Preparation and Characterization of Melamine–Benzoguanamine–Formaldehyde Resins and Their Flame-Retardant Properties in Impregnated Paper for Low Pressure Laminates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
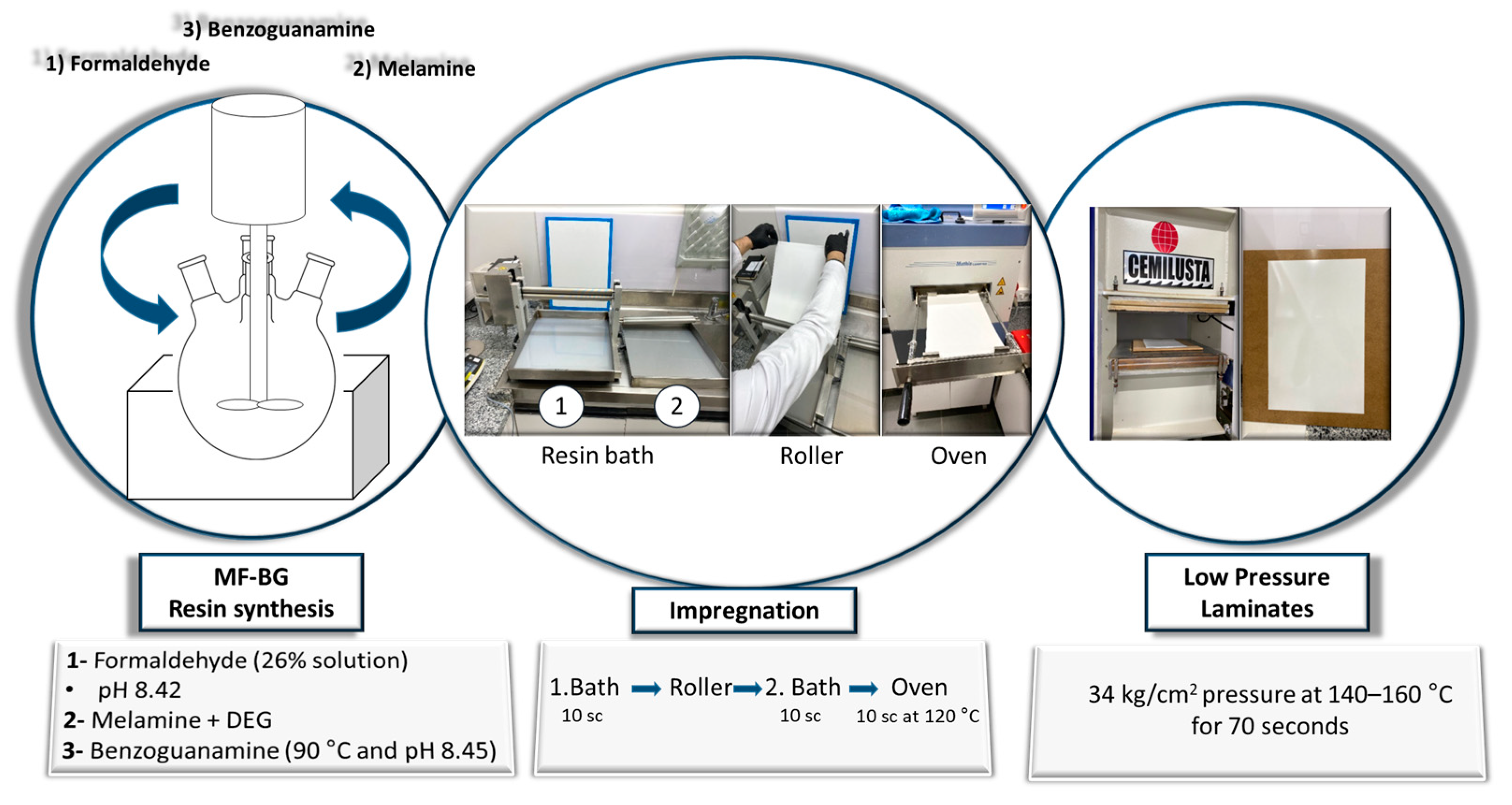
2.2. Synthesis of Resins
2.3. Physical Properties of the Resin
2.4. Paper Impregnation
2.5. Low-Pressure Laminates Production
2.6. Low-Pressure Laminate Characterization
2.7. Characterization
3. Results and Discussion
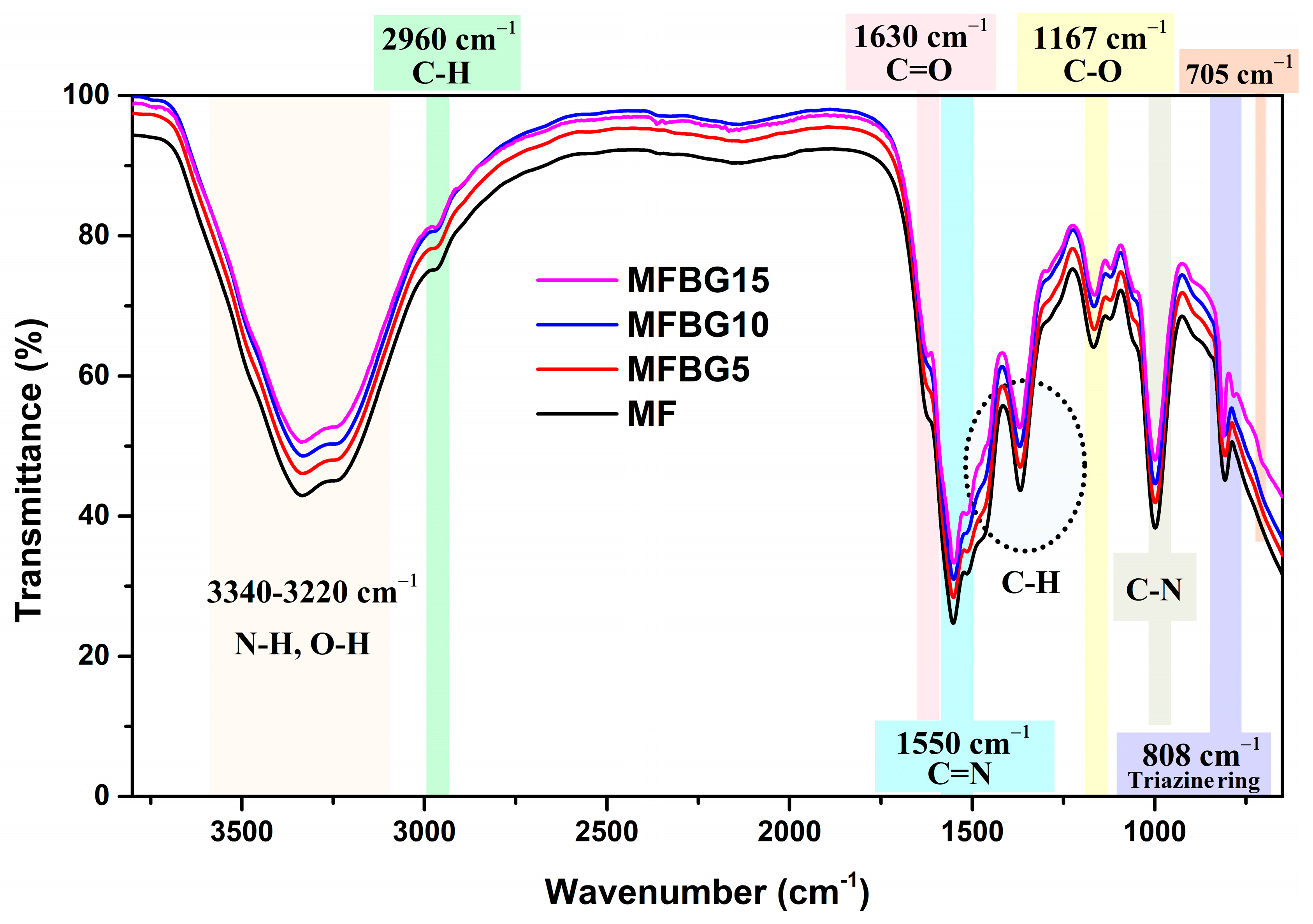
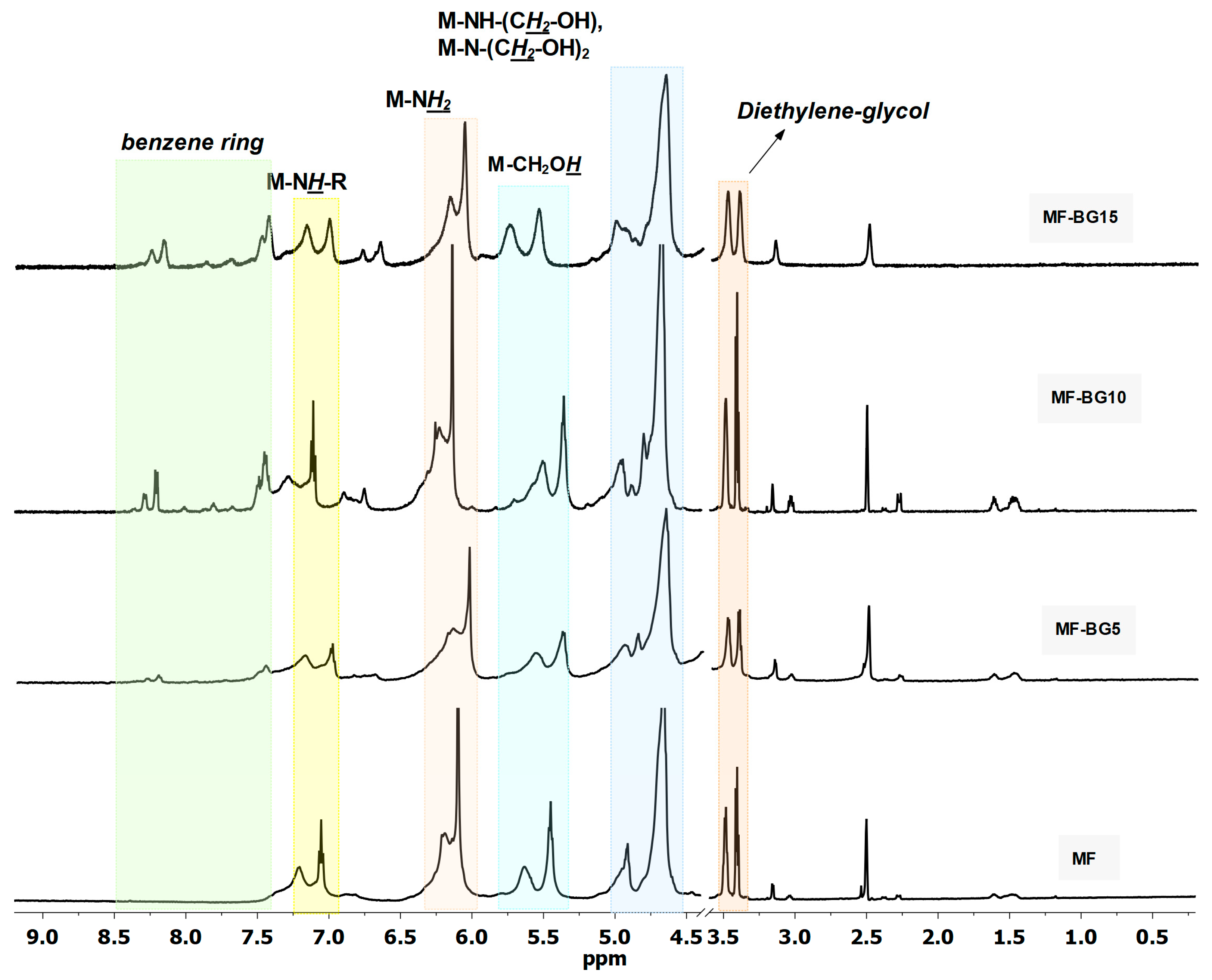
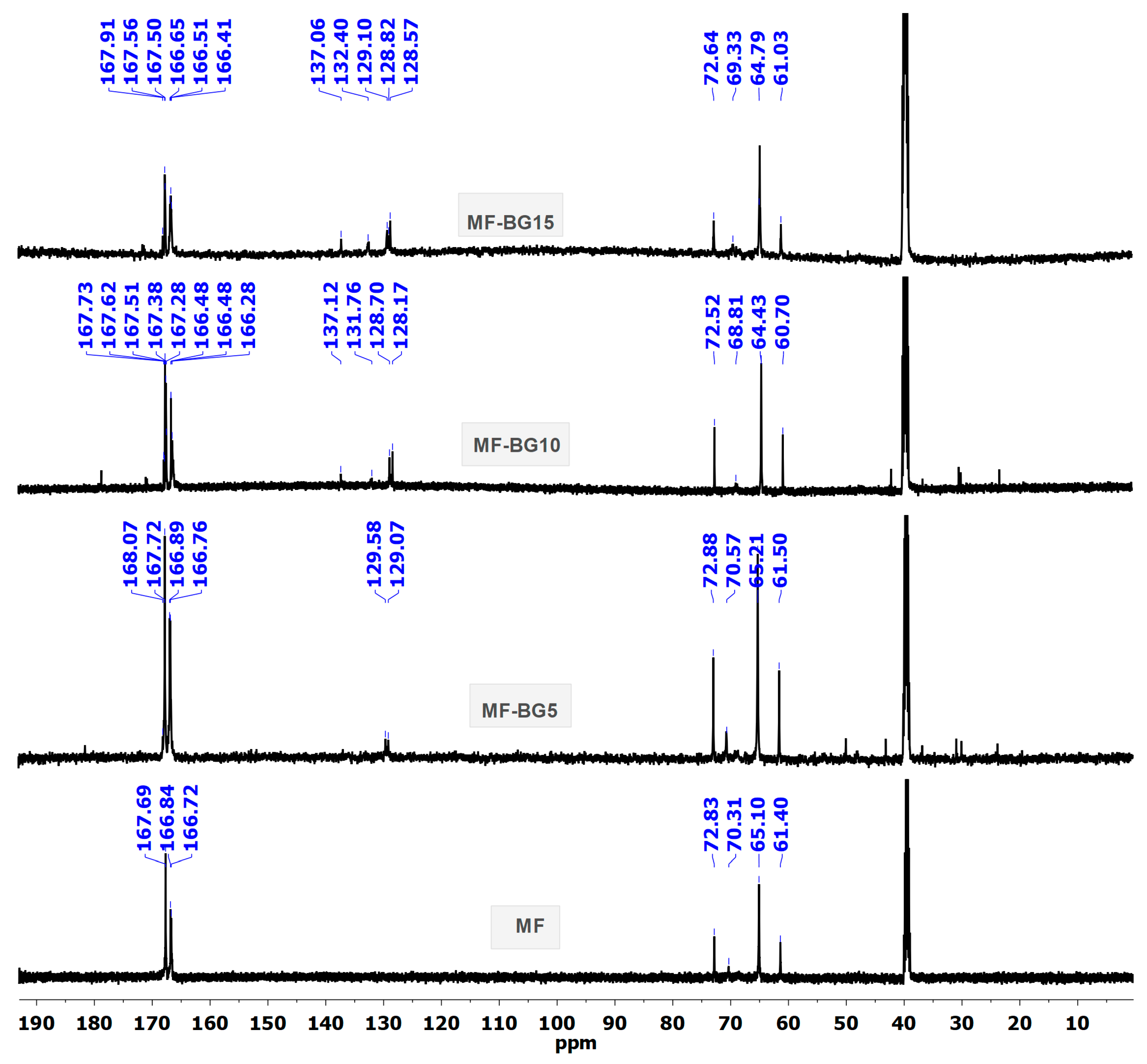
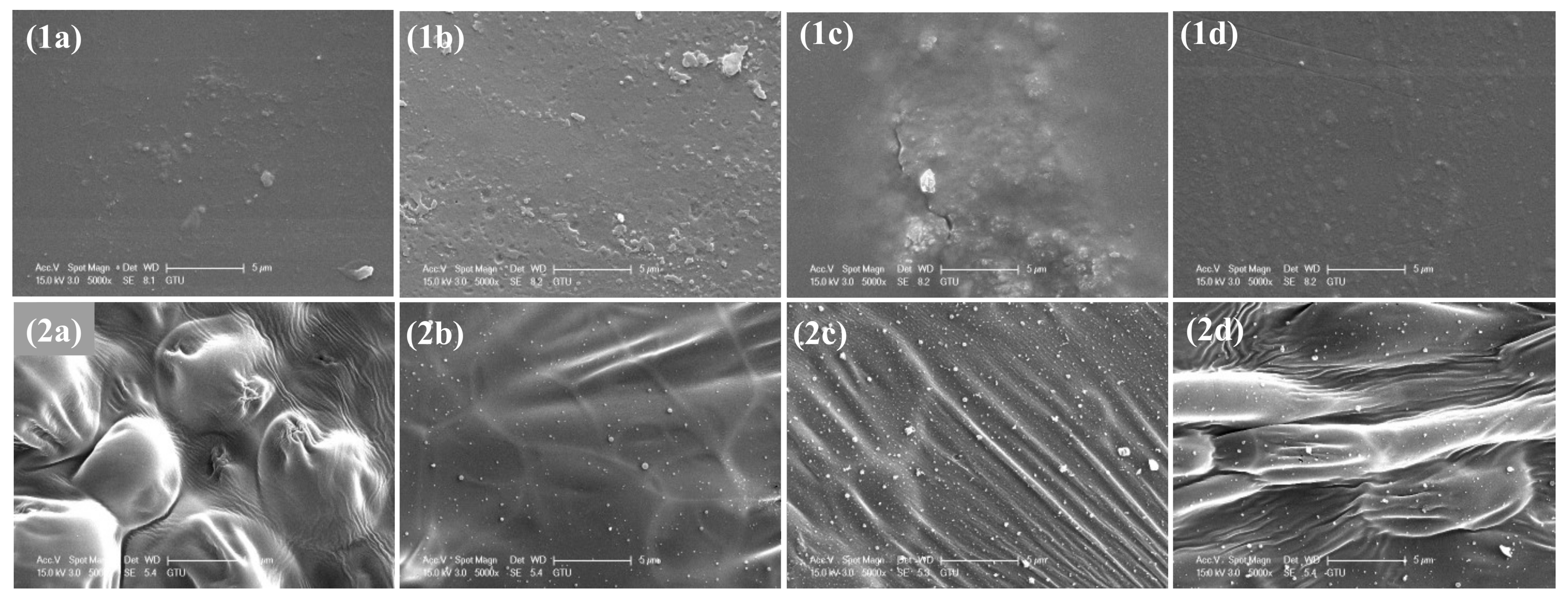
3.1. Characterization of Modified MF Resin
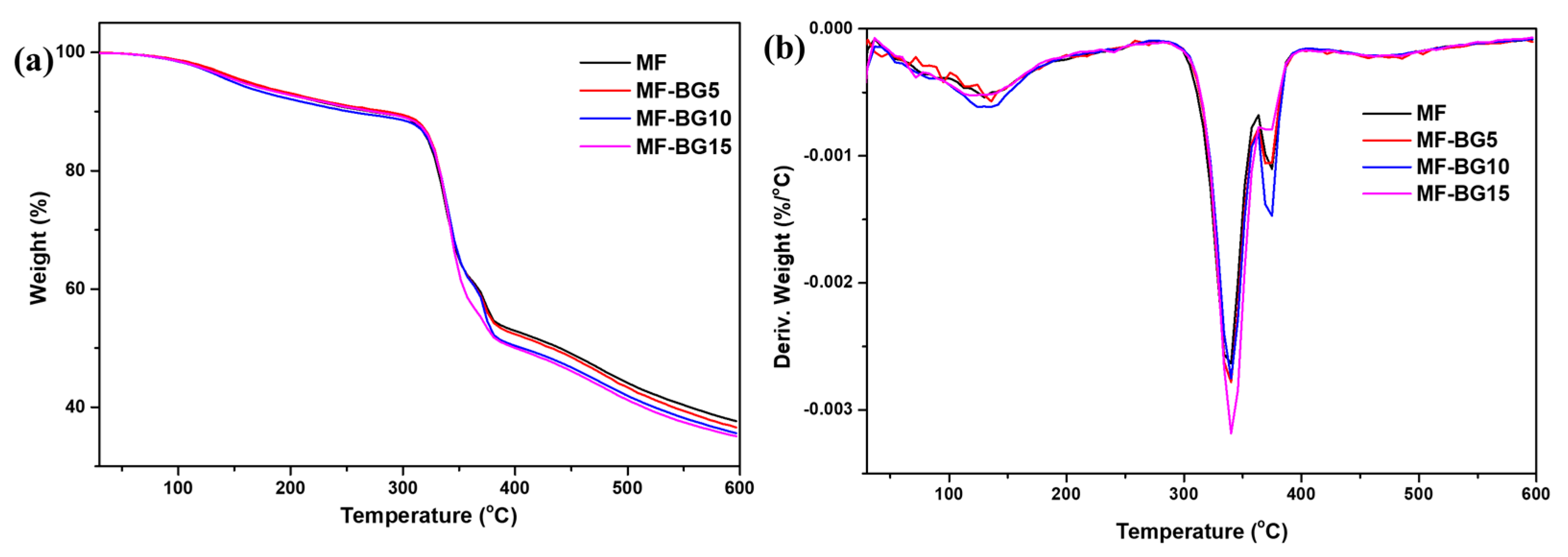
3.2. Thermal Analysis of MF-BG Resin Formulations
3.3. Flame-Retardant Performance Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, Z.; Wang, Y.; Wang, Z.; Emon, A.I.; Peng, H.; Hassan, M.; Narayanasamy, B.; Luo, F. Design of partial-discharge-free busbar for more-electric aircraft application with low pressure condition. In Proceedings of the 2021 IEEE Applied Power Electronics Conference and Exposition (APEC), Phoenix, AZ, USA, 14–17 June 2021; pp. 1178–1182. [Google Scholar] [CrossRef]
