The Study of Combination of Biodegradable Packaging and Biocoating with Lactic Acid Bacteria as a Green Alternative for Traditional Packaging in Gouda Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocoating
2.2.1. Biopackaging
2.2.2. Coating Solutions
2.2.3. Experimental Design
2.3. Chemical and Physical Analysis
2.3.1. Moisture Content and Weight Loss
- m1—initial cheese weight, g;
- m2—cheese weight during ripening, g.
2.3.2. pH Value
2.3.3. Water Activity
2.3.4. Colour Change
- —value of sample colour components measured before packaging;
- —value of sample colour components measured after storage.
2.3.5. Texture
2.4. Sensory Evaluation
2.5. Microbiological Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Gouda Cheese Protection
3.1.1. Physicochemical Profile of Biocoated Gouda Cheese
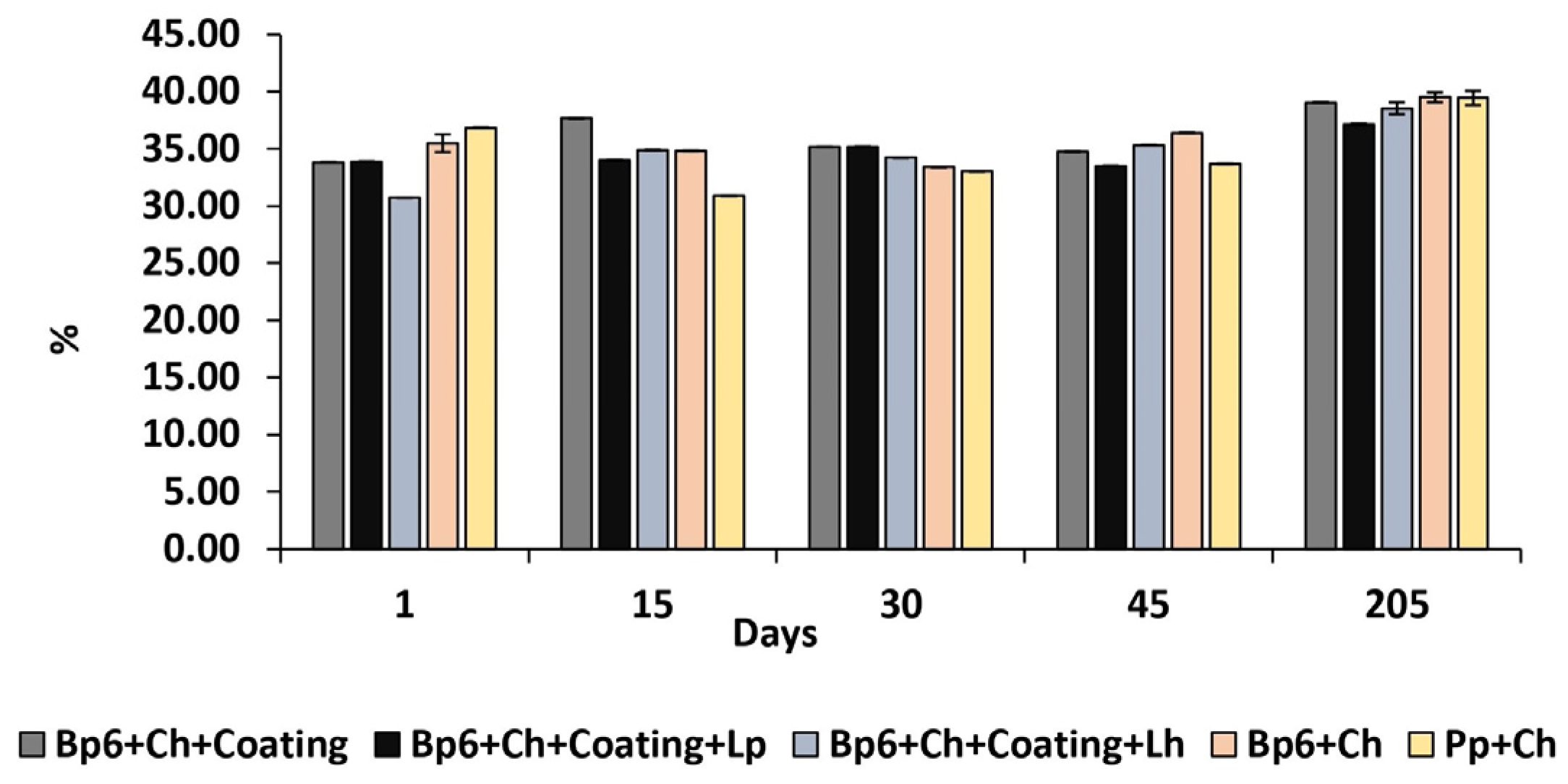
3.1.2. Moisture Content
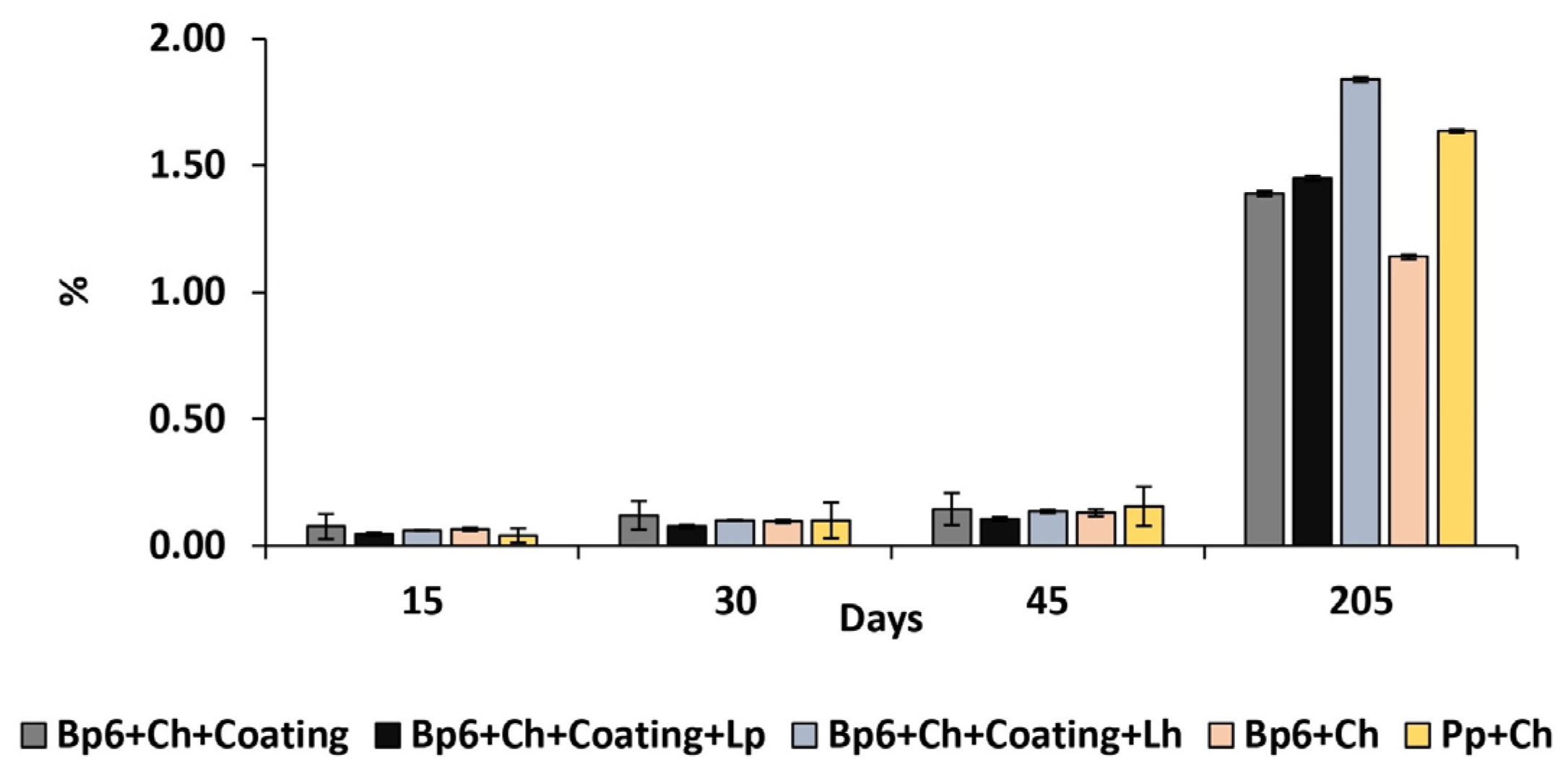
3.1.3. Weight Loss
3.1.4. pH
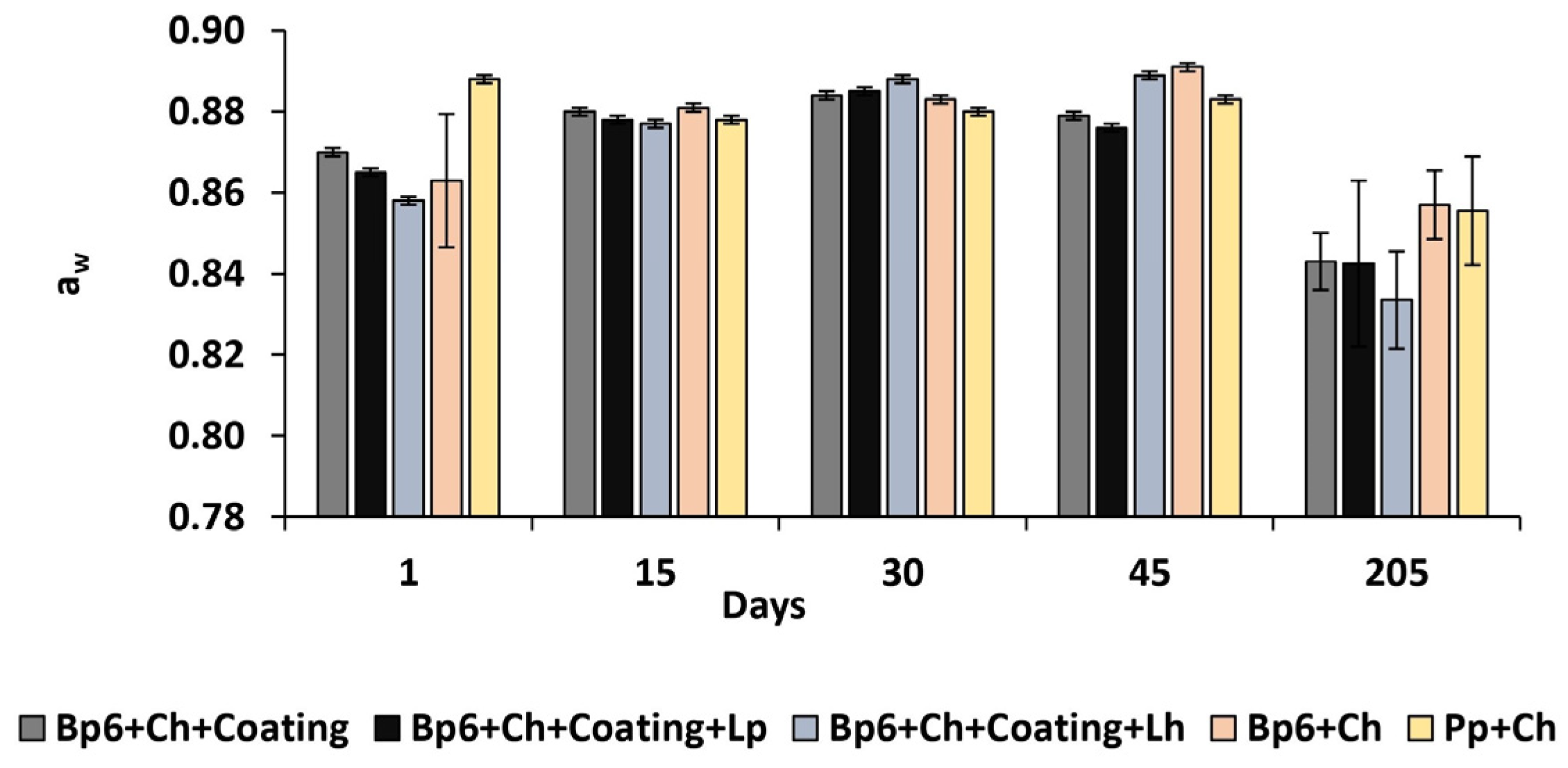
3.1.5. Water Activity
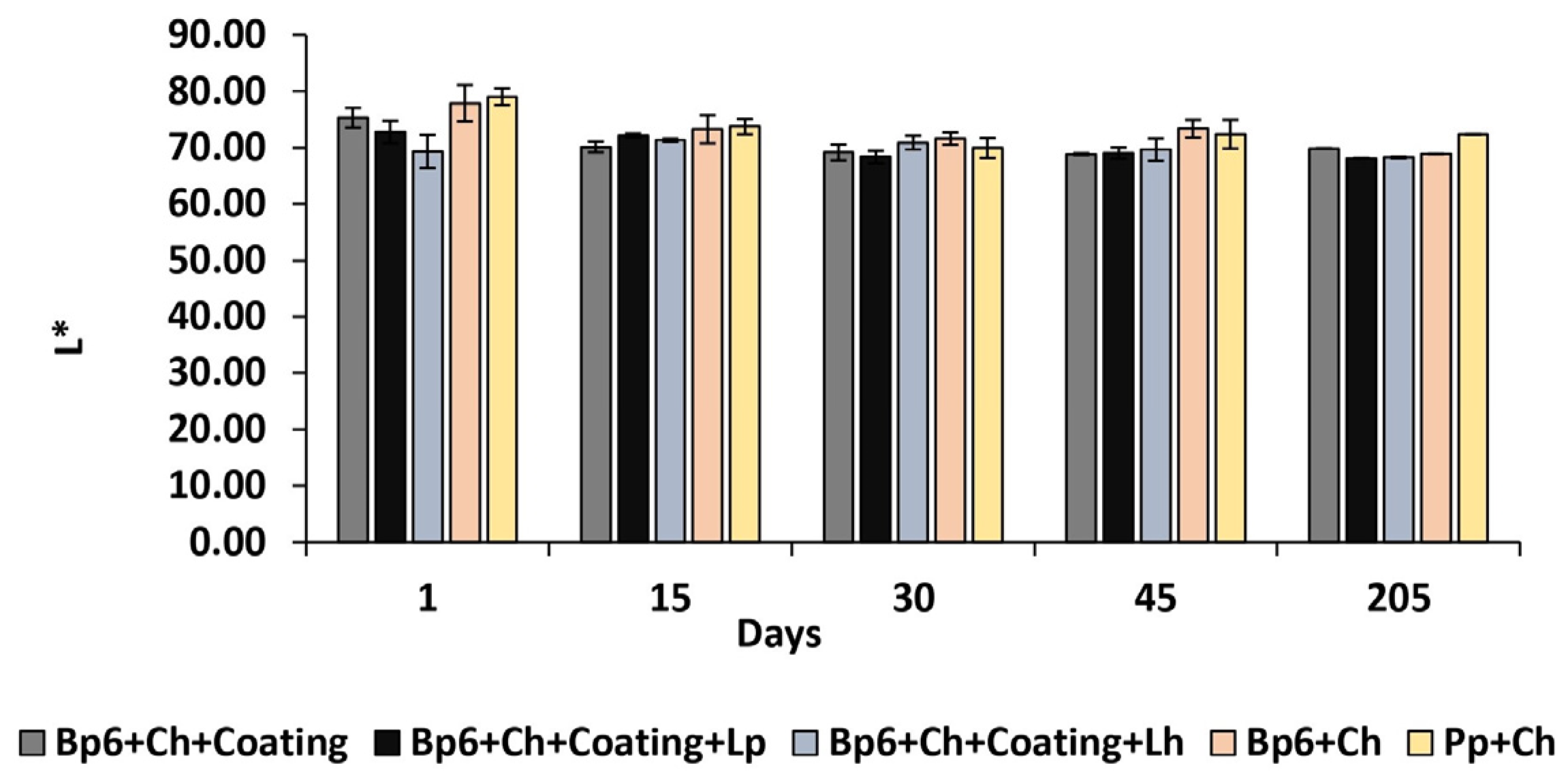
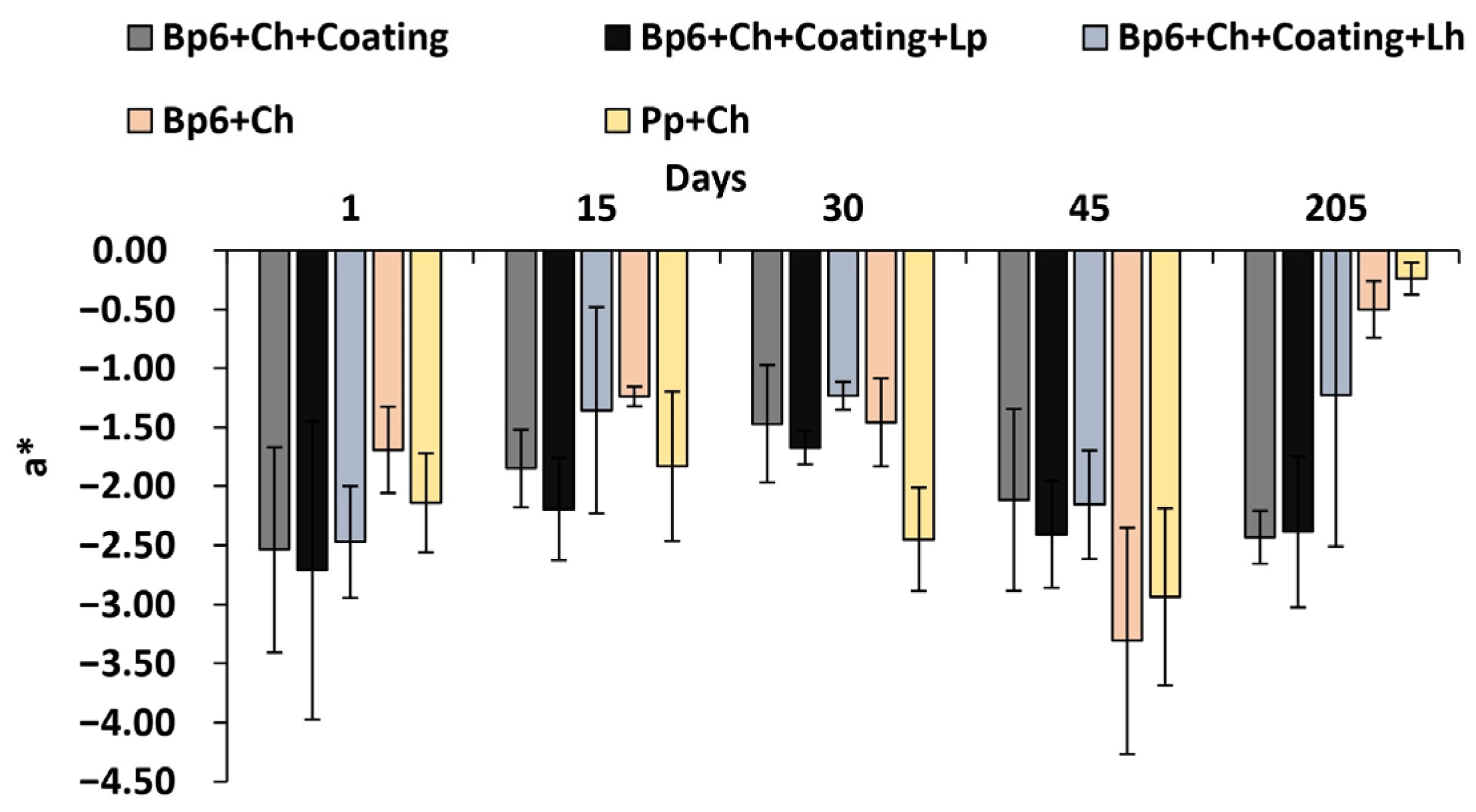
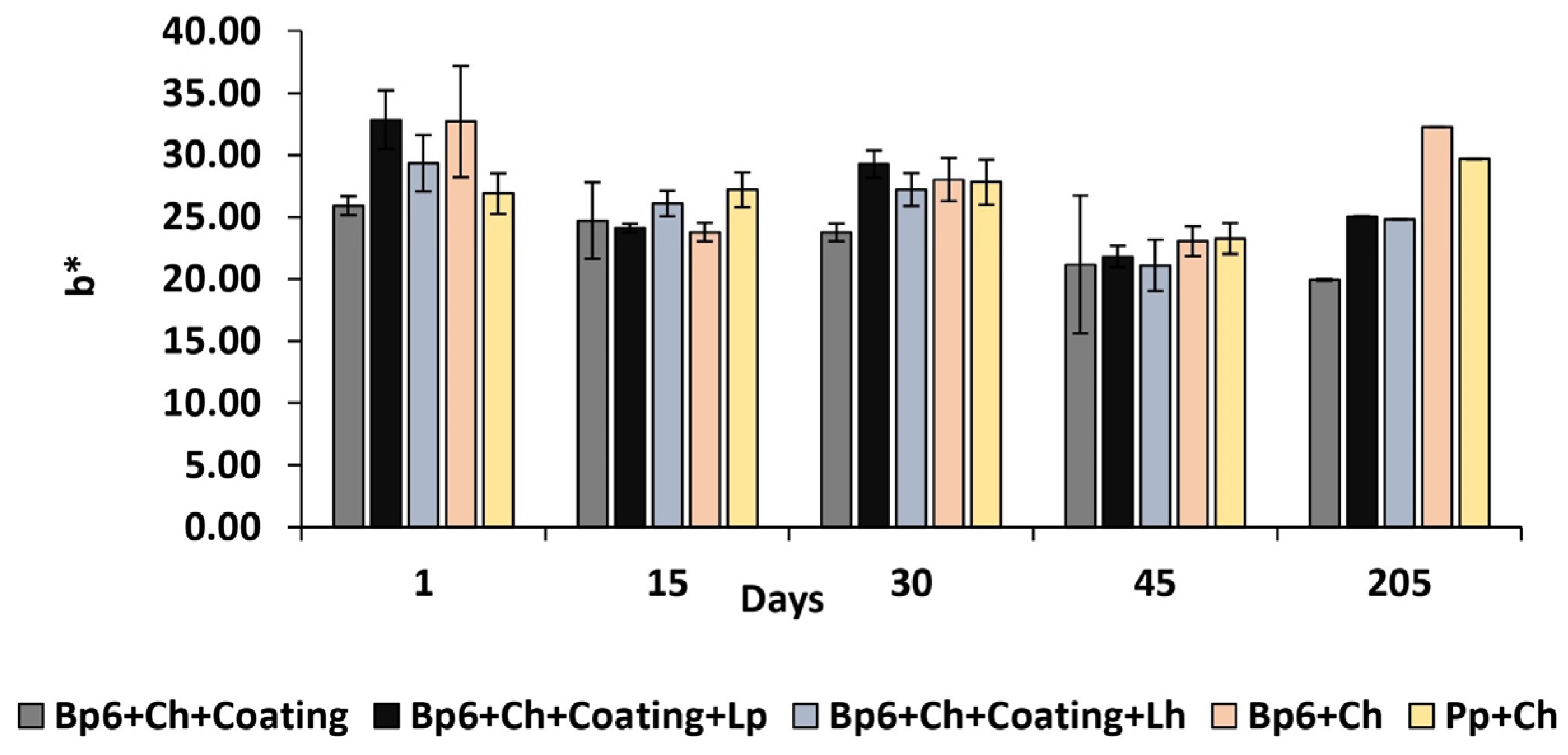
3.1.6. Colour Change
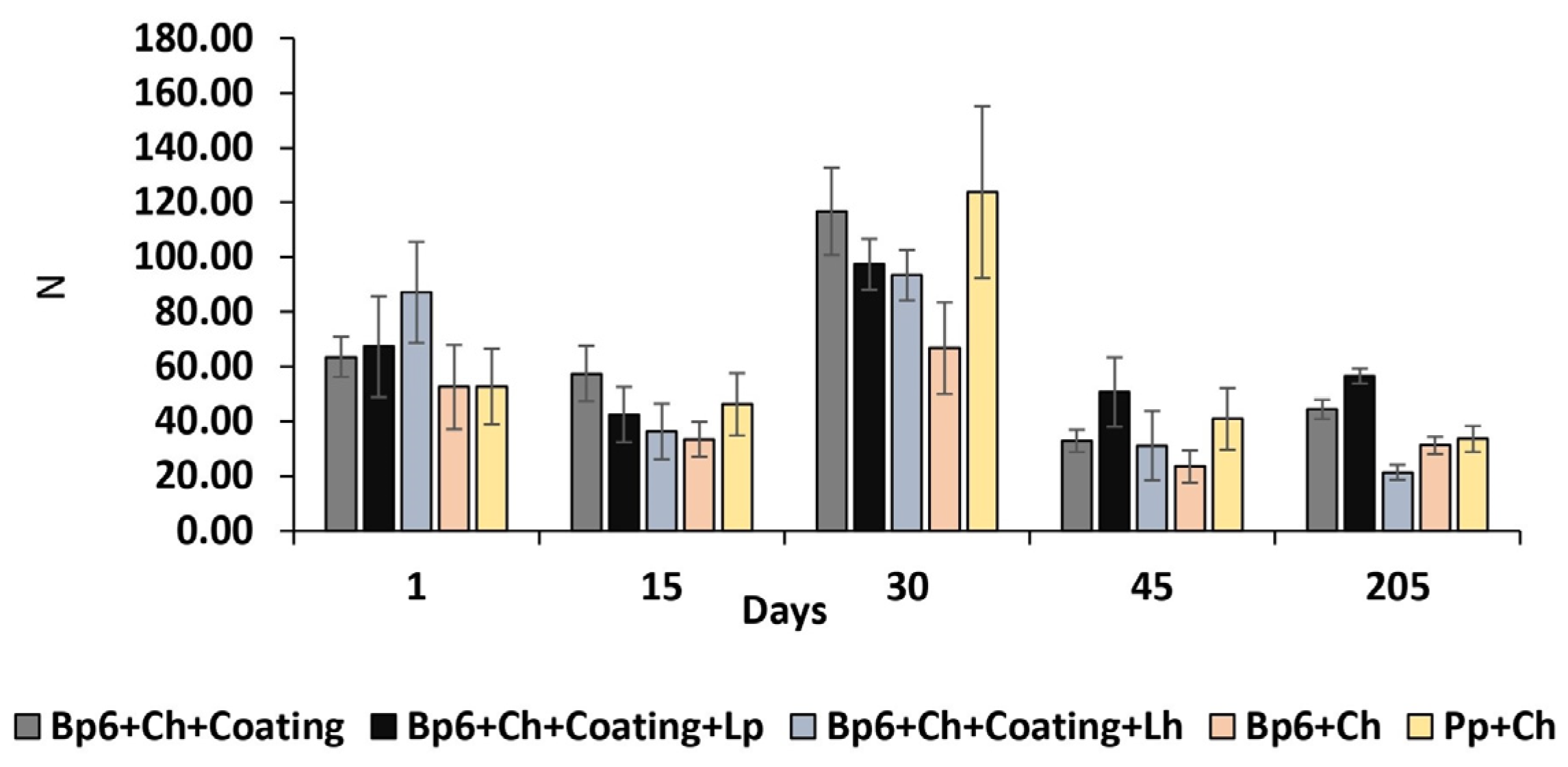
3.1.7. Texture
3.2. Sensory Evaluation
3.3. Microbiological Profile of Biocoated Gouda Cheese
| Parameter | Sample | Day 1 | Day 15 | Day 30 | Day 45 | Day 205 |
|---|---|---|---|---|---|---|
| LAB, log10 CFU g−1 | Bp6 + Ch + Coating | 8.58 ± 0.05 CDbcd | 9.33 ± 0.62 Ec | 9.69 ± 0.06 AEc | 9.69 ± 0.04 AEbce | 8.27 ± 0.37 BCDde |
| Bp6 + Ch + Coating + Lp | 8.65 ± 0.09 BCDEb | 9.75 ± 0.18 AEce | 9.81 ± 0.0.4 AEc | 9.84 ± 0.09 AEac | 8.29 ± 0.07 ABCDde | |
| Bp6 + Ch + Coating + Lh | 8.80 ± 0.06 BCDEb | 10.19 ± 0.03 AEe | 10.16 ± 0.0.6 AEd | 10.14 ± 0.0.3 AEabde | 8.25 ± 0.18 ABCDabde | |
| Bp6 + Ch | 7.76 ± 0.71 BCDdc | 9.68 ± 0.05 AEce | 9.76 ± 0.13 AEc | 9.83 ± 0.04 AEc | 8.39 ± 0.27 BCDab | |
| Pp + Ch | 8.32 ± 0.28 BCDbcd | 9.64 ± 0.19 AEce | 9.80 ± 0.01 AEc | 9.85 ± 0.03 AEac | 8.07 ± 0.01 BCDab | |
| Yeast, log10 CFU g−1 | Bp6 + Ch + Coating | 2.12 ± 0.05 c | ||||
| Bp6 + Ch + Coating + Lp | 1.40 ± 0.94 b | |||||
| Bp6 + Ch + Coating + Lh | 0.39 ± 0.12 a | |||||
| Bp6 + Ch | 1.36 ± 0.10 b | |||||
| Pp + Ch | 1.31 ± 0.87 b | |||||
| Mould, log10 CFU g−1 | Bp6 + Ch + Coating | 2.50 ± 0.03 a | ||||
| Bp6 + Ch + Coating + Lp | 2.39 ± 0.35 a | |||||
| Bp6 + Ch + Coating + Lh | 1.91 ± 0.12 b | |||||
| Bp6 + Ch | 3.40 ± 0.25 c | |||||
| Pp + Ch | 1.69 ± 0.39 b | |||||
| Enterobacteriaceae, log10 CFU g−1 | Bp6 + Ch + Coating | |||||
| Bp6 + Ch + Coating + Lp | ||||||
| Bp6 + Ch + Coating + Lh | ||||||
| Bp6 + Ch | 0.58 ± 0.64 a | |||||
