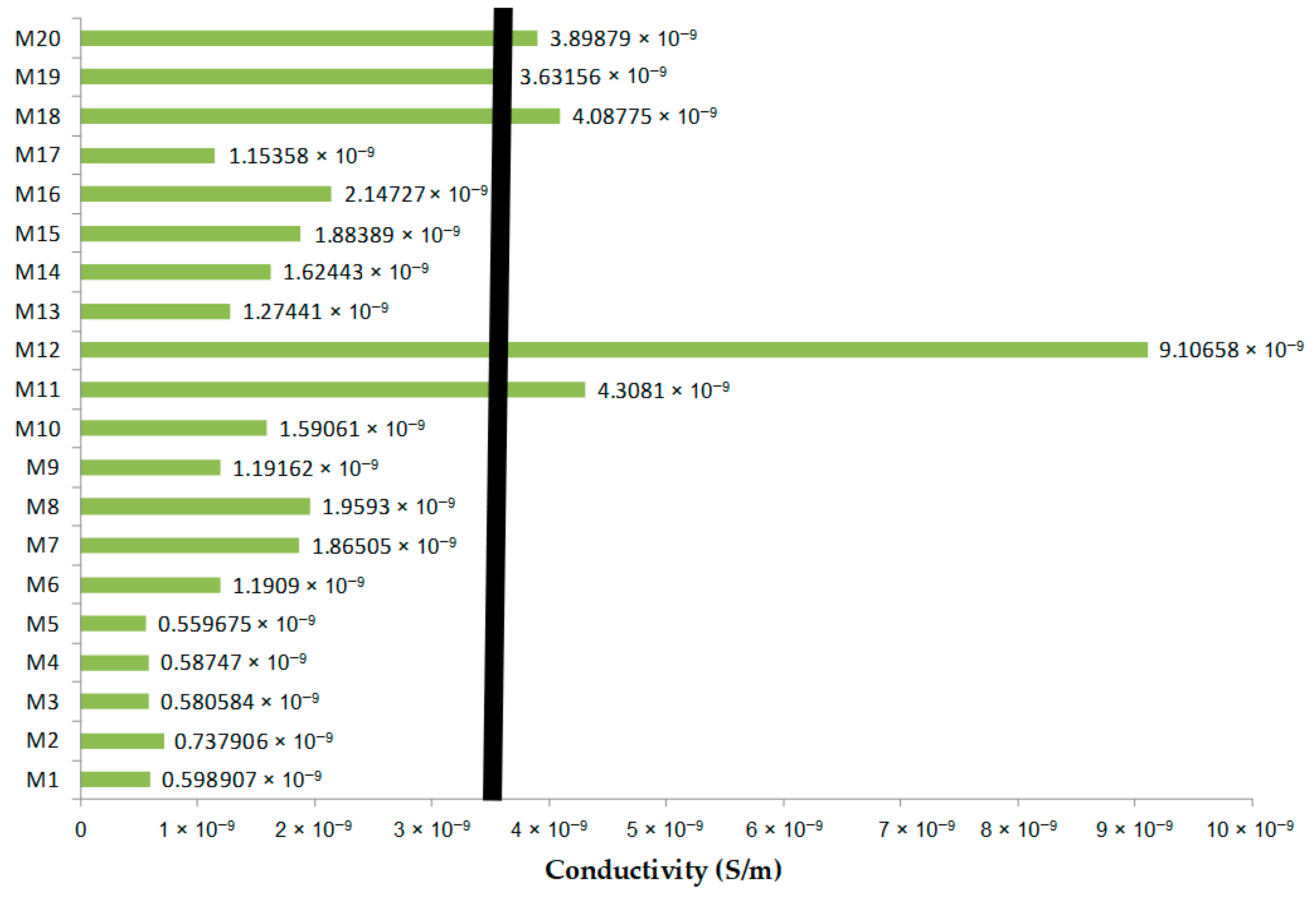
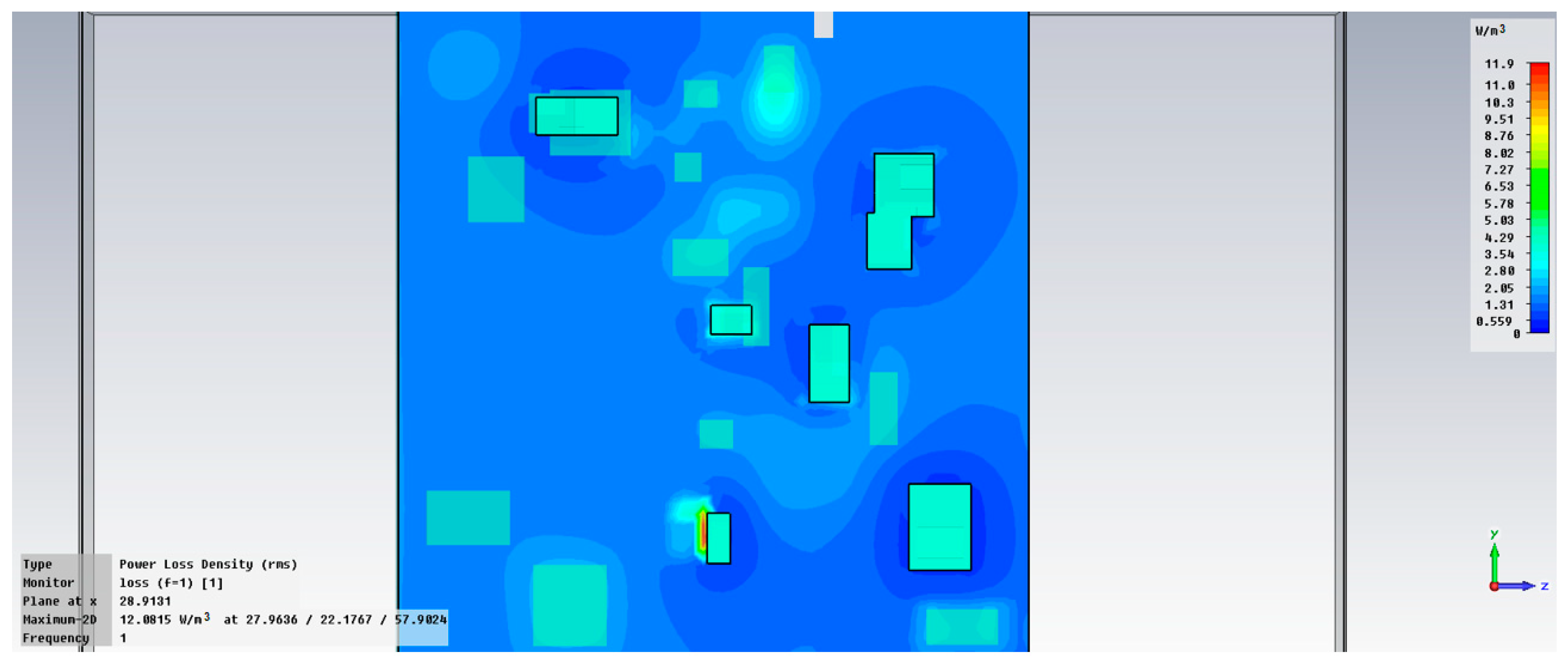
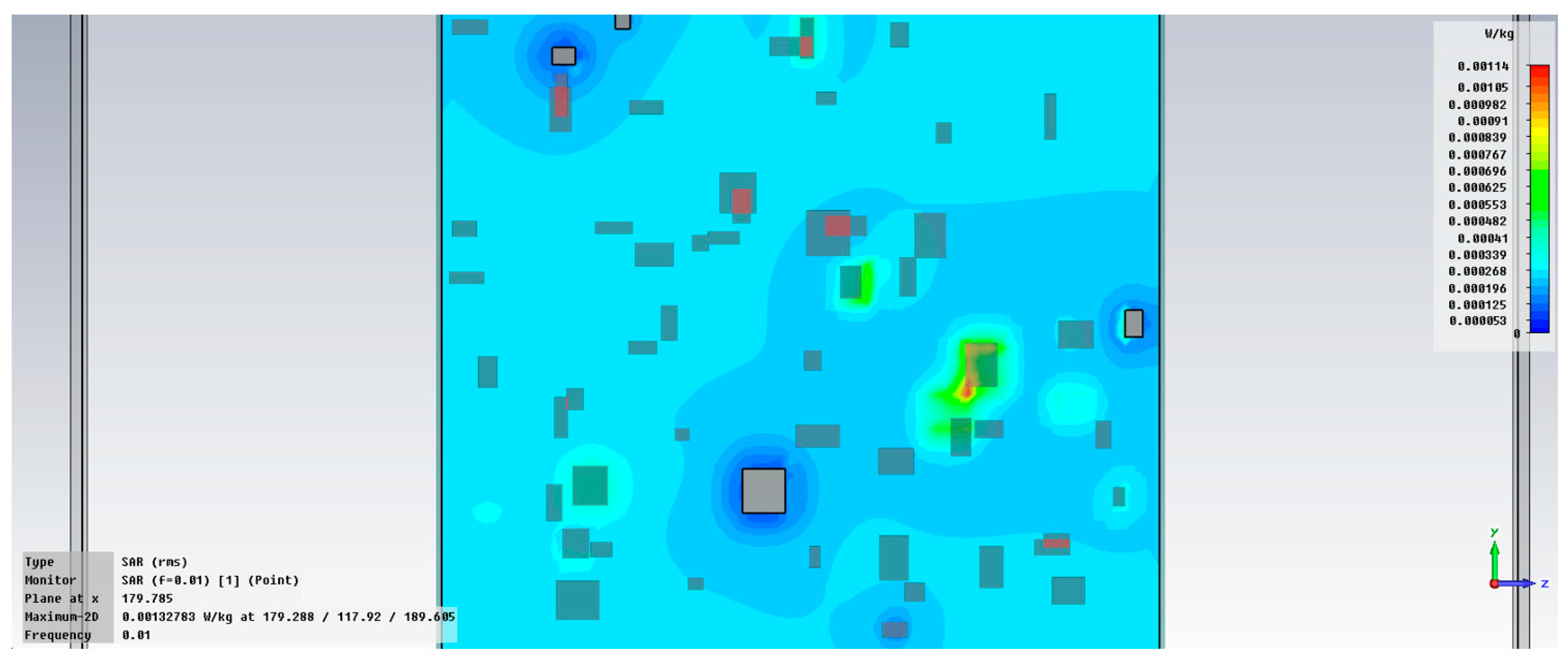
3.5. Comparative Analysis of Dielectric Parameters for M1–M20 Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMC Electrically Conductive Paints. Available online: https://www.solianiemc.com/en/cp/emc-electrically-conductive-paints/?gad_source=1&gclid=Cj0KCQjwjLGyBhCYARIsAPqTz1-DOF1Sw7J8wFzhbDIZLBd4b8Z5UxsiopHr2oeQ_mM4k1MuQkk-btgaAsFvEALw_wcB (accessed on 30 May 2024).