- Błażejewski, W.; Barcikowski, M.; Stosiak, M.; Warycha, J.; Stabla, P.; Smolnicki, M.; Bury, P.; Towarnicki, K.; Lubecki, M.; Paczkowska, K. A novel design of a low-pressure composite vessel with inspection opening–design, manufacturing and testing. Alex. Eng. J. 2024, 91, 442–456. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Luo, Y.; Zhang, N.; Qi, W.; Jiang, E.; Bao, J.; Zhang, X.; Zheng, W.; An, B.; et al. Low-pressure loose GO composite membrane intercalated by CNT for effective dye/salt separation. Sep. Purif. Technol. 2021, 256, 117839. [Google Scholar] [CrossRef]
- Sanchis, M.R.; Calvo, O.; Fenollar, O.; Garcia, D.; Balart, R. Surface modification of a polyurethane film by low pressure nitrogen plasma for improved adhesion to polyethylene foam for automotive industry laminates. Plasma Process. Polym. 2007, 4 (Suppl. S1), S1091–S1097. [Google Scholar] [CrossRef]
- Khonakdar Dazmiri, M.; Valizadeh Kiamahalleh, M.; Valizadeh Kiamahalleh, M.; Mansouri, H.R.; Moazami, V. Revealing the impacts of recycled urea–formaldehyde wastes on the physical–mechanical properties of MDF. Eur. J. Wood Wood Prod. 2019, 77, 293–299. [Google Scholar] [CrossRef]
- Pizzi, A.; Ibeh, C.C. Phenol-formaldehyde resins. In Handbook of Thermoset Plastics; William Andrew Publishing: Norwich, NY, USA, 2022; pp. 13–40. [Google Scholar] [CrossRef]
- Weldemhret, T.G.; Park, Y.T.; Song, J.I. Recent progress in surface engineering methods and advanced applications of flexible polymeric foams. Adv. Colloid Interface Sci. 2024, 326, 103132. [Google Scholar] [CrossRef]