| Pp + Ch | 1.32 ± 0.28 b |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravani, M.; Ehsani, A.; Aliakbarlu, J.; Ghasempour, Z. Gouda cheese spoilage prevention: Biodegradable coating induced by Bunium persicum essential oil and lactoperoxidase system. Food Sci. Nutr. 2019, 7, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kõrge, K.; Laos, K. The influence of different packaging materials and atmospheric conditions on the properties of pork rinds. J. Appl. Packag. Res. 2019, 11, 1–11. [Google Scholar]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- MEl-Sayed, S.; Youssef, A.M. Eco-friendly biodegradable nanocomposite materials and their recent use in food packaging applications: A review. Sustain. Food Technol. 2023, 1, 215–227. [Google Scholar] [CrossRef]
- Kleeberg, I.; Hetz, C.; Kroppenstedt, R.M.; Müller, R.J.; Deckwer, W.D. Biodegradation of aliphatic-aromatic copolyesters by Thermomonospora fusca and other thermophilic compost isolates. Appl. Env. Microbiol. 1998, 64, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Rieger, B.; Künkel, A.; Coates, G.; Reichardt, R.; Dinjus, E.; Zevaco, T. Synthetic Biodegradable Polymers; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. 2019. Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 18 December 2023).
- Ivanković, A.; Zeljko, K.; Talić, S.; Martinović Bevanda, A. Biodegradable packaging in the food industry. Arch. Für Leb. 2017, 68, 23–52. [Google Scholar]
- Nair, S.S.; Trafiałek, J.; Kolanowski, W. Edible Packaging: A Technological Update for the Sustainable Future of the Food Industry. Appl. Sci. 2023, 13, 8234. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Karaman, A.D.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent Advances in Dairy Packaging. Food Rev. Int. 2015, 31, 295–318. [Google Scholar] [CrossRef]
- Ramos, O.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible films and coatings from whey proteins: A review on formulation, and on mechanical and bioactive properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Shen, X.; Hou, J.; Guo, M. Effects of polymerized whey protein prepared directly from cheese whey as fat replacer on physiochemical, texture, microstructure and sensory properties of low-fat set yogurt. LWT 2019, 115, 108268. [Google Scholar] [CrossRef]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Mohamed, F.; Bleckwedel, J.; Medina, R.; Vuyst, L.D.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in northern Argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Siedler, S.; Rau, M.H.; Bidstrup, S.; Vento, J.M.; Aunsbjerg, S.D.; Bosma, E.F.; Mcnair, L.M.; Beisel, C.L.; Neves, A.R. Competitive Exclusion Is a Major Bioprotective Mechanism of Lactobacilli against Fungal Spoilage in Fermented Milk Products. Appl. Environ. Microbiol. 