- EMF and RFI Shielding with Conductive Paints. Available online: https://www.antala.uk/emf-and-rfi-shielding-with-conductive-paints/ (accessed on 30 May 2024).
- Conductive Paints and Coatings. Available online: https://limitless-shielding.com/conductive-paints-and-coatings/ (accessed on 30 May 2024).
- ECOS Paints. Available online: https://ecospaints.net/blog/what-to-know-about-electromagnetic-shielding-materials (accessed on 30 May 2024).
- Conductive Translucent Paint. Available online: https://hollandshielding.com/en/conductive-translucent-paint?gad_source=1&gclid=CjwKCAjw9cCyBhBzEiwAJTUWNZC2A5l8HgsLs4KaJrIJbPz6e8rSoxmem-6Wv9nuq7kMi8Koz6ZhDhoCCHAQAvD_BwE (accessed on 30 May 2024).
- Conductive Paints. Available online: https://mgchemicals.com/category/conductive-paint/ (accessed on 30 May 2024).
- Electric Paint. Available online: https://www.bareconductive.com/collections/electric-paint (accessed on 30 May 2024).
- Electrically Conductive Paints. Available online: https://www.euro-technologies.eu/en/product/electrically-conductive-paints/ (accessed on 30 May 2024).
- Künniger, T.; Heeb, M.; Arnold, M. Antimicrobial efficacy of silver nanoparticles in transparent wood coatings. Eur. J. Wood Wood Prod. 2014, 72, 285–288. [Google Scholar] [CrossRef]
- Marolt, T.; Sever Škapin, A.; Bernard, J.; Živec, P.; Gaberšček, M. Photocatalytic activity of anatase-containing facade coatings. Surf. Coat. Technol. 2011, 206, 1355–1361. [Google Scholar] [CrossRef]
- Solano, R.; Patino-Ruiz, D.; Herrera, A. Preparation of modified paints with nano-structured additives and its potential applications. Nanomater. Nanotechnol. 2020, 10, 1847980420909188. [Google Scholar] [CrossRef]
- Héritier, P.A. The Impact of Particulate Matters and Nanoparticles on Thermoplastic Polymer Coatings and Paint Layers. Polymers 2022, 14, 2477. [Google Scholar] [CrossRef] [PubMed]
- González-Barriuso, M.; Soriano, L. Conductive paints development through nanotechnology. Prog. Org. Coat. 2016, 95, 85–90. [Google Scholar]
- Tudose, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Popescu, M.; Khomenko, V.; Butenko, O.; Chernysh, O.; Kenanakis, G.; et al. Novel Water-Based Paints for Composite Materials Used in Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Tudose, I.V.; Mouratis, K.; Ionescu, O.N.; Romanitan, C.; Pachiu, C.; Pricop, E.; Khomenko, V.H.; Butenko, O.; Chernysh, O.; Barsukov, V.Z.; et al. Carbon Allotropes-Based Paints and Their Composite Coatings for Electromagnetic Shielding Applications. Nanomaterials 2022, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Barsukov, V.; Senyk; Kuryptia, Y.; Butenko, O.; Chernysh, O.; Khomenko, V.; Suchea, M.; Koudoumas, E. Composite paints for electromagnetic shielding. AIP Conf. Proc. 2023, 2803, 020001. [Google Scholar] [CrossRef]
- Khomenko, V.; Butenko, O.; Chernysh, O.; Barsukov, V.; Suchea, M.P.; Koudoumas, E. Electromagnetic Shielding of Composite Films Based on Graphite, Graphitized Carbon Black and Iron-Oxide. Coatings 2022, 12, 665. [Google Scholar] [CrossRef]
- Demirci, G.; Okuyucu, S.; Hoda, N.; Topel, Ö. Assessment of Electromagnetic Shielding Efficacy of Magnetic Metal Oxide Nanoparticles and their Application in Electromagnetic Shielding Paints. Chem. Eur. 2024, 9, e202304078. [Google Scholar] [CrossRef]
- Barsukov, V.; Senyk, I.; Kryukova, O.; Butenko, O. Composite Carbon-Polymer Materials for Electromagnetic Radiation Shielding. Mater. Today Proc. 2018, 5, 15909–15914. [Google Scholar] [CrossRef]
- Folgueras, L.; Alves, M.; Rezende, M.C. Electromagnetic Radiation Absorbing Paints Based on Carbonyl Iron and Polyaniline. In Proceedings of the 2009 SBMO/IEEE MTT-S International Microwave and Optoelectronics Conference (IMOC), Belem, Brazil, 3–6 November 2009. [Google Scholar] [CrossRef]
- Graphene Based Paint-Like Composites for Electromagnetic Shielding in the GHz Frequency Range. Available online: https://www.phantomsnet.net/Graphene_Conf/2014/Abstracts/[email protected]_graphene%20based%20layers%20for%20electromagnetic%20shielding.pdf (accessed on 30 May 2024).
