In Situ Fabrication of Ti-xNb Alloys by Conventional Powder Metallurgy
Abstract
1. Introduction
2. Experimental Procedure
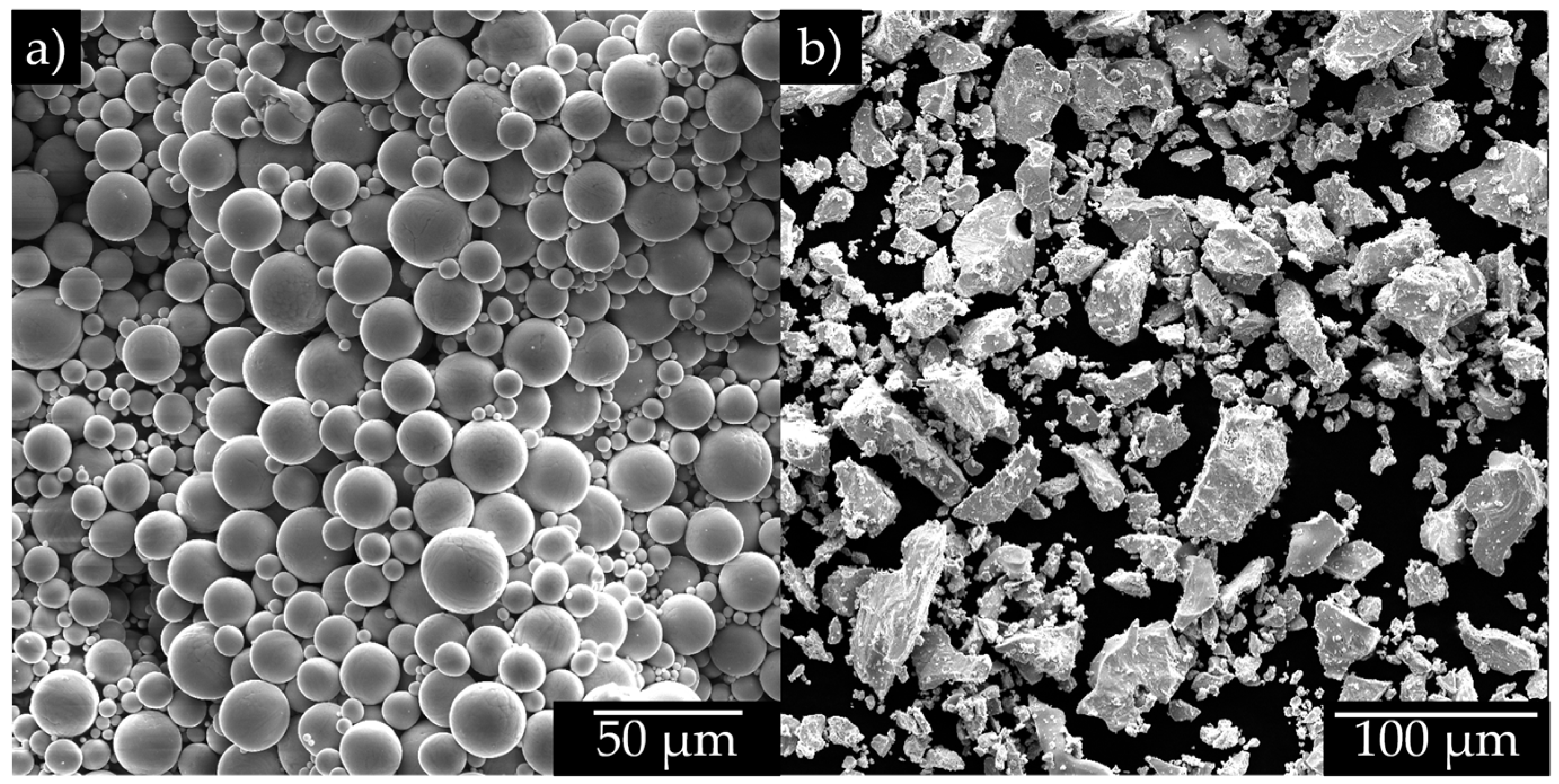
2.1. Sample Preparation
2.2. Sintering Process
2.3. Material Characterization
2.4. Compression Tests
2.5. Corrosion Test
3. Results and Discussion
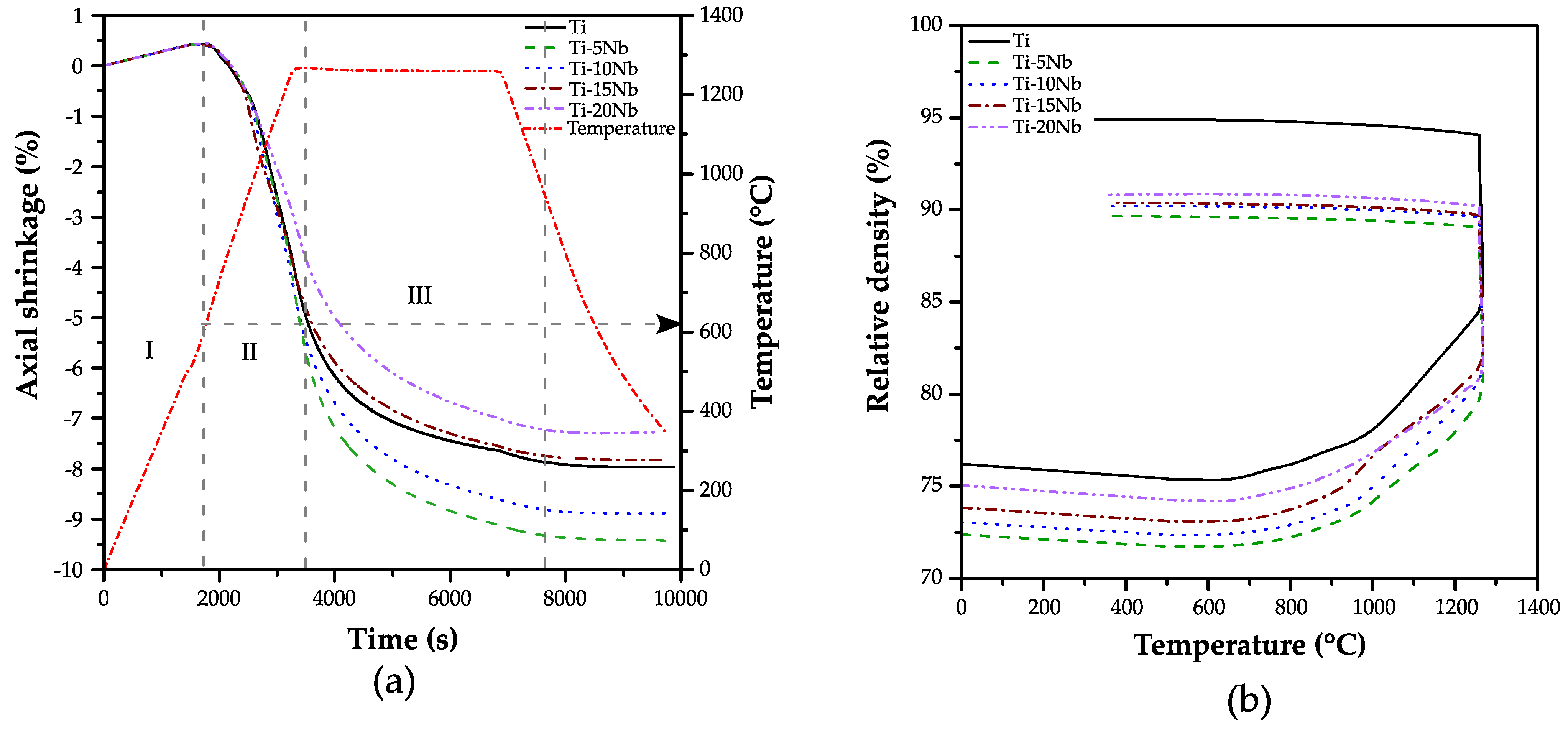
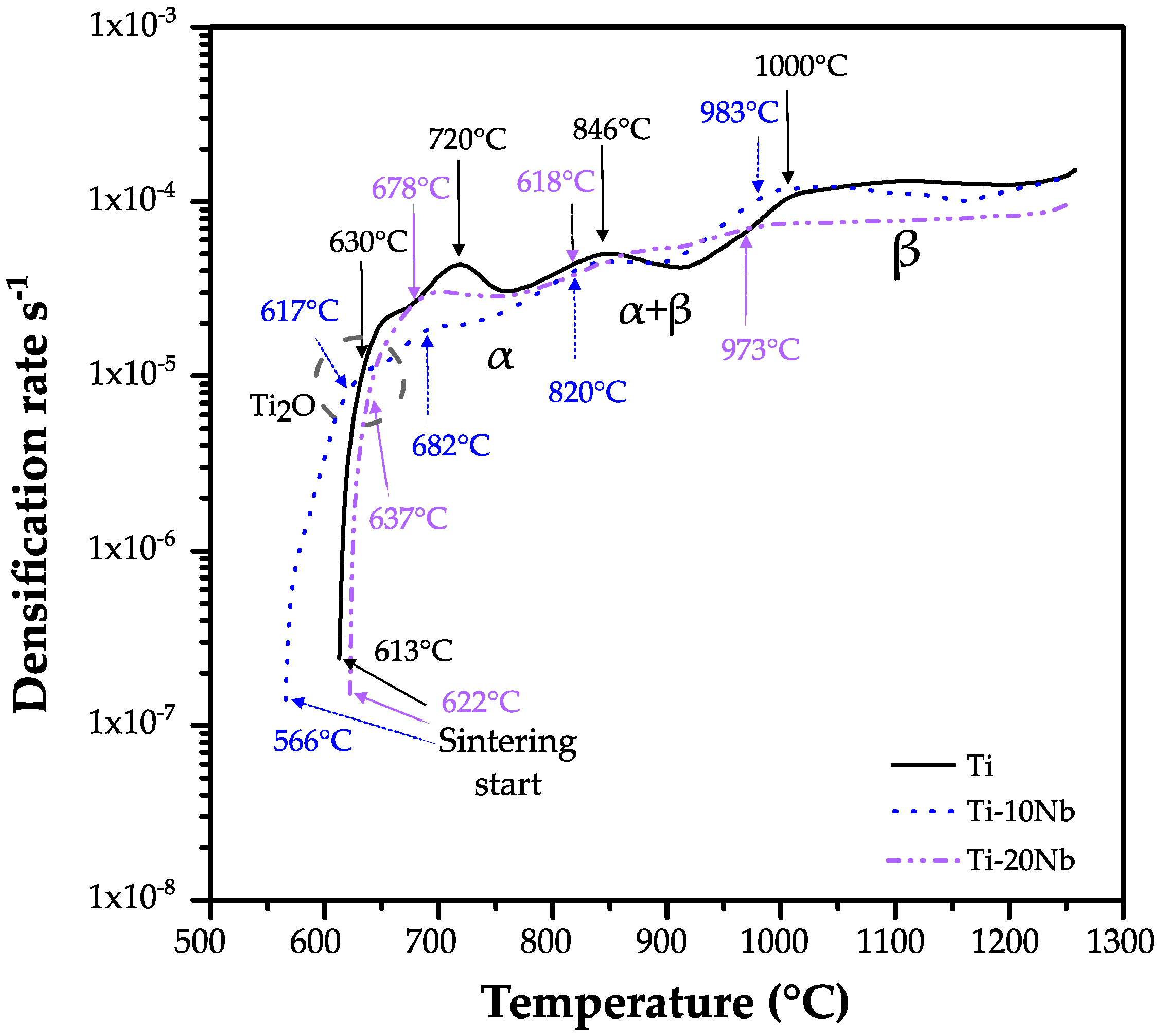
3.1. Dilatometry Analysis
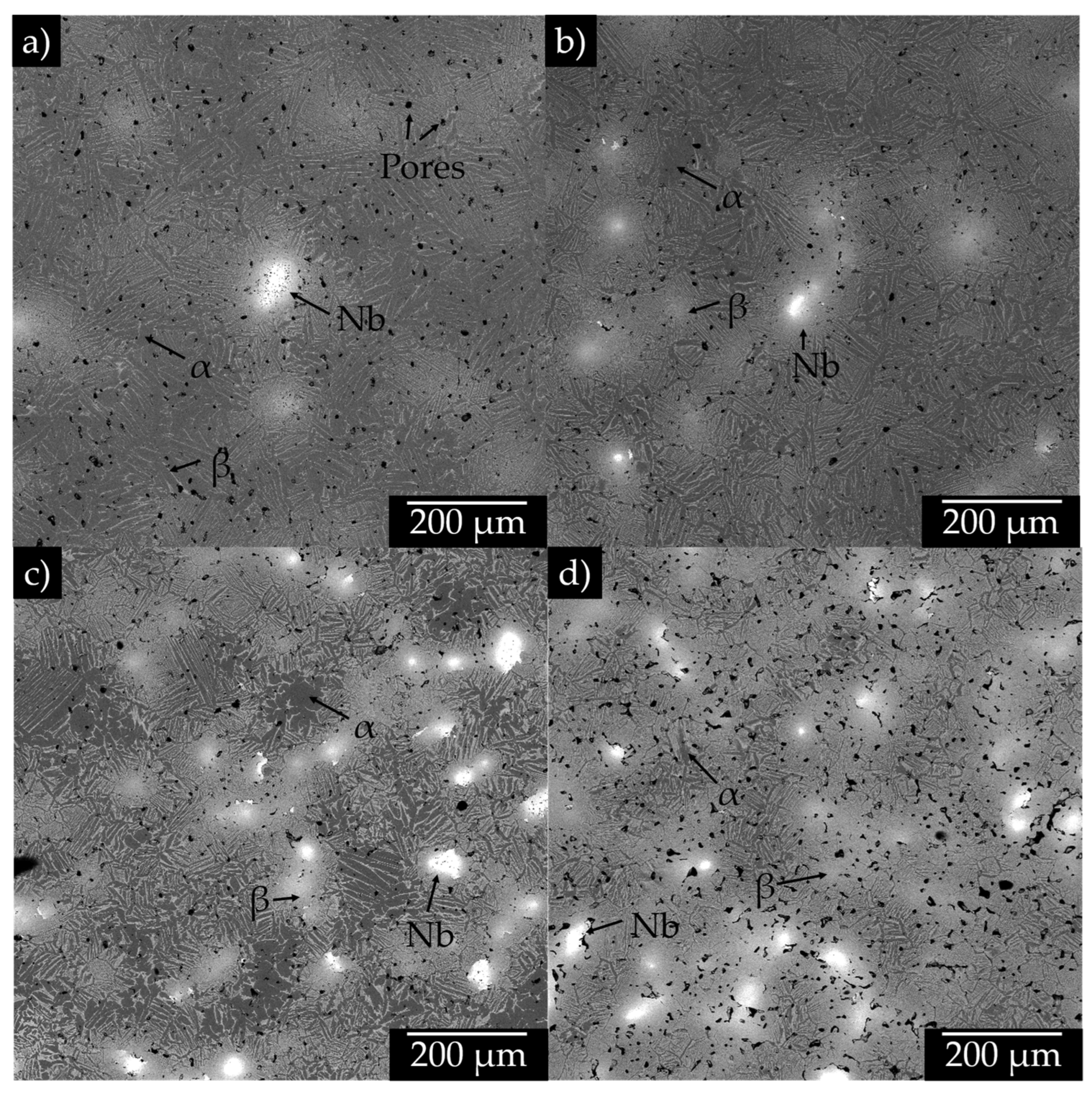
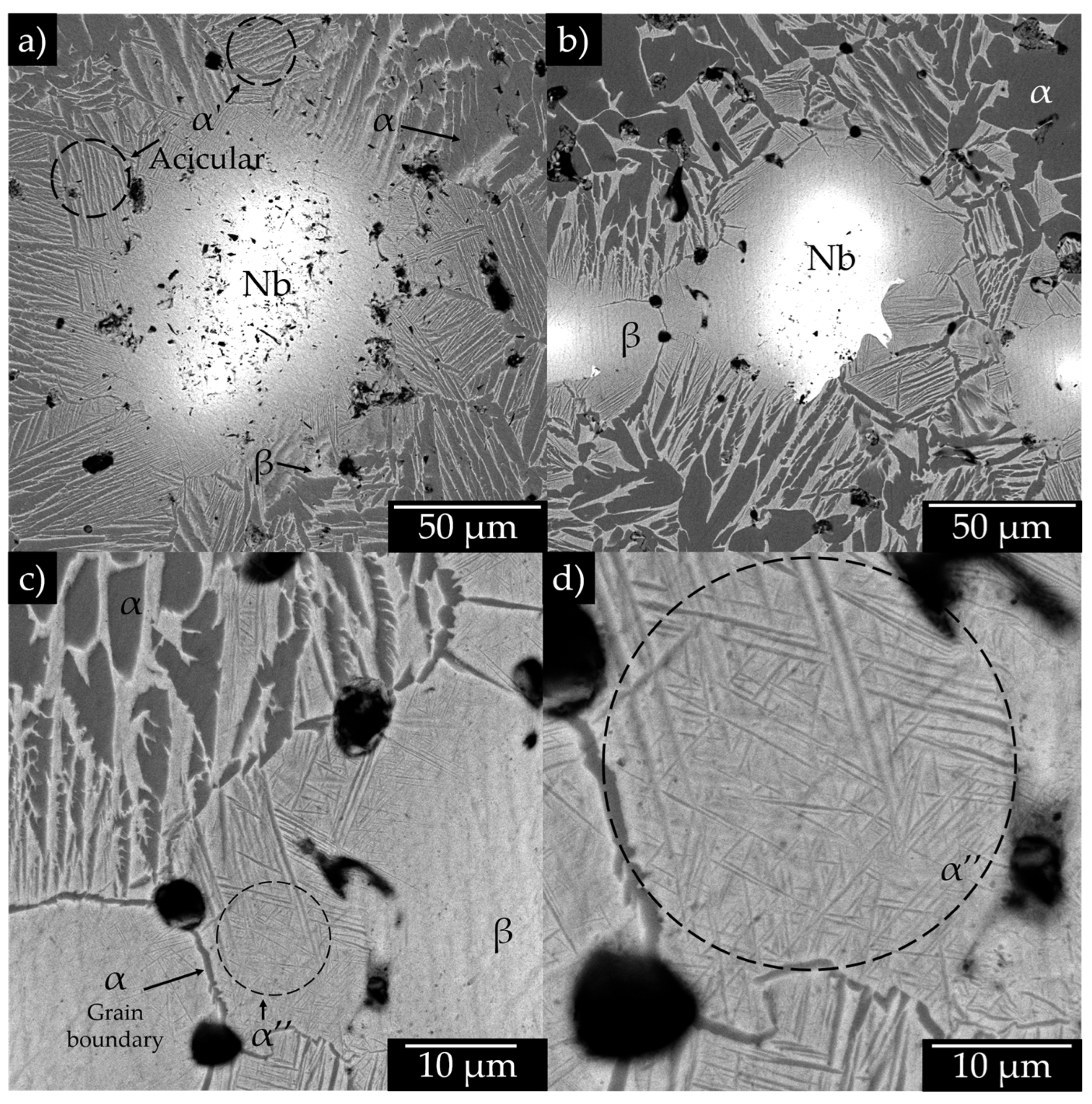
3.2. Microstructural Analysis
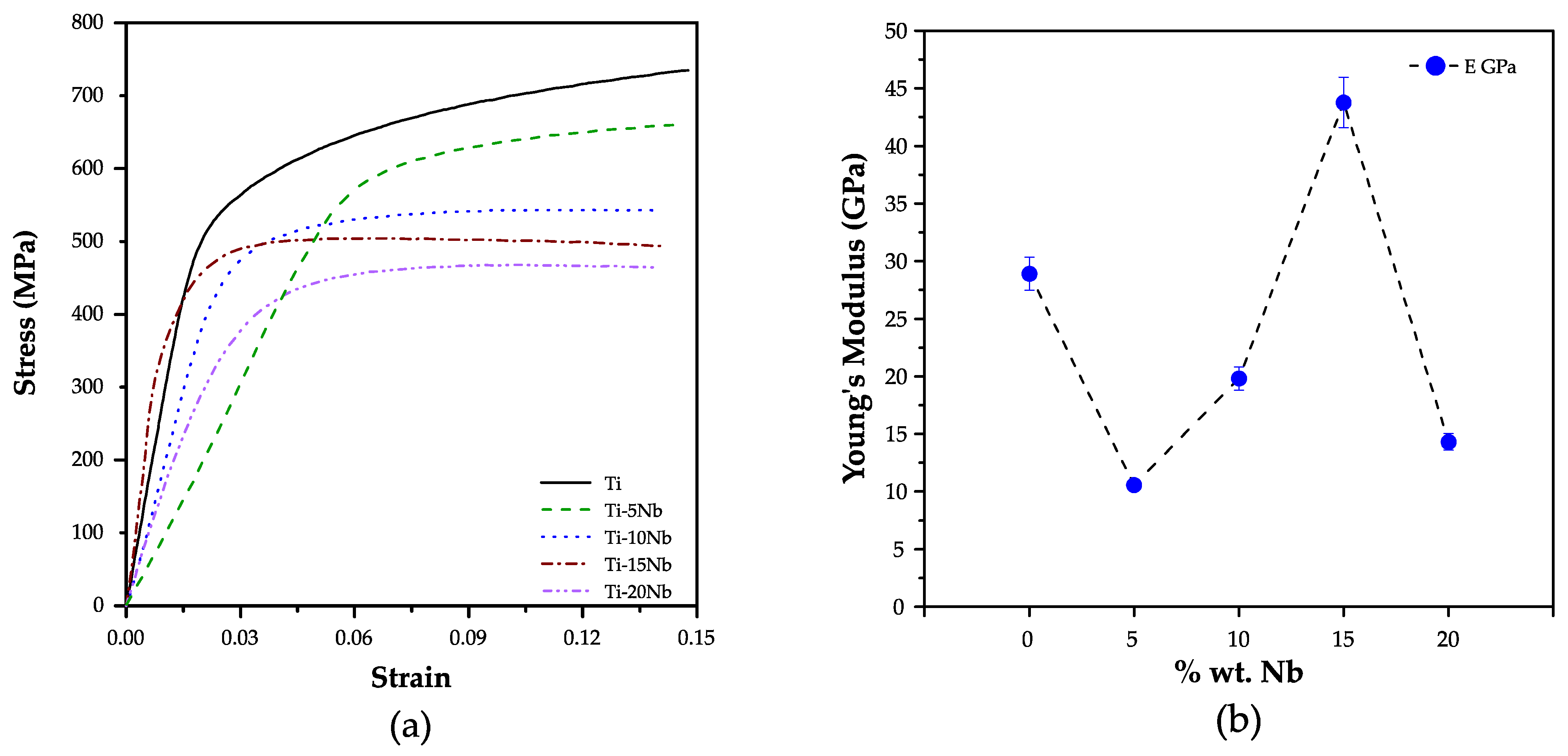
3.3. Compression Analysis
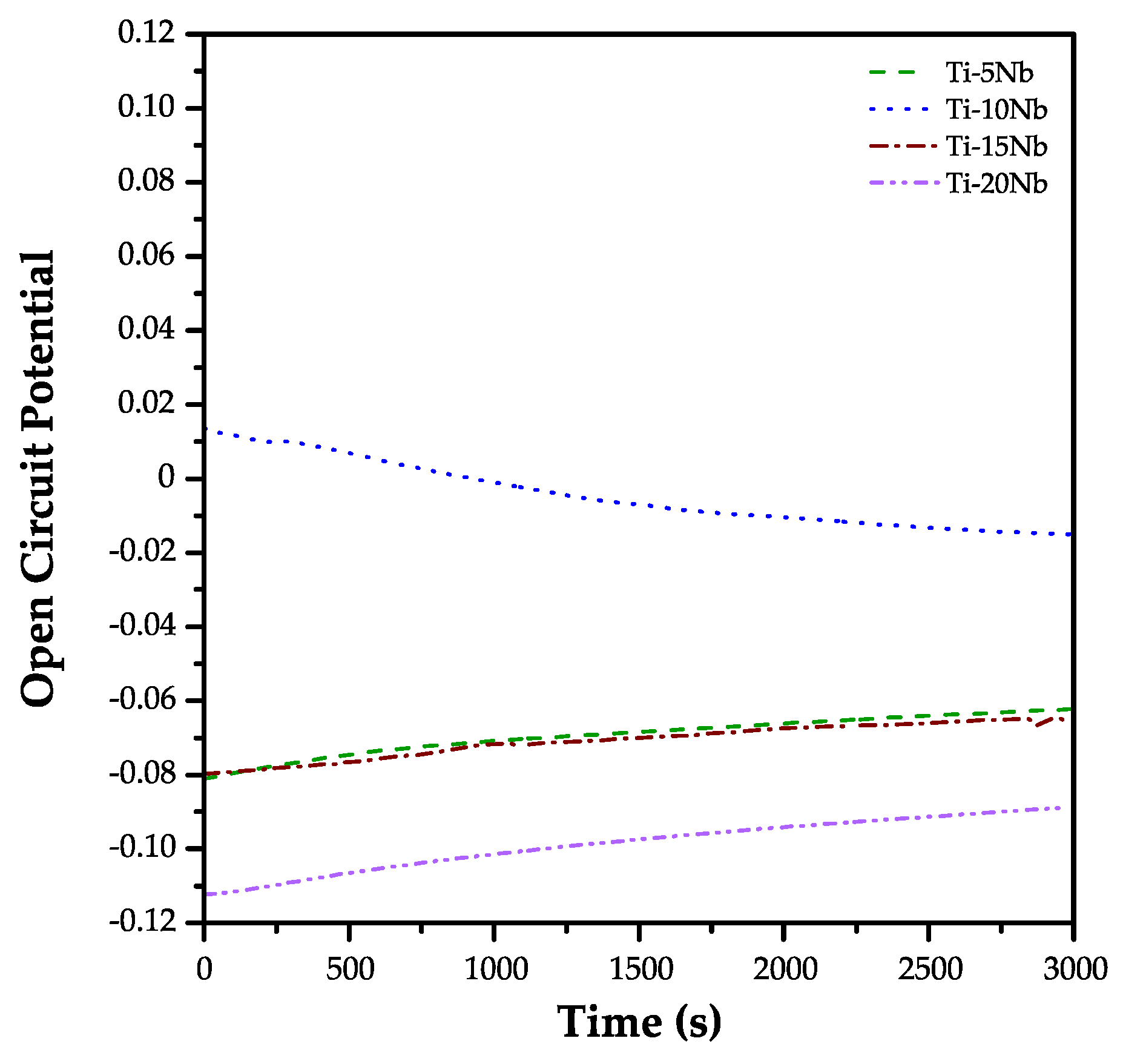
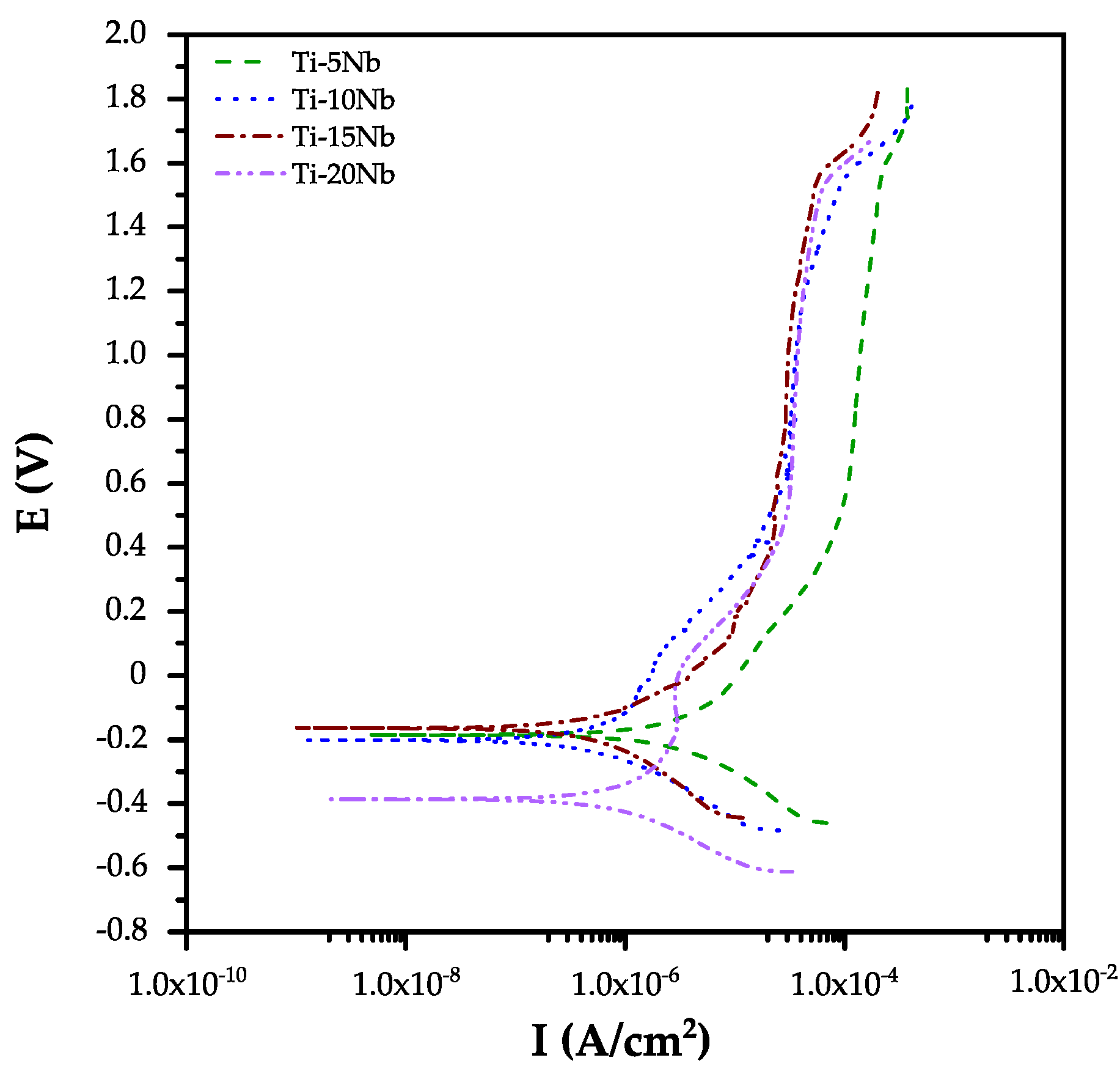
3.4. Electrochemical Behavior Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-W.; Wang, L.; Zhang, L.-C. Towards load-bearing biomedical titanium-based alloys: From essential requirements to future developments. Prog. Mater. Sci. 2024, 144, 101277. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Niinomi, M.; Akahori, T. Changes in mechanical properties of Ti alloys in relation to alloying additions of Ta and Hf. Mater. Sci. Eng. A 2008, 483–484, 153–156. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. A 2004, 371, 283–290. [Google Scholar] [CrossRef]
- Gupta, A.; Khatirkar, R.; Singh, J. A review of microstructure and texture evolution during plastic deformation and heat treatment of β-Ti alloys. J. Alloys Compd. 2022, 899, 163242. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, X.; Liu, Q.; Liu, L. Evaluation of the potential cytotoxicity of metals associated with implanted biomaterials (I). Prep. Biochem. Biotechnol. 2008, 39, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Evaluation of the mechanical properties of powder metallurgy Ti-6Al-7Nb alloy. J. Mech. Behav. Biomed. Mater. 2017, 67, 110–116. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, H.Z.; Li, Z.; Wang, H.J.; Liu, B. Constitutive modeling and processing map for elevated temperature flow behaviors of a powder metallurgy titanium aluminide alloy. J. Mater. Process. Technol. 2009, 209, 5363–5370. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Anene, F.A.; Aiza Jaafar, C.N.; Zainol, I.; Azmah Hanim, M.A.; Suraya, M.T. Biomedical materials: A review of titanium-based alloys. Proc. Inst. Mech. Eng. Part C J. Mechan. Eng. Sci. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Amigó, V.; Salvador, M.D.; Romero, F.; Solves, C.; Moreno, J.F. Microstructural evolution of Ti–6Al–4V during the sintering of microspheres of Ti for orthopedic implants. J. Mater. Process. Technol. 2003, 141, 117–122. [Google Scholar] [CrossRef]
- Bellemans, J. Osseointegration in porous coated knee arthroplasty: The influence of component coating type in sheep. Acta Orthop. 1999, 70 (Suppl. 288), i-35. [Google Scholar] [CrossRef]
- Oh, I.-H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Olmos, L.; Bouvard, D.; Lemus-Ruiz, J.; Jiménez, O. Processing and properties of highly porous Ti6Al4V mimicking human bones. J. Mater. Res. 2018, 33, 650–661. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of porous materials by spark plasma sintering: A review. Materials 2019, 12, 541. [Google Scholar] [CrossRef]