2020, 86, e02312-19. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite, A.; Mileriene, J.; Songisepp, E.; Rud, I.; Muizniece-Brasava, S.; Ciprovica, I.; Axelsson, L.; Lutter, L.; Aleksandrovas, E.; Tammsaar, E.; et al. Application of Edible Coating Based on Liquid Acid Whey Protein Concentrate with Indigenous Lactobacillus helveticus for Acid-Curd Cheese Quality Improvement. Foods 2022, 11, 3353. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite, A.; Mileriene, J.; Kasparaviciene, B.; Aleksandrovas, E.; Songisepp, E.; Rud, I.; Axelsson, L.; Muizniece-Brasava, S.; Ciprovica, I.; Paskevicius, A.; et al. Screening for Antifungal Indigenous Lactobacilli Strains Isolated from Local Fermented Milk for Developing Bioprotective Fermentates and Coatings Based on Acid Whey Protein Concentrate for Fresh Cheese Quality Maintenance. Microorganisms 2023, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, S.; Abdul Malek, S.; Kamdem, R.E.; Yam, K. A Versatile and Inexpensive Technique for Measuring Color of Foods. Food Technol. 2000, 54, 48–51. [Google Scholar]
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chem. 2016, 192, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Bodyfelt, F.W.; Tobias, J.; Trout, G.M. The Sensory Evaluation of Dairy Products; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mileriene, J.; Serniene, L.; Henriques, M.; Gomes, D.; Pereira, C.; Kondrotiene, K.; Kasetiene, N.; Lauciene, L.; Sekmokiene, D.; Malakauskas, M. Effect of liquid whey protein concentrate–based edible coating enriched with cinnamon carbon dioxide extract on the quality and shelf life of Eastern European curd cheese. J. Dairy Sci. 2021, 104, 1504–1517, Epub ahead of print 2021. [Google Scholar] [CrossRef] [PubMed]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 6611:2004; Milk and Milk Products—Enumeration of Colony-Forming Units of Yeasts and/or Moulds—Colony-Count Technique at 25 Degrees C. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2, Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- Lei, T.; Sun, D.-W. Developments of nondestructive techniques for evaluating quality attributes of cheeses: A review. Trends Food Sci. Technol. 2019, 88, 527–542. [Google Scholar] [CrossRef]
- Sharaby, M.R.; Soliman, E.A.; Abdel-Rahman, A.B.; Osman, A.; Khalil, R. Novel pectin-based nanocomposite film for active food packaging applications. Sci. Rep. 2022, 12, 20673. [Google Scholar] [CrossRef] [PubMed]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ma, D.; Qin, W.; Liu, Y. Physical and Antibacterial Properties of Sodium Alginate—Sodium Carboxymethylcellulose Films Containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hao, X.; Wang, H.; Li, X.; Liu, L.; Yang, W.; Zhao, M.; Wang, L.; Massounga Bora, A.F. The effects of Lactobacillus plantarum combined with inulin on the physicochemical properties and sensory acceptance of low-fat Cheddar cheese during ripening. Int. Dairy. J. 2021, 115, 104947. [Google Scholar] [CrossRef]