- Kim, Y.; Lee, B.W. A Study on MXene/Graphene Composite Coatings for Electromagnetic Shielding Paints. Meet. Abstr. 2023, MA2023-02, 1042. [Google Scholar] [CrossRef]
- Paints and Varnishes. Determination of Density. Part 4: Cylinder Method under Pressure: SR EN ISO 2811-2002. Available online: https://magazin.asro.ro/ro/standard/191719 (accessed on 30 May 2024).
- SR ISO 6504-3:2003; Paints and Varnishes. Determination of Coverage Power. ISO: Geneva, Switzerland, 2003. Available online: https://magazin.asro.ro/ro/standard/77636 (accessed on 30 May 2024).
- BS EN 29117:1992; Paints and Varnishes. Determination of Through-Dry State and Through-Dry Time. ISO: Geneva, Switzerland, 1992. Available online: https://standards.iteh.ai/catalog/standards/cen/5972906c-2cb8-4c79-8484-ad3b4903d282/en-29117-1992 (accessed on 30 May 2024).
- ASTM E2009-08(2014)e1; Standard Test Methods for Oxidation Onset Temperature of Hydro-Carbons by Differential Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2014. Available online: https://www.astm.org/e2009-08r14e01.html (accessed on 30 May 2024).
- Optics and Optical Instruments: ISO 10109:2014, Revised in 2024 for Environmental Testing. Available online: https://www.iso.org/standard/82186.html (accessed on 30 May 2024).
- CST Studio Suite Software. Available online: https://www.3ds.com/products/simulia/electromagnetic-simulation (accessed on 20 June 2024).
- Duan, Z.; Linton, D.; Scanlon, W. Investigating the Permittivity of Three-Dimensional Metal Particles Embedded in a Dielectric Medium with Random Distributions. In Proceedings of the 2nd International Congress on Advanced Electromagnetic Materials in Microwaves and Optics, Pamplona, Spain, 21–26 September 2008; pp. 217–219. [Google Scholar]
- Ghodgaonkar, D.K.; Varadan, V.V.; Varadan, V.K. Free-Space Measurement of Complex Permittivity and Complex Permeability of Magnetic Materials at Microwave Frequencies. IEEE Trans. Instrum. Meas. 1990, 39, 387–394. [Google Scholar] [CrossRef]
- Automotive Electromagnetic Compatibility (EMC) Test Standards. Available online: https://learnemc.com/automotive-emc-test-standards (accessed on 30 May 2024).
- Patent WO 2009/095654 A1. Available online: https://patentimages.storage.googleapis.com/0a/3e/61/befbb893b347a6/WO2009095654A1.pdf (accessed on 20 June 2024).
- Plastic Metallization. Available online: https://www.teprosa.de/en/metallization/ (accessed on 30 May 2024).
- Metallization of Plastics. Available online: https://www.cybershieldinc.com/wp-content/uploads/2013/05/Cybershield-Metallization-of-Plastics.pdf (accessed on 30 May 2024).
- Metallization Solutions. Available online: https://impactcoatings.com/metallization/ (accessed on 30 May 2024).
- The Rise of Water-Based Coatings. Available online: https://www.pcimag.com/articles/112266-the-rise-of-water-based-coatings (accessed on 20 June 2024).
- Global EMI & RFI Shielding Materials Market Overview. Available online: https://www.marketresearchfuture.com/reports/emi-rfi-shielding-materials-market-7687?utm_term=&utm_campaign=&utm_source=adwords&utm_medium=ppc&hsa_acc=2893753364&hsa_cam=20373674291&hsa_grp=150985931723&hsa_ad=665909881832&hsa_src=g&hsa_tgt=dsa-2089395925824&hsa_kw=&hsa_mt=&hsa_net=adwords&hsa_ver=3&gad_source=1 (accessed on 20 June 2024).