- Binder, W.H.; Dunky, M. Melamine–formaldehyde resins. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Kohlmayr, M.; Zuckerstätter, G.; Kandelbauer, A. Modification of melamine-formaldehyde resins by substances from renewable resources. J. Appl. Polym. Sci. 2012, 124, 4416–4423. [Google Scholar] [CrossRef]
- Magina, S.; Ferra, J.; Cruz, P.; Nogueira, H.I.; Portugal, I.; Evtuguin, D.V. Fluorinated polyhedral oligomeric silsesquioxane nanoparticles to boost the dirt repellence of high pressure laminates. Chem. Eng. J. 2016, 301, 362–370. [Google Scholar] [CrossRef]
- Henriques, A.; Almeida, M.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Magalhães, F.D. Improving hydrophobic and oleophobic performances of high-pressure laminates. Eur. J. Wood Wood Prod. 2018, 76, 1685–1695. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Teischinger, A. Dynamic mechanical properties of decorative papers impregnated with melamine formaldehyde resin. Eur. J. Wood Wood Prod. 2009, 68, 179–187. [Google Scholar] [CrossRef]
- Antunes, A.; Henriques, A.; Lima, F.; Ferra, J.; Martins, J.; Carvalho, L.; Magalhaes, F.D. Postformable and self-healing finish foil based on polyurethane-impregnated paper. Ind. Eng. Chem. Res. 2016, 55, 12376–12386. [Google Scholar] [CrossRef]