- Diezhandino, I.; Fernández, D.; Sacristán, N.; Combarros-Fuertes, P.; Prieto, B.; Fresno, J.M. Rheological, textural, colour and sensory characteristics of a Spanish blue cheese (Valdeón cheese). LWT-Food Sci. Technol. 2016, 65, 1118–1125. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess. Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Kõrge, K.; Šeme, H.; Bajić, M.; Likozar, B.; Novak, U. Reduction in Spoilage Microbiota and Cyclopiazonic Acid Mycotoxin with Chestnut Extract Enriched Chitosan Packaging: Stability of Inoculated Gouda Cheese. Foods 2020, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P.; Noomen, A.; Geurts, T.J. Dutch-Type Varieties. In Cheese: Chemistry, Physics and Microbiology: Major Cheese Groups; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1993; Volume 2, pp. 39–82. [Google Scholar]
- Jafarzadeh, S.; Salehabadi, A.; Mohammadi Nafchi, A.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Burgess, C.; O’Connell-Motherway, M.; Sybesma, W.; Hugenholtz, J.; Van Sinderen, D. Riboflavin production in Lactococcus lactis: Potential for in situ production of vitamin-enriched foods. Appl. Environ. Microbiol. 2004, 70, 5769–5777. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Ó.L.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Lopes-da-Silva, J.A.; Pintado, M.E.; Malcata, F.X. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J. Dairy. Sci. 2012, 95, 6282–6292. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT-Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Cai, H.; Bijl, E.; Sala, G.; Scholten, E. Relationship between the perception of complex textural attributes and the bolus properties of cheese. Food Hydrocoll. 2024, 150, 109713. [Google Scholar] [CrossRef]
- Shiota, M.; Iwasawa, A.; Suzuki-Iwashima, A.; Iida, F. Effects of Flavor and Texture on the Sensory Perception of Gouda-Type Cheese Varieties during Ripening Using Multivariate Analysis. J. Food Sci. 2015, 80, C2740–C2750. [Google Scholar] [CrossRef] [PubMed]
- Luan, D.; Li, S.; Wang, Y.; Wang, Y. Studying the non-thermal effects of microwave on amino acids in sterilized rainbow trout (Oncorhynchus mykiss) fillets using a double side approximating method. Food Res. Int. 2023, 173, 113352. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Font de Valdez, G.; Gigante, M.L.; Grosso, C.R.F. Whey protein coating bead improves the survival of the probiotic Lactobacillus rhamnosus CRL 1505 to low, p.H. Lett. Appl. Microbiol. 