- Macias, R.; Garnica-Gonzalez, P.; Olmos, L.; Jimenez, O.; Chavez, J.; Vazquez, O.; Alvarado-Hernandez, F.; Arteaga, D. Sintering Analysis of Porous Ti/xTa Alloys Fabricated from Elemental Powders. Materials 2022, 15, 6548. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti–29Nb–13Ta–4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Filip, R.; Kubiak, K.; Ziaja, W.; Sieniawski, J. The effect of microstructure on the mechanical properties of two-phase titanium alloys. J. Mater. Process. Technol. 2003, 133, 84–89. [Google Scholar] [CrossRef]
- Motyka, M. Martensite formation and decomposition during traditional and AM processing of two-phase titanium alloys—An overview. Metals 2021, 11, 481. [Google Scholar] [CrossRef]
- Zhang, J.; Tasan, C.C.; Lai, M.J.; Dippel, A.-C.; Raabe, D. Complexion-mediated martensitic phase transformation in Titanium. Nat. Commun. 2017, 8, 14210. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Wang, D.; Zhang, H. Microstructural and mechanical properties of β-type Ti–Nb–Sn biomedical alloys with low elastic modulus. Metals 2019, 9, 712. [Google Scholar] [CrossRef]
- Karre, R.; Niranjan, M.K.; Dey, S.R. First principles theoretical investigations of low Young’s modulus beta Ti–Nb and Ti–Nb–Zr alloys compositions for biomedical applications. Mater. Sci. Eng. C 2015, 50, 52–58. [Google Scholar] [CrossRef]
- Zhuravleva, K.; Bönisch, M.; Scudino, S.; Calin, M.; Schultz, L.; Eckert, J.; Gebert, A. Phase transformations in ball-milled Ti–40Nb and Ti–45Nb powders upon quenching from the ß-phase region. Powder Technol. 2014, 253, 166–171. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Chen, R.-R.; Yang, Y.; Guo, J.-J.; Ding, H.-S.; Su, Y.-Q.; Fu, H.-Z. Microstructure and mechanical properties of Ti43Al6Nb alloys with different zirconium contents. Rare Met. 2023, 42, 2047–2056. [Google Scholar] [CrossRef]
- Macias, R.; Garnica-Gonzalez, P.; Villalobos-Brito, J.; Fernandez-Salvador, C.; Alanis-Fuerte, I.; Olmos, L.; Jimenez, O.; Chávez, J. Effect of niobium on corrosion resistance of 75Ti-x-25Ta-xNb alloy. MRS Adv. 2024, 1–4. [Google Scholar] [CrossRef]
- Singh, N.; Srikanth, K.P.; Gopal, V.; Rajput, M.; Manivasagam, G.; Prashanth, K.G.; Chatterjee, K.; Suwas, S. In Situ Production of Low-Modulus Ti-Nb Alloys by Selective Laser Melting and their Functional Assessment Toward Orthopedic Applications. J. Mater. Chem. B 2024, 12, 5982–5993. [Google Scholar] [CrossRef]
- Vishnu, D.S.M.; Sure, J.; Liu, Y.; Kumar, R.V.; Schwandt, C. Electrochemical synthesis of porous Ti-Nb alloys for biomedical applications. Mater. Sci. Eng. C 2019, 96, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, D.S.M.; Sure, J.; Schwandt, C. Effect of Nb on phase composition, microstructure and corrosion resistance of electrochemically synthesized porous Ti-xNb-13Zr for use as a bioalloy. J. Alloy. Met. Syst. 2024, 6, 100072. [Google Scholar] [CrossRef]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2021, 5, 371–395. [Google Scholar] [CrossRef] [PubMed]
- ASTM D695-02; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM G3-89; Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing. ASTM: West Conshohocken, PA, USA, 2010.