- Ranjbaran, S.; Nazerian, M.; Kermanian, H.; Koosha, M.; Garmaroody, E.R. High strength papers impregnated with urea/melamine formaldehyde resin/nanosilica nanocomposite coatings: The effects of paper type, blend ratio and nano-content. Mater. Today Commun. 2020, 25, 101300. [Google Scholar] [CrossRef]
- Henriques, A.; Paiva, N.; Bastos, M.; Martins, J.; Carvalho, L.; Magalhaes, F.D. Improvement of storage stability and physicochemical properties by addition of benzoguanamine in melamine-formaldehyde resin synthesis. J. Appl. Polym. Sci. 2017, 134, 45185. [Google Scholar] [CrossRef]
- Chen, X.; Afreen, S.; Yu, X.; Dong, C.; Kong, Q. Modified melamine-formaldehyde resins improve tensile strength along with antifouling and flame retardancy in impregnation of cellulose paper. RSC Adv. 2019, 9, 36788–36795. [Google Scholar] [CrossRef] [PubMed]
- August, G.N. Melamine-formaldehyde-benzoguanamine Resin and Process for Preparing the same. U.S. Patent No. 3,367,917, 6 February 1968. [Google Scholar]
- Pizzi, A. Melamine-formaldehyde adhesives. In Handbook of Adhesive Technology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 2. [Google Scholar] [CrossRef]
- EN 438–2; Decorative High Pressure Laminates (HPL)—Sheets Based on Thermosetting Resins (Usually Called Laminates)—Part 2: Determination of Properties. European Committee for Standard: Brussels, Belgium, 2019.