2012, 54, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Blaiotta, G.; La Gatta, B.; Di Capua, M.; Di Luccia, A.; Coppola, R.; Aponte, M. Effect of chestnut extract and chestnut fiber on viability of potential probiotic Lactobacillus strains under gastrointestinal tract conditions. Food Microbiol. 2013, 36, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Al-Gamal, M.S.; Ibrahim, G.A.; Sharaf, O.M.; Radwan, A.A.; Dabiza, N.M.; Youssef, A.M.; El-ssayad, M.F. The protective potential of selected lactic acid bacteria against the most common contaminants in various types of cheese in Egypt. Heliyon 2019, 5, e01362. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Wong, C.H.; Li, D. Postbiotics enhance the functionality of a probiotic edible coating for salmon fillets and the probiotic stability during simulated digestion. Food Packag. Shelf Life 2022, 34, 100954. [Google Scholar] [CrossRef]
- Cheong, E.Y.L.; Sandhu, A.; Jayabalan, J.; Le, T.T.K.; Nhiep, N.T.; Ho, H.T.M.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Beristain-Bauza, S. del C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity of whey protein films supplemented with Lactobacillus sakei cell-free supernatant on fresh beef. Food Microbiol. 2017, 62, 207–211. [Google Scholar] [CrossRef]



| Sample No. | Material | Thickness, µm | Water Vapor Transmission Rate g/m2 24 h (ASTM F 1249-20; 38 °C, 90% RH) | Visual Evaluation of Fungi Growth |
|---|---|---|---|---|
| 1 | PropaFresh, Innovia | 35 ± 2 | 3.89 | + |
| 2 | BOPP Propafilm P2GAF, Innovia | 35 ± 2 | 15.18 | + |
| 3 | CERAMIS PLA with SiO2 coating, Amcor | 45 ± 2 | 32.67 | + |
| 4 | Biodegradable cheese storage sheets (derived from wood-based cellulose fibres), Formaticum | 25 ± 2 | 437.47 | + |
| 5 | PLA with Nature Flex coating, Mixpack | 35 ± 2 | 35.50 | + |
| 6 | DECOLINE® DCL-BD—biodegradable polyolefin multi-layer shrink film, Dekofilm Polska, Emilianów, Poland (shrinkage 93 °C is MD 22/TD 22) | 20 ± 1 | 29.20 | − |
| 7 | Conventional packaging BK3550K, Sealed Air, EVA/PE/EPC/PVDC (Ethylene VinylAcetate/Polyethylene/Ethylene Propylene Copolymer/PolyVinylideneChloride) | 52 ± 2 | 17.00 | + |
| L* | a* | b* | ΔE* | pH | aw | Moisture | Texture | Sensory Evaluation | Weight Loss | LAB | Mould | Yeast | Enterobacteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.05 | 0.001 | 0.001 | - | - | - |
| Treatment | 0.001 | 0.014 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | - | 0.243 | 0.001 | 0.001 | 0.05 | 0.001 |
| Day × Treatment | 0.001 | 0.018 | 0.001 | 0.003 | 0.001 | 0.001 | 0.001 | 0.001 | - | 0.988 | 0.29 | - | - | - |
| Parameter | Sample | Day 1 | Day 15 | Day 30 | Day 45 | Day 205 |
|---|---|---|---|---|---|---|