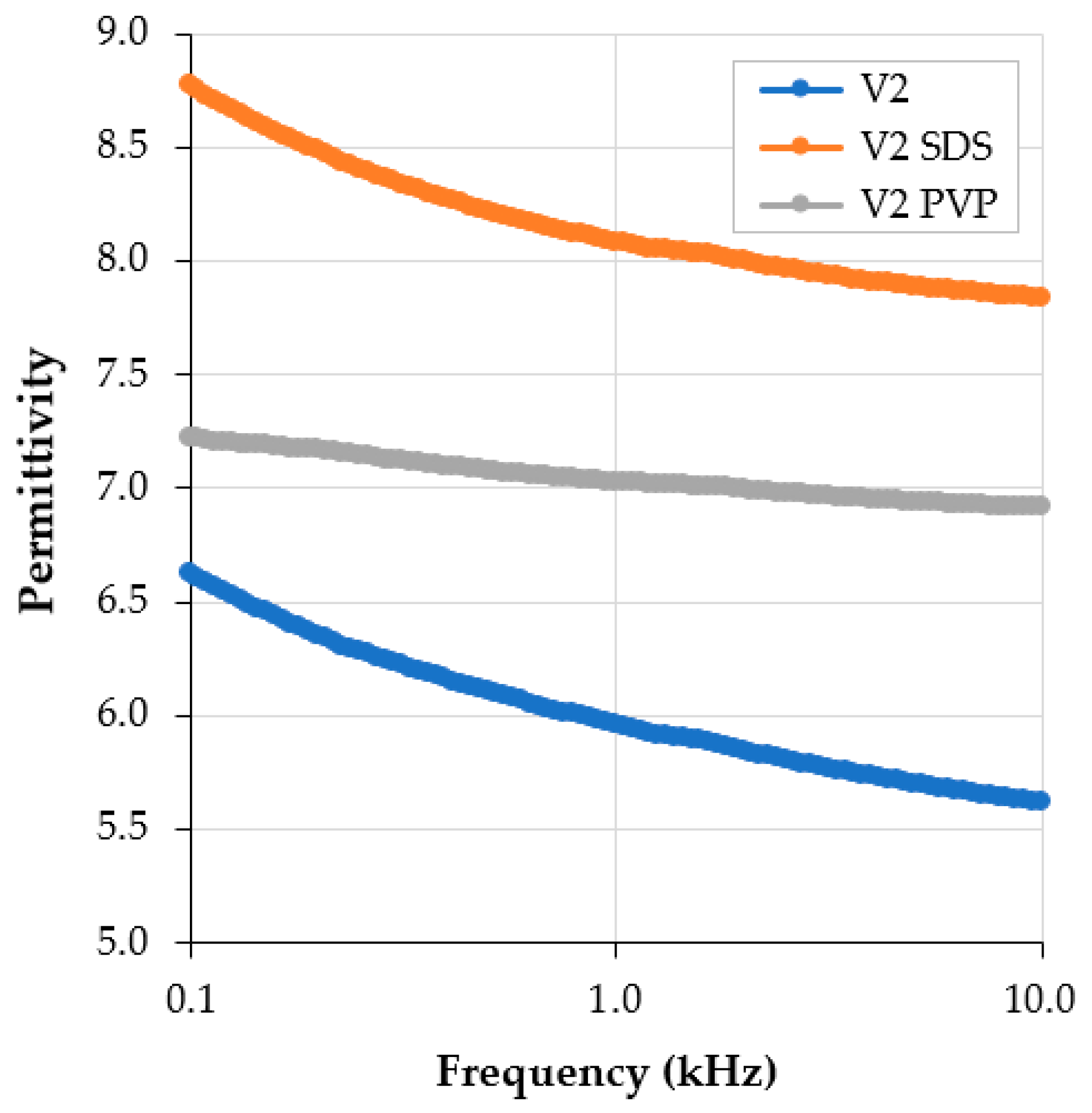
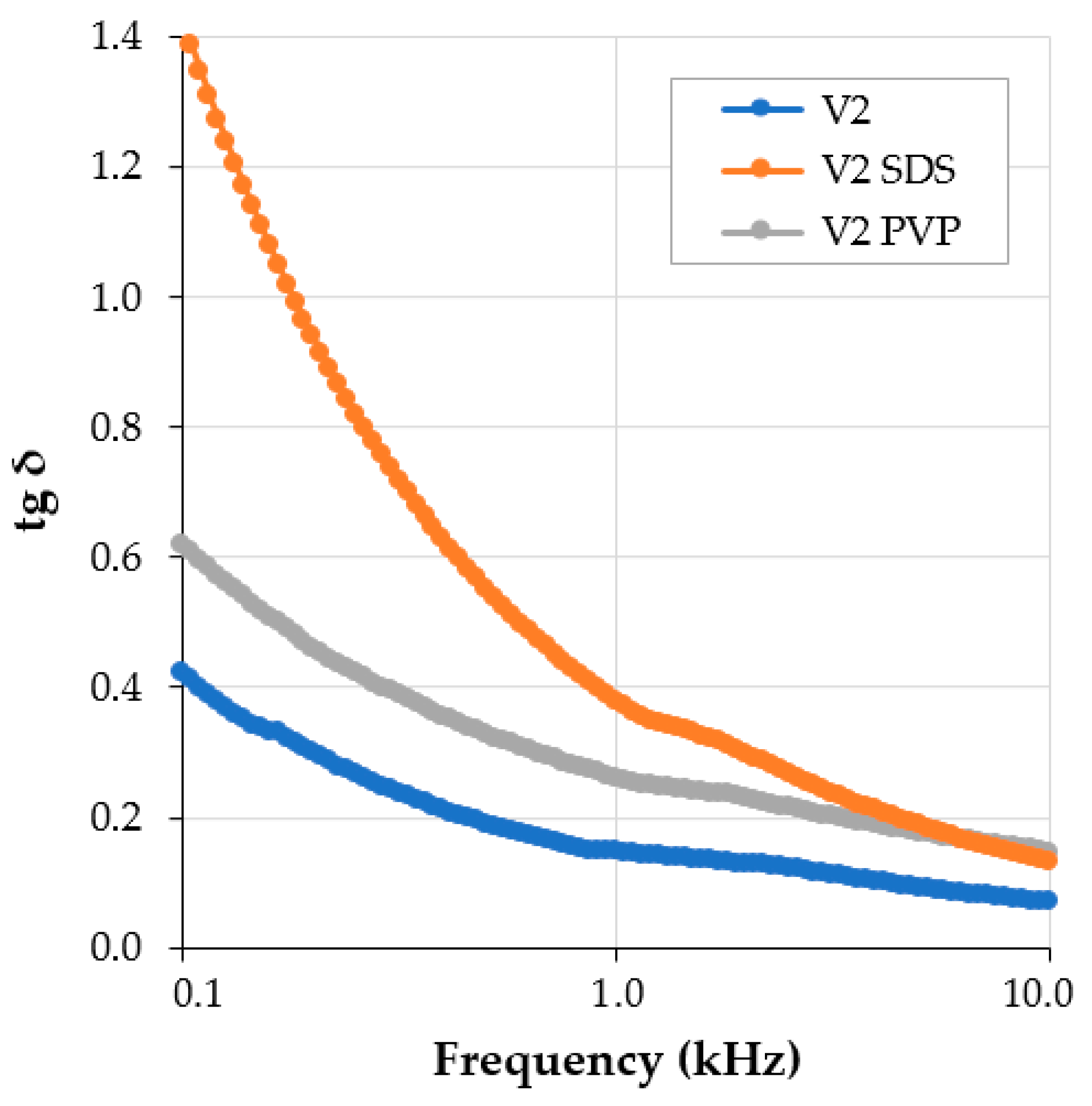

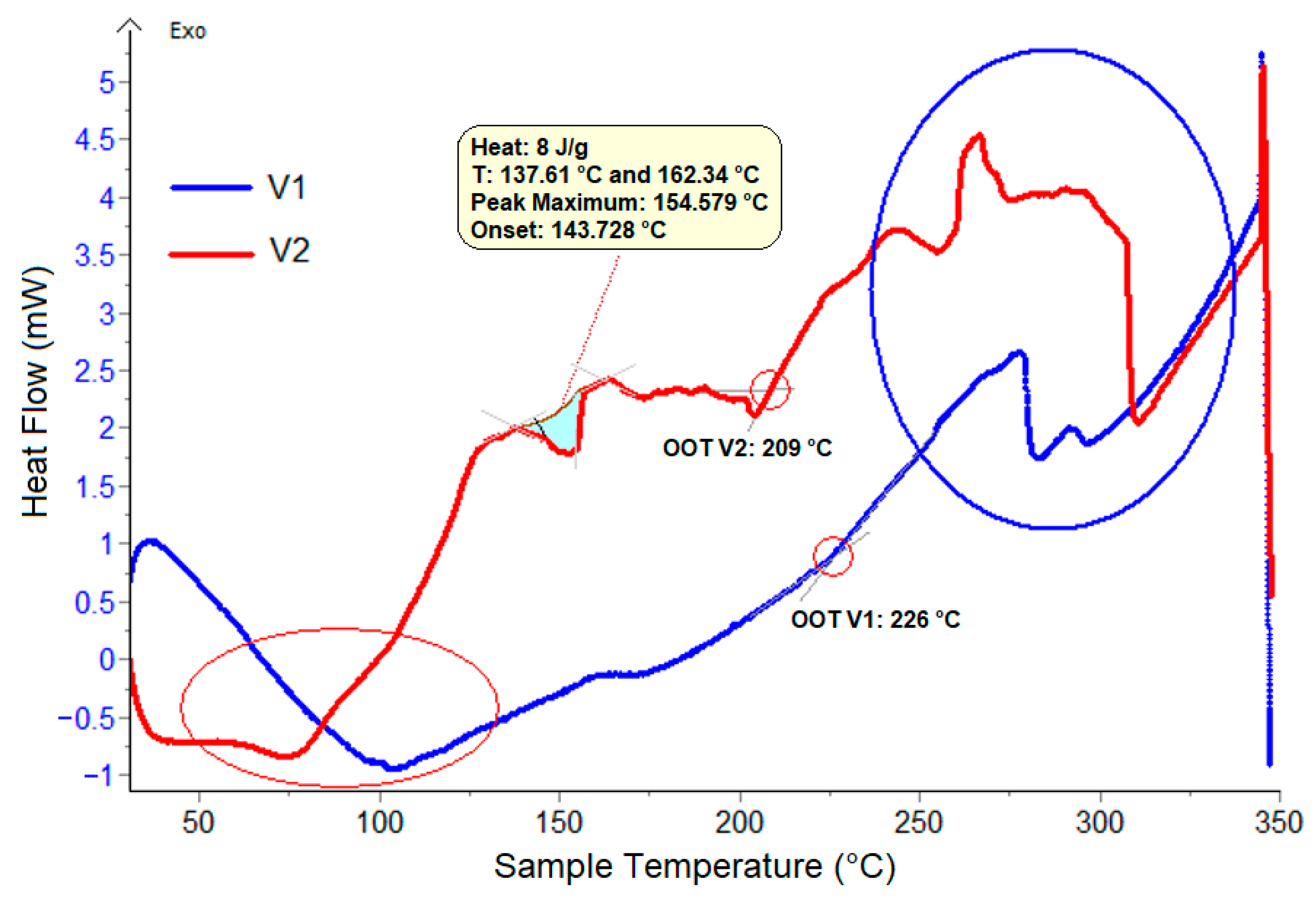


| No. | Characteristic | Unit | Value | Analysis Method |
|---|---|---|---|---|
| 1. | Appearance | – | homogeneous- thixotropic | Visual examination |
| 2. | Density, at 20 °C | g/cm3 | 1.48/1.72 ± 0.05 | SR EN ISO 2811– 2002 [23] |
| 3. | Covering power | number of layers | 1–2 | SR ISO 6504–3:2003 [24] |
| 4. | Specific consumption | g/m2 per layer | 130–180/150–200 | Function of roughness area |
| 5. | Appearance | – | continuous film, matt | Visual examination |
| 6. | Drying time: at touch; while recovering | hours | 4/3 10/9 | BS EN 29117:1992 [25] |
| No. | Characteristic | Unit | Value | Analysis Method |