- EN 14323; Wood-Based Panels—Melamine Faced Boards for İnterior Uses—Test Methods. European Committee for Standard: Brussels, Belgium, 2021.
- Badila, M.; Kohlmayr, M.; Zikulnig-Rusch, E.M.; Dolezel-Horwath, E.; Kandelbauer, A. Improving the cleanability of melamine-formaldehyde-based decorative laminates. J. Appl. Polym. Sci. 2014, 131, 40964. [Google Scholar] [CrossRef]
- EN ISO 4589-2; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. European Committee for Standard: Brussels, Belgium, 2017.
- Nemanič, V.; Zajec, B.; Žumer, M.; Figar, N.; Kavšek, M.; Mihelič, I. Synthesis and characterization of melamine–formaldehyde rigid foams for vacuum thermal insulation. Appl. Energy 2014, 114, 320–326. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.Z.; Xie, J.Q.; He, S.B. Synthesis and characterization of microencapsulated dicyclopentadiene with melamine–formaldehyde resins. Colloid Polym. Sci. 2007, 285, 781–791. [Google Scholar] [CrossRef]
- Antunes, A.; Duarte, M.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Partial replacement of melamine by benzoguanamine in MUF resins towards improved flexibility of agglomerated cork panels. Int. J. Adhes. Adhes. 2018, 87, 142–150. [Google Scholar] [CrossRef]
- Slonim, I.Y.; Alekseyeva, S.G.; Arshava, B.M.; Matvelashvili, G.S.; Romanov, N.M.; Potseluyeva, N.V.; Bashta, N.I. An NMR study of the synthesis of benzoguanamine-formaldehyde resins. Polym. Sci. USSR 1985, 27, 2843–2851. [Google Scholar] [CrossRef]