| Moisture | Bp6 + Ch + Coating | 33.82 ± 0.01 Aa | 37.68 ± 0.01 Ba | 35.17 ± 0.01 Ca | 34.73 ± 0.01 Da | 39.05 ± 0.01 Eb |
| Bp6 + Ch + Coating + Lp | 33.89 ± 0.01 Aa | 33.99 ± 0.01 Bb | 35.2 ± 0.01 Cb | 33.51 ± 0.01 Db | 37.13 ± 0.08 Eacde | |
| Bp6 + Ch + Coating + Lh | 30.7 ± 0.01 BCDEb | 34.88 ± 0.01 AEc | 34.23 ± 0.01 ABDEc | 35.32 ± 0.01 ACEc | 38.55 ± 0.51 ABCDbd | |
| Bp6 + Ch | 35.49 ± 0.79 CEc | 34.82 ± 0.01 CDEd | 33.4 ± 0.01 ABDEd | 36.39 ± 0.01 BCEd | 39.5 ± 0.45 ABCDbc | |
| Pp + Ch | 36.85 ± 0.01 Ad | 30.89 ± 0.01 Be | 33.03 ± 0.01 Ce | 33.69 ± 0.01 De | 39.46 ± 0.64 Eb | |
| Weight loss | Bp6 + Ch + Coating | - | 0.08 ± 0.05 Ea | 0.12 ± 0.06 AEc | 0.14 ± 0.06 AEa | 1.39 ± 0.01 ABCDcde |
| Bp6 + Ch + Coating + Lp | - | 0.05 ± 0.01 Aa | 0.07 ± 0.01 Bb | 0.1 ± 0.01 Ca | 1.45 ± 0.01 Dde | |
| Bp6 + Ch + Coating + Lh | - | 0.06 ± 0.01 Ea | 0.1 ± 0.01 Aa | 0.13 ± 0.01 Aa | 1.84 ± 0.01 ABCDde | |
| Bp6 + Ch | - | 0.06 ± 0.01 Aa | 0.09 ± 0.01 Bb | 0.13 ± 0.01 Ca | 1.14 ± 0.01 Dabcde | |
| Pp + Ch | - | 0.04 ± 0.03 DEa | 0.1 ± 0.07 Ea | 0.15 ± 0.08 ABEa | 1.64 ± 0.01 ABCDabcde | |
| pH | Bp6 + Ch + Coating | 5.43 ± 0.03 CDEa | 5.49 ± 0.01 DEa | 5.5 ± 0.01 ADEbc | 5.62 ± 0.01 ABCcb | 5.62 ± 0.01 ABCde |
| Bp6 + Ch + Coating + Lp | 5.54 ± 0.01 BDEb | 5.51 ± 0.01 ACDEa | 5.55 ± 0.01 BDEbc | 5.59 ± 0.01 ABCc | 5.59 ± 0.01 ABCde | |
| Bp6 + Ch + Coating + Lh | 5.54 ± 0.01 BCDEb | 5.49 ± 0.01 ACDEa | 5.62 ± 0.01 ABa | 5.64 ± 0.01 ABb | 5.61 ± 0.01 ABde | |
| Bp6 + Ch | 5.56 ± 0.03 EBb | 5.54 ± 0.01 EBa | 5.59 ± 0.03 AEBab | 5.61 ± 0.01 AEBcb | 5.68 ± 0.01 ABabc | |
| Pp + Ch | 5.5 ± 0.01 BDEc | 5.63 ± 0.01 ACEb | 5.52 ± 0.01 BDEab | 5.6 ± 0.01 ACEcb | 5.68 ± 0.02 abc | |
| aw | Bp6 + Ch + Coating | 0.870 ± 0.001 BCDEa | 0.880 ± 0.001 ACEc | 0.884 ± 0.001 ABDEca | 0.879 ± 0.001 ACEa | 0.843 ± 0.007 ABCDcde |
| Bp6 + Ch + Coating + Lp | 0.865 ± 0.001 BCDEb | 0.878 ± 0.001 ACEb | 0.885 ± 0.001 ABDEca | 0.876 ± 0.001 ACEb | 0.843 ± 0.02 ABCDc | |
| Bp6 + Ch + Coating + Lh | 0.858 ± 0.001 BCDEc | 0.877 ± 0.001 ACDEb | 0.888 ± 0.001 ABEedba | 0.889 ± 0.001 ABEabe | 0.834 ± 0.01 ABCDEabcde | |
| Bp6 + Ch | 0.863 ± 0.02 BCDEd | 0.881 ± 0.001 AEc | 0.883 ± 0.001 ADEa | 0.891 ± 0.001 ABCEabe | 0.857 ± 0.008 ABCDac | |
| Pp + Ch | 0.888 ± 0.001 BCDEe | 0.878 ± 0.001 ADEb | 0.880 ± 0.001 ADEedcb | 0.883 ± 0.001 ABCEc | 0.856 ± 0.01 ABCDac | |
| L* | Bp6 + Ch + Coating | 75.33 ± 1.74 BCDEc | 70.14 ± 0.97 Aba | 69.12 ± 1.39 Abd | 68.88 ± 0.18 Ade | 69.88 ± 0.01 Aa |
| Bp6 + Ch + Coating + Lp | 72.8 ± 2.01 CDEba | 72.17 ± 0.31 CDEabe | 68.39 ± 1.09 ABb | 69.03 ± 1.01 ABd | 68.11 ± 0.07 ABb | |
| Bp6 + Ch + Coating + Lh | 69.33 ± 2.95 Aeba | 71.33 ± 0.28 Aa | 70.91 ± 1.2 Ad | 69.66 ± 1.97 Ad | 68.27 ± 0.16 Ac | |