- Mihalcea, E.; Hernández, H.V.; Olmos, L.; Jimenez, O. Semi-solid sintering of Ti6Al4V/CoCrMo composites for biomedical applications. Mater. Res. 2019, 22, e20180391. [Google Scholar] [CrossRef]
- Pontau, A.E.; Lazarus, D. Diffusion of titanium and niobium in bcc Ti-Nb alloys. Phys. Rev. B 1979, 19, 4027. [Google Scholar] [CrossRef]
- Panelli, R.; Ambrozio Filho, F. A study of a new phenomenological compacting equation. Powder Technol. 2001, 114, 255–261. [Google Scholar] [CrossRef]
- Heckel, R.W. Density-pressure relationships in powder compaction. Trans. Metal. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Hui, Q.; Xue, X.; Kou, H.; Lai, M.; Tang, B.; Li, J. Kinetics of the ω phase transformation of Ti-7333 titanium alloy during continuous heating. J. Mater. Sci. 2013, 48, 1966–1972. [Google Scholar] [CrossRef]
- Alanis-Fuerte, I.; Garnica-González, P.; López-Martínez, E.; Vergara-Hernández, H.J.; Vázquez-Gómez, O. Effect of Cold-rolling and Heating Rate on Austenite Formation in a Low–Carbon Steel. ISIJ Int. 2022, 62, 227–236. [Google Scholar] [CrossRef]
- Moffat, D.L.; Kattner, U.R. The stable and metastable Ti-Nb phase diagrams. Metall. Trans. A 1998, 19, 2389–2397. [Google Scholar] [CrossRef]
- Han, M.-K.; Kim, J.-Y.; Hwang, M.-J.; Song, H.-J.; Park, Y.-J. Effect of Nb on the microstructure, mechanical properties, corrosion behavior, and cytotoxicity of Ti-Nb alloys. Materials 2015, 8, 5986–6003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, P.; Dehghan-Manshadi, A.; Kent, D.; Ehtemam-Haghighi, S.; Jowers, C.; Bermingham, M.; Li, T.; Cooper-White, J.; Dargusch, M.S. Sintering and biocompatibility of blended elemental Ti-xNb alloys. J. Mech. Behav. Biomed. Mater. 2020, 104, 103691. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, Q.; Wang, J.; Li, D.; Song, Z.; Yu, J. Promotion of osseointegration between implant and bone interface by titanium alloy porous scaffolds prepared by 3D printing. ACS Biomater. Sci. Eng. 2020, 6, 5181–5190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ruan, J.; Chang, L.; Yang, H.; Ruan, W. Porous Nb-Ti-Ta alloy scaffolds for bone tissue engineering: Fabrication, mechanical properties and in vitro/vivo biocompatibility. Mater. Sci. Eng. C 2017, 78, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Farrahnoor, A.; Zuhailawati, H. Effects of hydroxyapatite addition on the bioactivity of Ti-Nb alloy matrix composite fabricated via powder metallurgy process. Mater. Today Commun. 2021, 27, 102209. [Google Scholar] [CrossRef]
- Welsch, G.; Boyer, R.; Collings, E.W. (Eds.) Materials Properties Handbook: Titanium Alloys; ASM International: Novelty, OH, USA, 1993. [Google Scholar]
- Moreno, J.G.; Bönisch, M.; Panagiotopoulos, N.T.; Calin, M.; Papageorgiou, D.G.; Gebert, A.; Eckert, J.; Evangelakis, G.A.; Lekka, C.E. Ab initio and experimental study of phase stability of Ti-Nb alloys. J. Alloys Compd. 2017, 696, 481–489. [Google Scholar] [CrossRef]
- D’yakonova, N.B.; Lyasotskii, I.V.; Rodionov, Y.L. Orthorhombic martensite and the ω phase in quenched and deformed titanium alloys with 20–24 at% Nb. Russ. Metall. Met. 2007, 2007, 51–58. [Google Scholar] [CrossRef]
- Marczewski, M.; Wieczerzak, K.; Maeder, X.; Lapeyre, L.; Hain, C.; Jurczyk, M.; Nelis, T. Microstructure and mechanical properties of Ti-Nb alloys: Comparing conventional powder metallurgy, mechanical alloying, and high-power impulse magnetron sputtering processes for supporting materials screening. J. Mater. Sci. 2024, 59, 9107–9125. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Jin, Z. Thermodynamic assessment of the Nb-Ti system. Calphad 2001, 25, 305–317. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Koptyug, A.; Khrapov, D.; Ivanov, Y.F.; Mishurova, T.; Evsevleev, S.; Prymak, O.; Loza, K.; Epple, M.; Bruno, G.; et al. In situ synthesis of a binary Ti–10at% Nb alloy by electron beam melting using a mixture of elemental niobium and titanium powders. J. Mater. Process. Technol. 2020, 282, 116646. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Nie, H.; Willumeit, R.; Pyczak, F. Sintering behavior and mechanical properties of a metal injection molded Ti–Nb binary alloy as biomaterial. J. Alloys Compd. 2015, 640, 393–400. [Google Scholar] [CrossRef]
- Garnica, P.; Macías, R.; Chávez, J.; Bouvard, D.; Jiménez, O.; Olmos, L.; Arteaga, D. Fabrication and characterization of highly porous Ti6Al4V/xTa composites for orthopedic applications. J. Mater. Sci. 2020, 55, 16419–16431. [Google Scholar] [CrossRef]