- Xu, G.R.; Xu, M.J.; Li, B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym. Degrad. Stab. 2014, 109, 240–248. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Wang, X.; Wu, D. Synthesis, characterization and curing properties of a novel cyclolinear phosphazene-based epoxy resin for halogen-free flame retardancy and high performance. RSC Adv. 2012, 2, 5789–5799. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q.; Lyon, R.E.; Hu, Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym. Degrad. Stab. 2010, 95, 108–115. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q. Textile heat release properties measured by microscale combustion calorimetry: Experimental repeatability. Fire Mater. 2012, 36, 127–137. [Google Scholar] [CrossRef]
- Mao, Z.; Li, J.; Pan, F.; Zeng, X.; Zhang, L.; Zhong, Y.; Sui, X.; Xu, H. High-temperature auto-cross-linking cyclotriphosphaznene: Synthesis and application in flame retardance and antidripping poly (ethylene terephthalate). Ind. Eng. Chem. Res. 2015, 54, 3788–3799. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, Z.; Wang, Q. Flame retardant eugenol-based thiol-ene polymer networks with high mechanical strength and transparency. Chem. Eng. J. 2019, 368, 359–368. [Google Scholar] [CrossRef]



| Properties | Standard MF | MF-BG5 | MF-BG10 | MF-BG15 |