| Bp6 + Ch | 77.92 ± 3.23 Adc | 73.28 ± 2.5 Be | 71.59 ± 1.09 Bbd | 73.38 ± 1.55 Babc | 68.94 ± 0.05 Bd | |
| Pp + Ch | 79.07 ± 1.51 BCDEdc | 73.73 ± 1.34 ACe | 69.95 ± 1.78 ABbd | 72.4 ± 2.54 Aab | 72.35 ± 0.01 Ae | |
| a* | Bp6 + Ch + Coating | −2.538 ± 0.87 Aa | −1.85 ± 0.33 Abd | −1.47 ± 0.5 Aa | −2.12 ± 0.77 Aa | −2.43 ± 0.22 Aa |
| Bp6 + Ch + Coating + Lp | −2.71 ± 1.26 Aa | −2.19 ± 0.43 Ab | −1.67 ± 0.14 Aa | −2.41 ± 0.45 Aa | −2.39 ± 0.64 Aa | |
| Bp6 + Ch + Coating + Lh | −2.47 ± 0.47 Aa | −1.36 ± 0.87 Abd | −1.23 ± 0.12 Aa | −2.16 ± 0.46 Aa | −1.23 ± 1.29 Aab | |
| Bp6 + Ch | −1.69 ± 0.37 DEa | −1.24 ± 0.08 Dd | −1.46 ± 0.37 Da | −3.31 ± 0.96 ABCEa | −0.5 ± 0.24 ADb | |
| Pp + Ch | −2.14 ± 0.42 Ea | −1.83 ± 0.63 Ebd | −2.45 ± 0.44 Eb | −2.94 ± 0.75 BEa | −0.24 ± 0.14 ABCDb | |
| b* | Bp6 + Ch + Coating | 25.92 ± 0.76 Ab | 24.72 ± 3.07 Aabd | 23.76 ± 0.7 Ad | 21.18 ± 5.55 Aa | 19.93 ± 0.07 Aa |
| Bp6 + Ch + Coating + Lp | 32.84 ± 2.36 BCDEa | 24.11 ± 0.35 ACa | 29.27 ± 1.11 ABDEe | 21.81 ± 0.86 ACEa | 25.05 ± 0.02 ACDb | |
| Bp6 + Ch + Coating + Lh | 29.33 ± 2.27 BDEab | 26.09 ± 1.03 ADabd | 27.22 ± 1.32 De | 21.09 ± 2.07 ABCEa | 24.81 ± 0.02 ADc | |
| Bp6 + Ch | 32.7 ± 4.47 Aa | 23.78 ± 0.74 Ba | 28.03 ± 1.74 ABe | 23.04 ± 1.2 Ba | 32.24 ± 0.02 Ad | |
| Pp + Ch | 26.9 ± 1.63 Db | 27.19 ± 1.4 Ddb | 27.81 ± 1.81 De | 23.26 ± 1.24 ABCEa | 29.67 ± 0.01 De | |
| Colour change (∆E*) | Bp6 + Ch + Coating | - | 5.95 ± 2.54 Aa | 6.89 ± 2.54 Aa | 9.03 ± 4.53 Aa | 7.77 ± 1.64 Acb |
| Bp6 + Ch + Coating + Lp | - | 9.19 ± 2.47 ABa | 6.32 ± 2.55 Aa | 11.96 ± 2.38 Ba | 9.84 ± 1.72 ABc | |
| Bp6 + Ch + Coating + Lh | - | 4.24 ± 3.22 Da | 3.62 ± 2.86 Db | 9.06 ± 2.16 ADa | 4.48 ± 1.14 Db | |
| Bp6 + Ch | - | 7.21 ± 2.09 Aa | 5.53 ± 1.46 Aa | 8.11 ± 3.06 Aa | 6.85 ± 2.19 Acb | |
| Pp + Ch | - | 6.01 ± 2.11 Aa | 9.52 ± 2.63 Ac | 7.81 ± 3.01 Aa | 7.66 ± 1.53 Acb | |
| Texture (N) | Bp6 + Ch + Coating | 63.56 ± 7.33 CDabc | 57.50 ± 10.15 CDb | 116.78 ± 16.03 ABDEd | 32.87 ± 4.07 ABCdb | 44.38 ± 3.5 Cc |
| Bp6 + Ch + Coating + Lp | 67.3 ± 11.31 BDECabc | 42.45 ± 13.33 Cbe | 97.39 ± 14.71 BDEde | 50.73 ± 18.14 Cd | 56.56 ± 23.55 Ccde | |
| Bp6 + Ch + Coating + Lh | 87.14 ± 18.45 BDEba | 36.33 ± 10.09 ACbe | 93.45 ± 9.26 BDEde | 31.11 ± 12.69 ACdb | 21.35 ± 2.73 ACab | |
| Bp6 + Ch | 52.52 ± 15.44 Dc | 33.45 ± 6.33 Ce | 66.75 ± 16.76 BDEe | 23.5 ± 5.89 ACb | 31.20 ± 3.19 Cb | |
| Pp + Ch | 52.75 ± 13.85 Cc | 46.22 ± 11.38 Cbe | 123.73 ± 31.47 ABDEd | 40.94 ± 11.27 Cdb | 33.57 ± 4.75 Cb |
| Parameter | Sample | Day 45 | Day 205 |
|---|---|---|---|
| Flavour | Bp6 + Ch + Coating | 7.2 ± 1.84 aA | 7.7 ± 1.41 aA |
| Bp6 + Ch + Coating + Lp | 6.8 ± 1.84 aA | 8.03 ± 1.01 aA | |