- Kent, D.; Wang, G.; Dargusch, M. Effects of phase stability and processing on the mechanical properties of Ti–Nb based β Ti alloys. J. Mech. Behav. Biomed. Mater. 2013, 28, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Ju, C.P.; Chern Lin, J.H. Structure–property relationship of cast Ti–Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Rabadia, C.D.; Liang, S.-X.; Sercombe, T.B.; Zhang, L.-C. Microstructural homogeneity and mechanical behavior of a selective laser melted Ti-35Nb alloy produced from an elemental powder mixture. J. Mater. Sci. Technol. 2021, 61, 221–233. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Liang, S.X.; Zhang, Y.S.; Wang, L.Q.; Sercombe, T.B.; Zhang, L.C. Comparison of microstructure and mechanical behavior of Ti-35Nb manufactured by laser powder bed fusion from elemental powder mixture and prealloyed powder. J. Mater. Sci. Technol. 2022, 105, 1–16. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Qin, P.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Selective laser melting of Ti–35Nb composite from elemental powder mixture: Microstructure, mechanical behavior and corrosion behavior. Mater. Sci. Eng. A 2019, 760, 214–224. [Google Scholar] [CrossRef]
- Zaveri, N.; Mahapatra, M.; Deceuster, A.; Peng, Y.; Li, L.; Zhou, A. Corrosion resistance of pulsed laser-treated Ti–6Al–4V implant in simulated biofluids. Electrochim. Acta 2008, 53, 5022–5032. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Q.; Jin, Y.; Li, M.; Yang, R.; Cui, X.; Gong, M. In vitro studying corrosion behavior of porous titanium coating in dynamic electrolyte. Mater. Sci. Eng. C 2017, 70, 1071–1075. [Google Scholar] [CrossRef]
- Alves, A.C.; Sendão, I.; Ariza, E.; Toptan, F.; Ponthiaux, P.; Pinto, A.M.P. Corrosion behaviour of porous Ti intended for biomedical applications. J. Porous Mater. 2016, 23, 1261–1268. [Google Scholar] [CrossRef]
- Ureña, J.; Tsipas, S.; Pinto, A.M.; Toptan, F.; Gordo, E.; Jiménez-Morales, A. Corrosion and tribocorrosion behaviour of β-type Ti-Nb and Ti-Mo surfaces designed by diffusion treatments for biomedical applications. Corros. Sci. 2018, 140, 51–60. [Google Scholar] [CrossRef]
- Barcia, O.E.; D’Elia, E.; Frateur, I.; Mattos, O.R.; Pébère, N.; Tribollet, B. Application of the impedance model of de Levie for the characterization of porous electrodes. Electrochim. Acta 2002, 47, 2109–2116. [Google Scholar] [CrossRef]
- Chen, Y.-I.; Chen, Y.-J.; Lai, C.-Y.; Chang, L.-C. Mechanical and anticorrosive properties of TiNbTa and TiNbTaZr films on Ti-6Al-4V alloy. Coatings 2022, 12, 1985. [Google Scholar] [CrossRef]


| Ti | Ti-5Nb | Ti-10Nb | Ti-15Nb | Ti-20Nb | |
|---|---|---|---|---|---|
| Theoretical density (g/cm3) | 4.5 | 4.70 | 4.90 | 5.11 | 5.31 |
| Green density (g/cm3) | 3.43 | 3.34 | 3.45 | 3.57 | 3.73 |
| Sintered density (g/cm3) | 4.27 | 4.13 | 4.26 | 4.37 | 4.41 |
| Relative green density | 0.762 | 0.724 | 0.730 | 0.738 | 0.750 |
| Relative Sintered density | 0.949 | 0.896 | 0.902 | 0.904 | 0.908 |
| Contraction % | 19.7 | 19.3 | 19.0 | 18.3 | 17.3 |
| Sample | Rs (Ω∙cm2) | CPE1-T (F∙cm−2∙sn−1) | CPE1-P | Ro (Ω∙cm2) | C1 (F∙cm−2) | Rp (Ω∙cm2) | |
|---|---|---|---|---|---|---|---|
| Ti-5Nb | 1.32 × 10−3 | 14.89 | 4.10 × 10−4 | 0.5557 | 5.65 | 6.68 × 10−6 | 18,730 |
| Ti-10Nb | 3.72 × 10−3 | 16.53 | 2.13 × 10−4 | 0.6716 | 15.89 | 8.93 × 10−6 | 60,737 |
| Ti-15Nb | 3.15 × 10−3 | 12.33 | 1.05 × 10−4 | 0.7763 | 294.6 | 3.19 × 10−6 | 47,541 |
| Ti-20Nb | 1.20 × 10−3 | 15.23 | 2.66 × 10−4 | 0.6338 | 8.22 | 6.52 × 10−6 | 83,251 |
| Ti-5Nb | Ti-10Nb | Ti-15Nb | Ti-20Nb | |
|---|---|---|---|---|
| Ba (mV) | 461.05 | 1053.9 | 234.17 | 1276.2 |
| Bc (mV) | 273.31 | 272.31 | 285.73 | 307.08 |
| Icorr (µA/cm2) | 4.7252 | 1.2475 | 0.7781 | 2.4642 |
| Ecorr (mV) | −185.46 | −201.98 | −163.78 | −386.15 |
| Corr Rate (µm/Y) | 100.15 | 23.345 | 14.161 | 42.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macias, R., Jr.; Garnica González, P.; Olmos, L.; Alanis-Fuerte, I.; Jimenez, O.; Alvarado-Hernández, F.; Velasco-Plascencia, M.; Ávila-Olivera, J.A. In Situ Fabrication of Ti-xNb Alloys by Conventional Powder Metallurgy. Coatings 2024, 14, 897. https://doi.org/10.3390/coatings14070897
Macias R Jr., Garnica González P, Olmos L, Alanis-Fuerte I, Jimenez O, Alvarado-Hernández F, Velasco-Plascencia M, Ávila-Olivera JA. In Situ Fabrication of Ti-xNb Alloys by Conventional Powder Metallurgy. Coatings. 2024; 14(7):897. https://doi.org/10.3390/coatings14070897
Chicago/Turabian StyleMacias, Rogelio, Jr., Pedro Garnica González, Luis Olmos, Ivon Alanis-Fuerte, Omar Jimenez, Francisco Alvarado-Hernández, Melina Velasco-Plascencia, and Jorge Alejandro Ávila-Olivera. 2024. "In Situ Fabrication of Ti-xNb Alloys by Conventional Powder Metallurgy" Coatings 14, no. 7: 897. https://doi.org/10.3390/coatings14070897
APA StyleMacias, R., Jr., Garnica González, P., Olmos, L., Alanis-Fuerte, I., Jimenez, O., Alvarado-Hernández, F., Velasco-Plascencia, M., & Ávila-Olivera, J. A. (2024). In Situ Fabrication of Ti-xNb Alloys by Conventional Powder Metallurgy. Coatings, 14(7), 897. https://doi.org/10.3390/coatings14070897