|---|---|---|---|---|
| Solid Content (%) | 54.00 | 54.55 | 54.70 | 54.85 |
| Viscosity (cPs) | 30 | 48 | 43 | 45 |
| Gel Time (sec) | 45 | 43 | 48 | 51 |
| Density (g/cm3) | 1230 | 1228 | 1225 | 1228 |
| pH | 9.30 | 9.30 | 9.40 | 9.31 |
| Water Tolerance (g/100 g) | 10–20 | 10–13.5 | 10–15 | 10–14 |
| Surface Tension(mN/m) | 54.60 | 57.18 | 58.10 | 57.16 |
| Test | Test Method | Standard Limits | Units | Standard MF | MF-BG 5 | MF-BG 10 | MF-BG 15 | |
|---|---|---|---|---|---|---|---|---|
| Resistance to Abrasion | TS EN 438-2 | Class 1 Class 2 Class 3A Class 3B Class 4 | <50 ≥50 ≥150 ≥250 ≥350 | Revolution | 50 | 50 | 50 | 50 |
| Resistance to Scratch | TS EN 14323 | Min. ≥ 4 N | Newton | 5 | 4.5 | 4.5 | 4 | |
| Resistance to stain | TS EN 14323 | Min. 3 | - | 5 | 5 | 5 | 5 | |
| Acid Value | Keas Special | Min. 4 | - | 5 | 2 | 2 | 2 | |
| Resistance to Dry Heat | TS EN 14323 | Min. 4 | - | 5 | 5 | 5 | 5 | |
| Resistance to Water Vapour | TS EN 14323 | Min. 4 | - | 5 | 5 | 5 | 5 | |
| Sample | T5%/°C | T10%/°C | Tmax (°C) |
|---|---|---|---|
| MF | 160 | 269 | 340 |
| MF-BG5 | 163 | 285 | 340 |
| MF-BG10 | 149 | 251 | 340 |
| MF-BG15 | 156 | 274 | 340 |
| Sample | TTI (s) | P-HRR (kW/m²) | THR (MJ/m²) | TSP (m²/m²) | av-CO (kg/kg) | av-CO2 (kg/kg) | LOI/% |
|---|---|---|---|---|---|---|---|
| MF | 4 | 127.13 | 0.78 | 0.29 | 0.04 | 0.45 | 35.2 |