|---|---|---|---|---|
| 1. | Appearance | – | homogeneous- thixotropic | Visual examination |
| 2. | Density, at 20 °C | g/cm3 | 1.88/2.66 ± 0.05 | SR EN ISO 2811– 2002 [23] |
| 3. | Covering power | number of layers | 1–2 | SR ISO 6504–3:2003 [24] |
| 4. | Specific consumption | g/m2 per layer | 350–500/450–650 | Function of roughness area |
| 5. | Appearance | – | continuous film, matt | Visual examination |
| 6. | Drying time: at touch; while recovering | hours | 3/2 9/8 | BS EN 29117:1992 [25] |
| Sample Code | Thickness (µm) |
|---|---|
| M6 | 19.96 |
| M8 | 25.87 |
| M10 | 20.49 |
| M12 | 26.13 |
| M15 | 26.20 |
| M19 | 27.58 |
| Sample | Ra (µm) | Rq (µm) | Rt (µm) |
|---|---|---|---|
| PC S with V1/Al | 2.95 | 3.73 | 25.65 |
| PP with V1/Al | 4.72 | 5.57 | 30.90 |
| PVC with V1/Al | 3.14 | 3.90 | 27.72 |
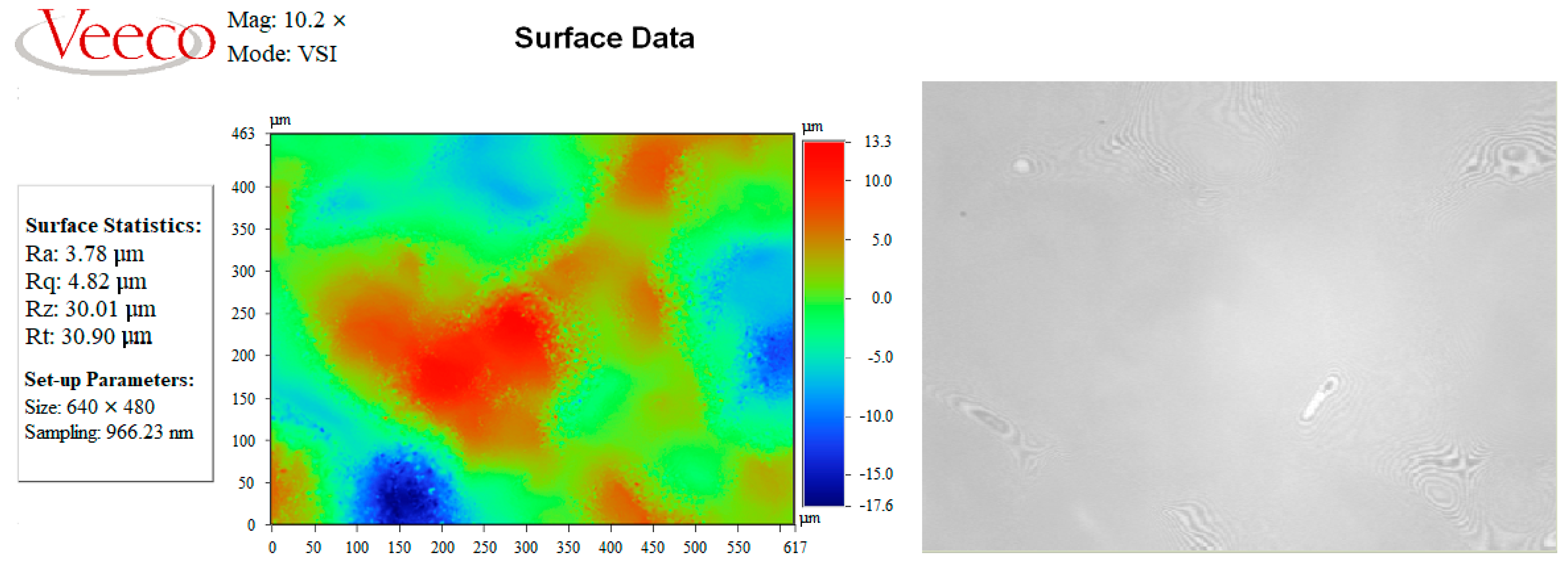
| PC S with V2/Al | 3.78 | 4.82 | 30.90 |
| PP with V2/Al | 4.06 | 5.00 | 36.05 |
| PVC with V2/Al | 4.00 | 4.99 | 35.52 |
| PC S with V1/Fe | 1.01 | 1.25 | 8.39 |
| PP with V1/Fe | 1.19 | 1.51 | 9.84 |
| PVC with V1/Fe | 1.05 | 1.32 | 8.81 |
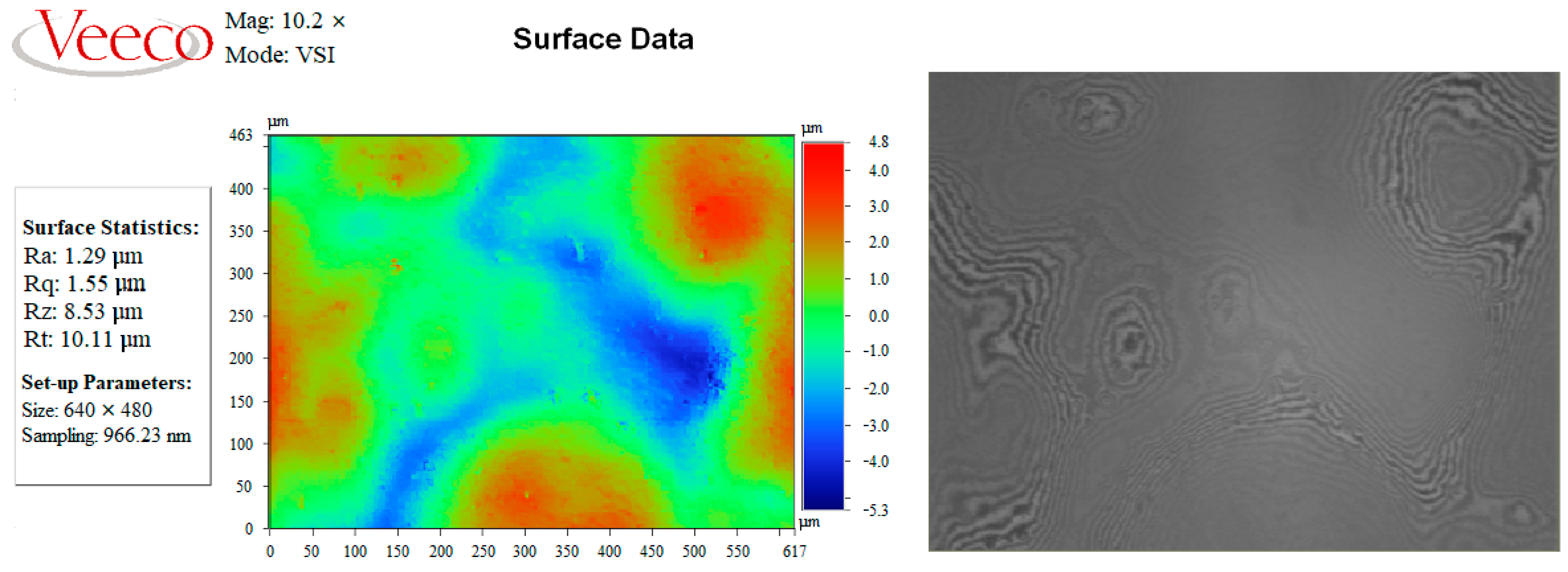
| PC S with V2/Fe | 1.29 | 1.55 | 10.11 |
| PP with V2/Fe | 1.47 | 2.02 | 12.83 |
| PVC with V2/Fe | 1.42 | 1.79 | 11.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, R.C.; Aradoaei, M.; Caramitu, A.R.; Lungu, M.V.; Schreiner, O.D.; Ion, I. Composite Paints with High Content of Metallic Microparticles for Electromagnetic Shielding Purposes. Coatings 2024, 14, 874. https://doi.org/10.3390/coatings14070874
Ciobanu RC, Aradoaei M, Caramitu AR, Lungu MV, Schreiner OD, Ion I. Composite Paints with High Content of Metallic Microparticles for Electromagnetic Shielding Purposes. Coatings. 2024; 14(7):874. https://doi.org/10.3390/coatings14070874
Chicago/Turabian StyleCiobanu, Romeo Cristian, Mihaela Aradoaei, Alina Ruxandra Caramitu, Magdalena Valentina Lungu, Oliver Daniel Schreiner, and Ioana Ion. 2024. "Composite Paints with High Content of Metallic Microparticles for Electromagnetic Shielding Purposes" Coatings 14, no. 7: 874. https://doi.org/10.3390/coatings14070874