| Bp6 + Ch + Coating + Lh | 5.8 ± 1.98 aA | 8.23 ± 1.11 aA | |
| Bp6 + Ch | 5.2 ± 2.4 aA | 8.13 ± 0.99 aA | |
| Pp + Ch | 4.35 ± 2.62 aA | 7.15 ± 0.07 aA | |
| Body and texture | Bp6 + Ch + Coating | 7.4 ± 1.27 aA | 6.97 ± 1.41 aA |
| Bp6 + Ch + Coating + Lp | 6.65 ± 2.2 aA | 8.33 ± 0.95 aA | |
| Bp6 + Ch + Coating + Lh | 7 ± 1.13 aA | 8.5 ± 0.78 aA | |
| Bp6 + Ch | 6.75 ± 0.78 aA | 7.87 ± 1.91 aA | |
| Pp + Ch | 5.35 ± 1.9 aA | 8 ± 1.13 aA | |
| Appearance | Bp6 + Ch + Coating | 7.85 ± 1.49 aA | 8.33 ± 1.04 aA |
| Bp6 + Ch + Coating + Lp | 7.9 ± 1.41 aA | 8.37 ± 1 aA | |
| Bp6 + Ch + Coating + Lh | 7.8 ± 1.56 aA | 8.53 ± 0.84 aA | |
| Bp6 + Ch | 7.9 ± 1.41 aA | 8.3 ± 1.15 aA | |
| Pp + Ch | 7.8 ± 1.23 aA | 8.35 ± 1.63 aA | |
| Total acceptability | Bp6 + Ch + Coating | 7.35 ± 1.56 aA | 7.47 ± 0.96 aA |
| Bp6 + Ch + Coating + Lp | 6.85 ± 1.94 aA | 8.19 ± 0.96 aA | |
| Bp6 + Ch + Coating + Lh | 6.48 ± 1.6 aA | 8.37 ± 0.84 aA | |
| Bp6 + Ch | 6.09 ± 1.66 aA | 8.04 ± 1.36 aA | |
| Pp + Ch | 5.1 ± 2.2 aA | 7.61 ± 0.58 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrovas, E.; Vasiliauskaitė, A.; Milerienė, J.; Muizniece-Brasava, S.; Ciprovica, I.; Songisepp, E.; Rud, I.; Axelsson, L.; Kasparavičienė, B.; Lutter, L.; et al. The Study of Combination of Biodegradable Packaging and Biocoating with Lactic Acid Bacteria as a Green Alternative for Traditional Packaging in Gouda Cheese. Coatings 2024, 14, 886. https://doi.org/10.3390/coatings14070886
Aleksandrovas E, Vasiliauskaitė A, Milerienė J, Muizniece-Brasava S, Ciprovica I, Songisepp E, Rud I, Axelsson L, Kasparavičienė B, Lutter L, et al. The Study of Combination of Biodegradable Packaging and Biocoating with Lactic Acid Bacteria as a Green Alternative for Traditional Packaging in Gouda Cheese. Coatings. 2024; 14(7):886. https://doi.org/10.3390/coatings14070886
Chicago/Turabian StyleAleksandrovas, Elvidas, Agnė Vasiliauskaitė, Justina Milerienė, Sandra Muizniece-Brasava, Inga Ciprovica, Epp Songisepp, Ida Rud, Lars Axelsson, Beatričė Kasparavičienė, Liis Lutter, and et al. 2024. "The Study of Combination of Biodegradable Packaging and Biocoating with Lactic Acid Bacteria as a Green Alternative for Traditional Packaging in Gouda Cheese" Coatings 14, no. 7: 886. https://doi.org/10.3390/coatings14070886
APA StyleAleksandrovas, E., Vasiliauskaitė, A., Milerienė, J., Muizniece-Brasava, S., Ciprovica, I., Songisepp, E., Rud, I., Axelsson, L., Kasparavičienė, B., Lutter, L., Malakauskas, M., & Šernienė, L. (2024). The Study of Combination of Biodegradable Packaging and Biocoating with Lactic Acid Bacteria as a Green Alternative for Traditional Packaging in Gouda Cheese. Coatings, 14(7), 886. https://doi.org/10.3390/coatings14070886







