Endurance to Multiple Factors of Water-Based Electrically Conductive Paints with Metallic Microparticles
Abstract
:1. Introduction
2. Preparation of Paints with Large Quantities of Metallic Microparticles
2.1. Materials and Methods
2.1.1. Raw Materials
- o
- Polyoxyethylene (25) octyl phenyl ether, butyl acrylate, vinyl acetate, dibutyl phthalate, sodium dodecyl sulfate. All these substances are procured from authorized distributors;
- o
- Microparticles powders (source: Laiwu Powder Material Co. Ltd., Shanghai, China).
2.1.2. Testing Equipment and Methods
3. Results and Discussion
3.1. Structural Analyses
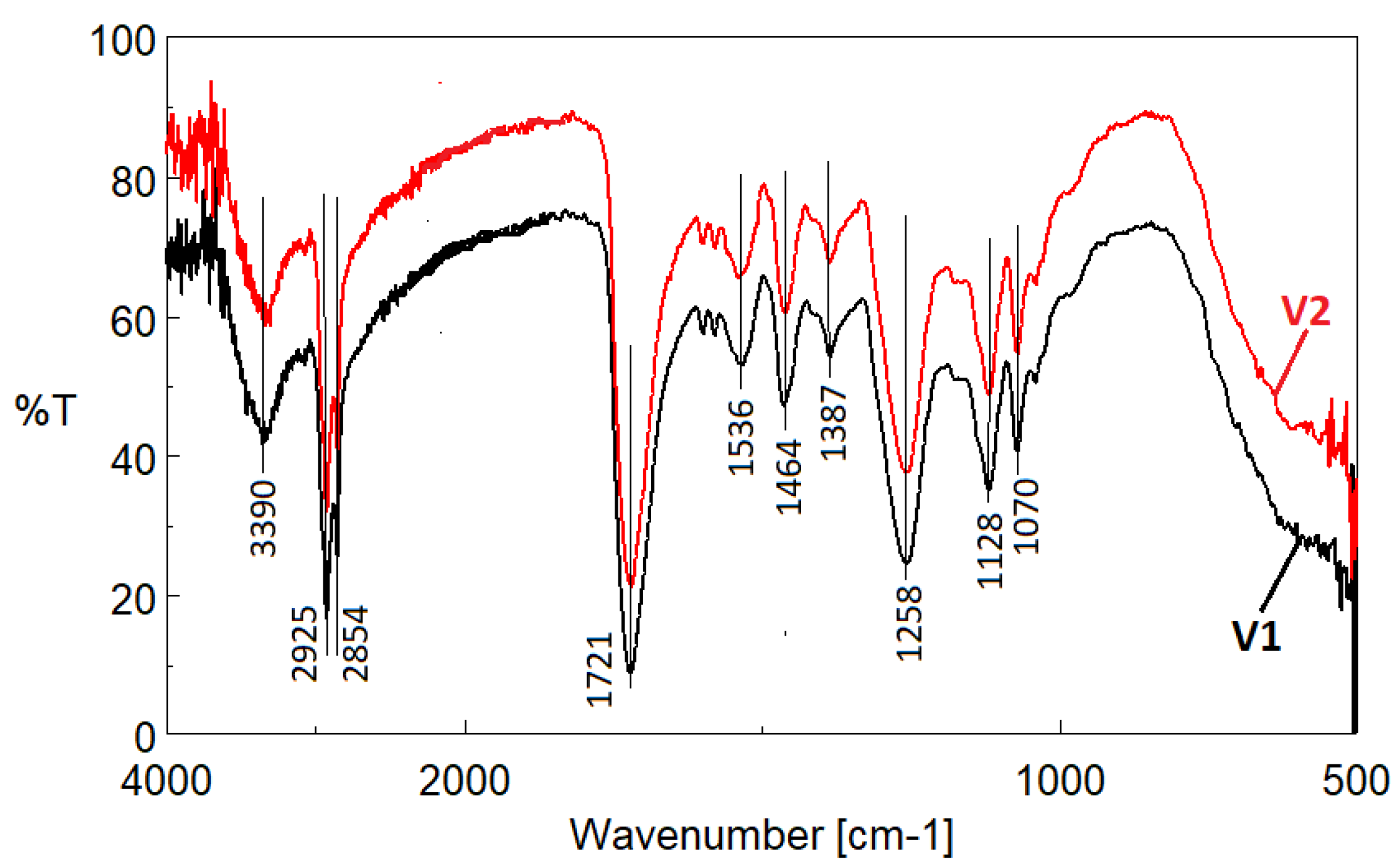
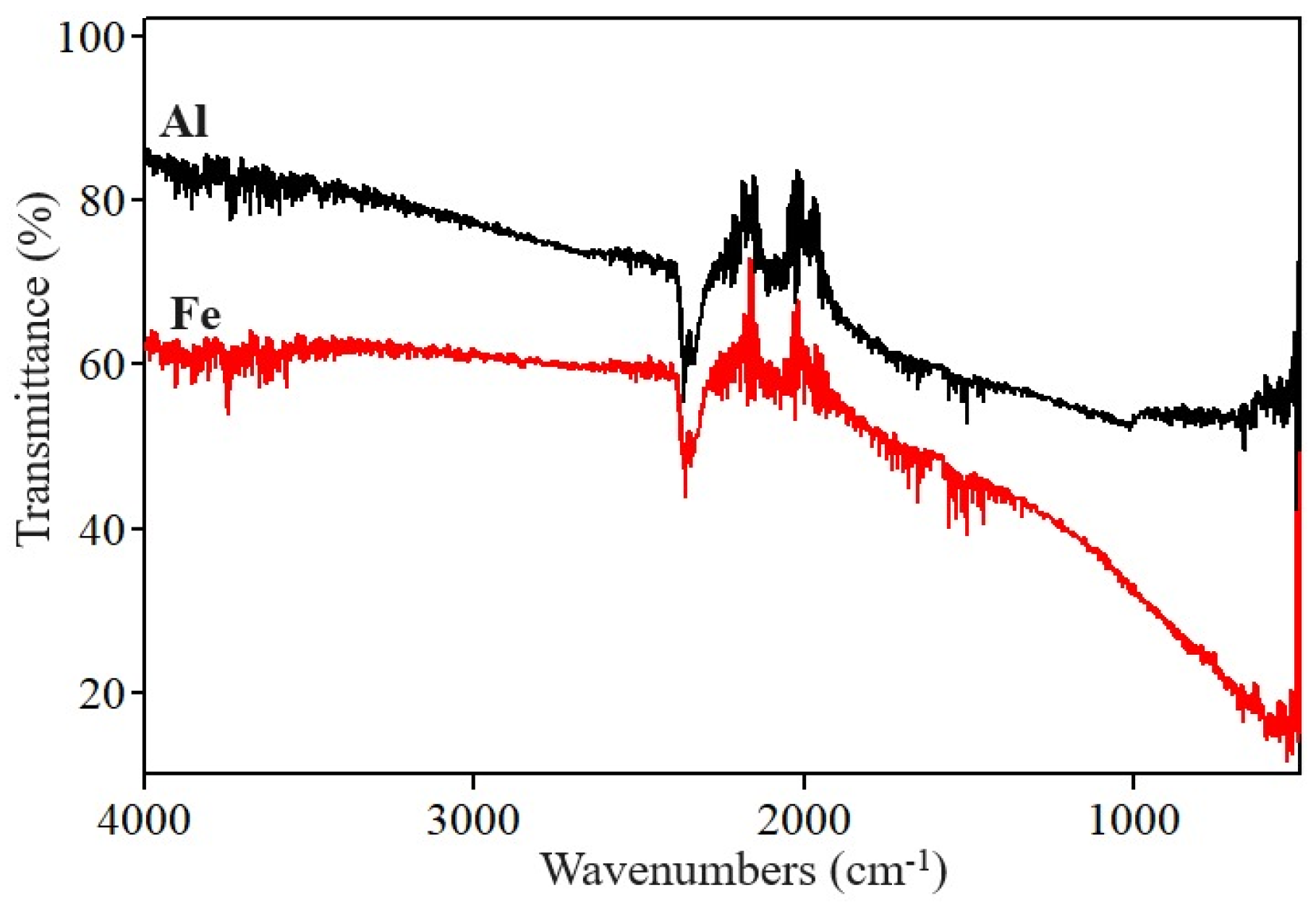
3.2. ATR/FTIR Analysis
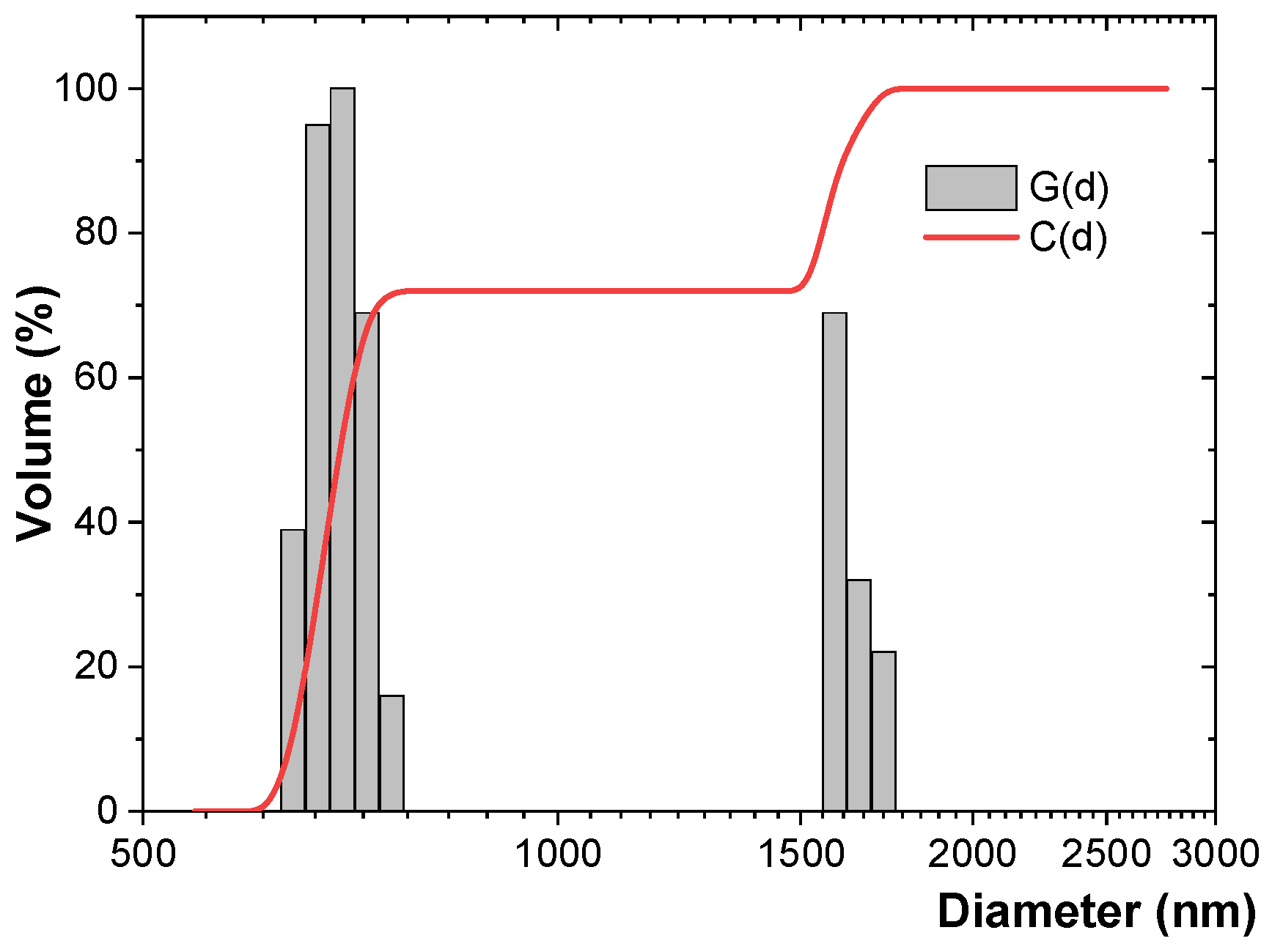
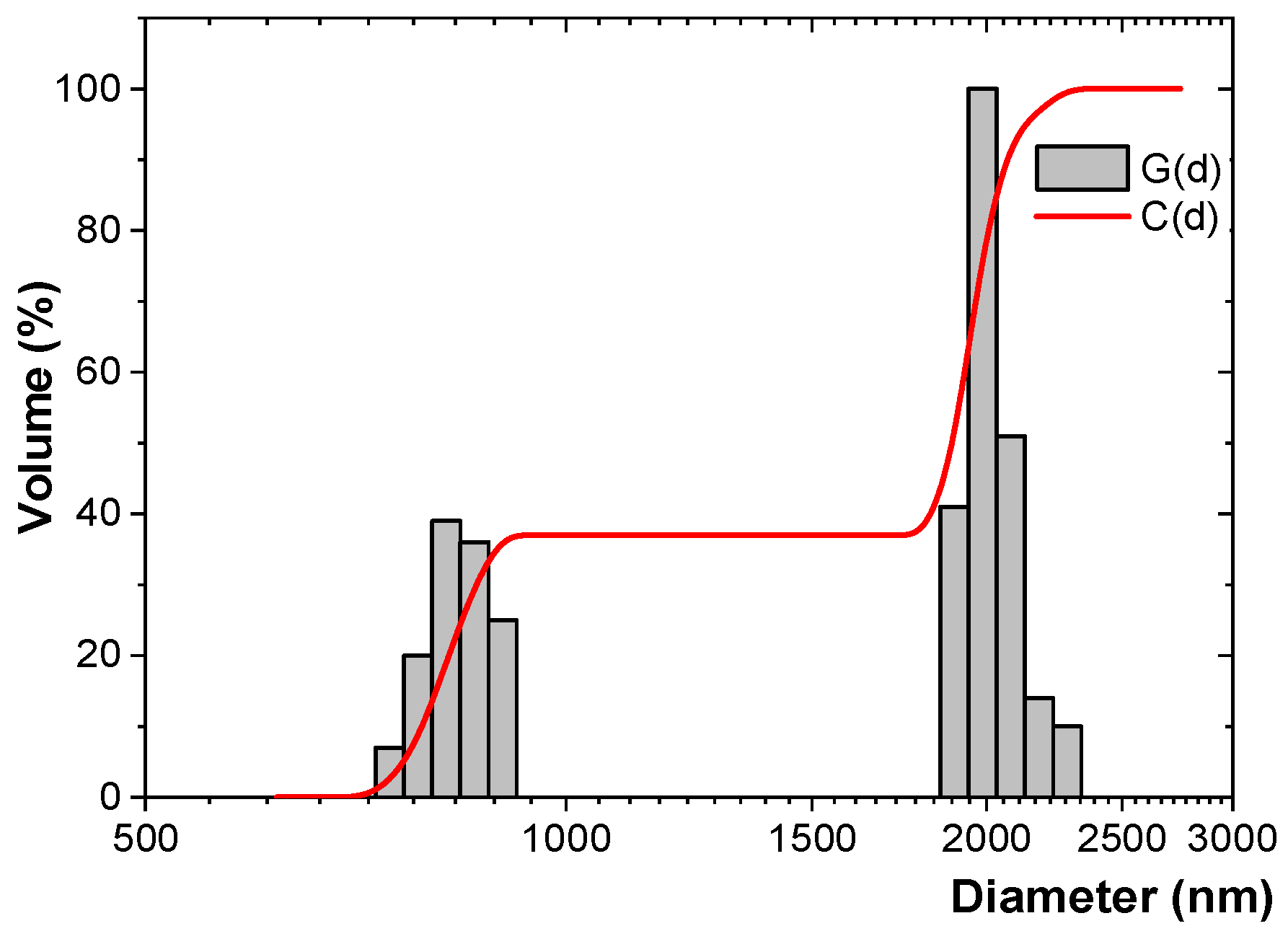
3.3. DLS Study
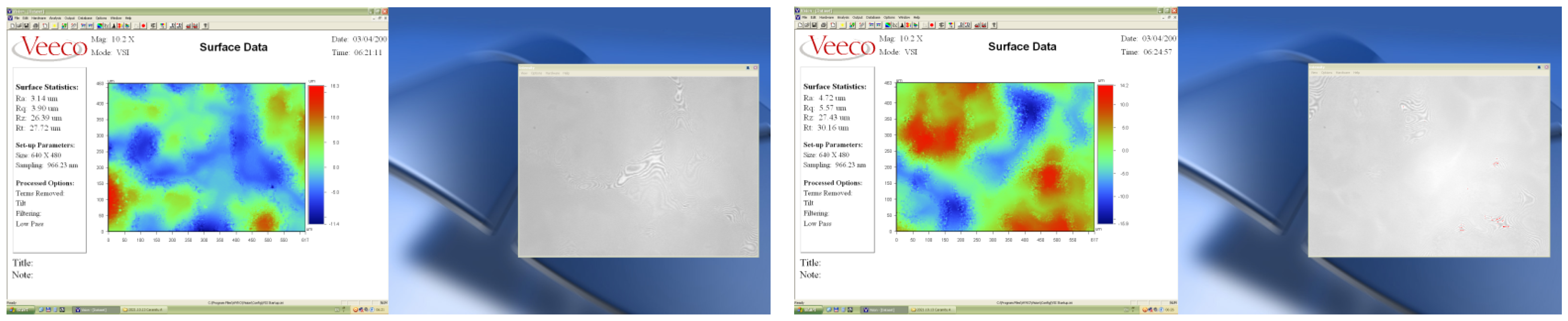
3.4. Determination of Thickness and Surface Topography (Roughness) of Paint Layers
3.5. Resistance to Water and Solvent (Isopropyl Alcohol)
- c—degree of swelling;
- m2,3,4—mass of inflated/swollen material (after each 72-h cycle); X1—mass of initial—dry material.