| MF-BG5 | 6 | 76.78 | 0.46 | 0.19 | 0.03 | 0.32 | 30.7 |
| MF-BG10 | 7 | 106.71 | 0.77 | 0.47 | 0.01 | 0.50 | 33.4 |
| MF-BG15 | 6 | 160.87 | 1.34 | 0.32 | 0.03 | 0.69 | 34.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çekiç, Y.; Duyar, H.; Hacıvelioğlu, F. Preparation and Characterization of Melamine–Benzoguanamine–Formaldehyde Resins and Their Flame-Retardant Properties in Impregnated Paper for Low Pressure Laminates. Coatings 2024, 14, 873. https://doi.org/10.3390/coatings14070873
Çekiç Y, Duyar H, Hacıvelioğlu F. Preparation and Characterization of Melamine–Benzoguanamine–Formaldehyde Resins and Their Flame-Retardant Properties in Impregnated Paper for Low Pressure Laminates. Coatings. 2024; 14(7):873. https://doi.org/10.3390/coatings14070873
Chicago/Turabian StyleÇekiç, Yusuf, Halil Duyar, and Ferda Hacıvelioğlu. 2024. "Preparation and Characterization of Melamine–Benzoguanamine–Formaldehyde Resins and Their Flame-Retardant Properties in Impregnated Paper for Low Pressure Laminates" Coatings 14, no. 7: 873. https://doi.org/10.3390/coatings14070873
APA StyleÇekiç, Y., Duyar, H., & Hacıvelioğlu, F. (2024). Preparation and Characterization of Melamine–Benzoguanamine–Formaldehyde Resins and Their Flame-Retardant Properties in Impregnated Paper for Low Pressure Laminates. Coatings, 14(7), 873. https://doi.org/10.3390/coatings14070873