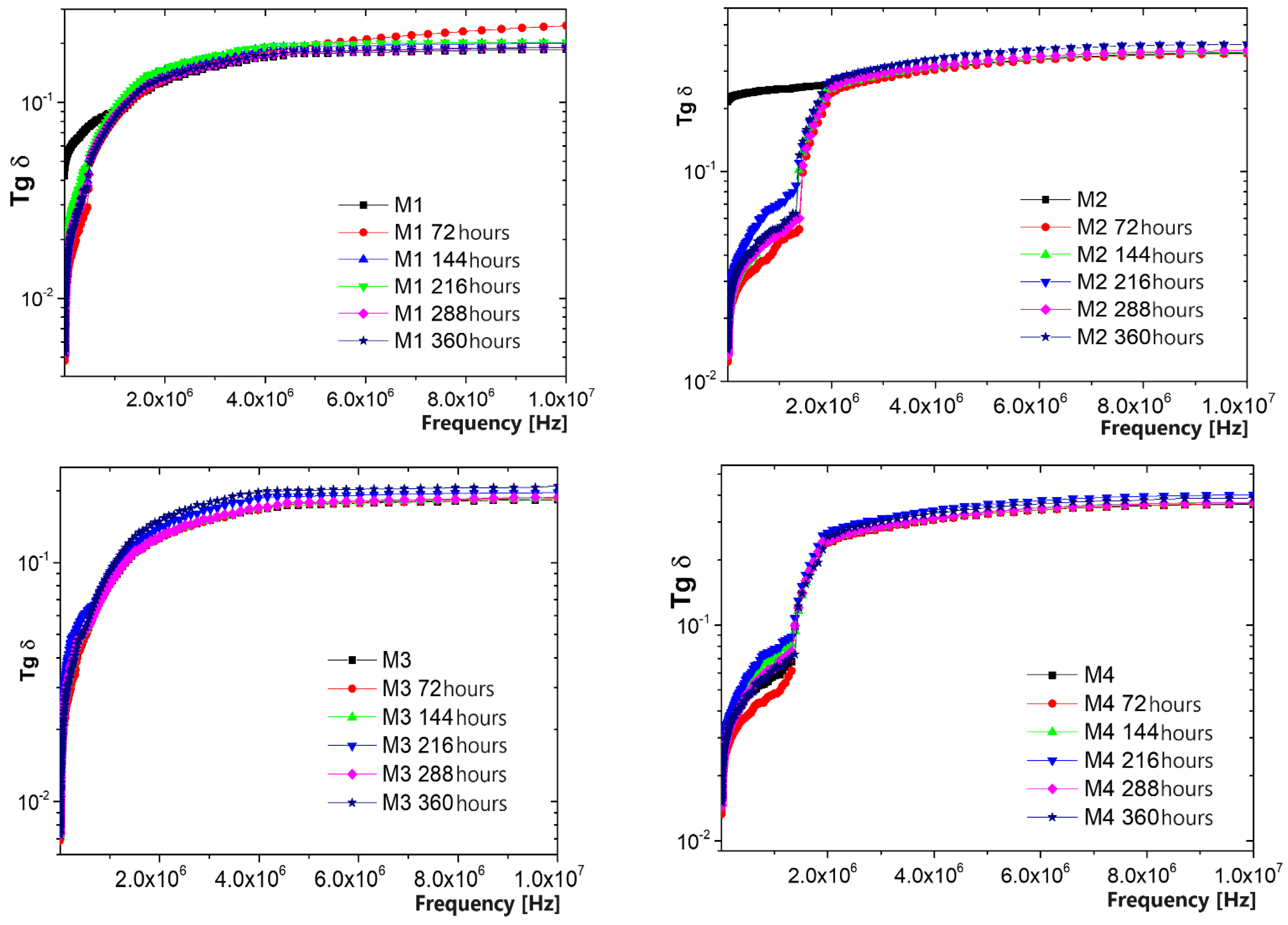
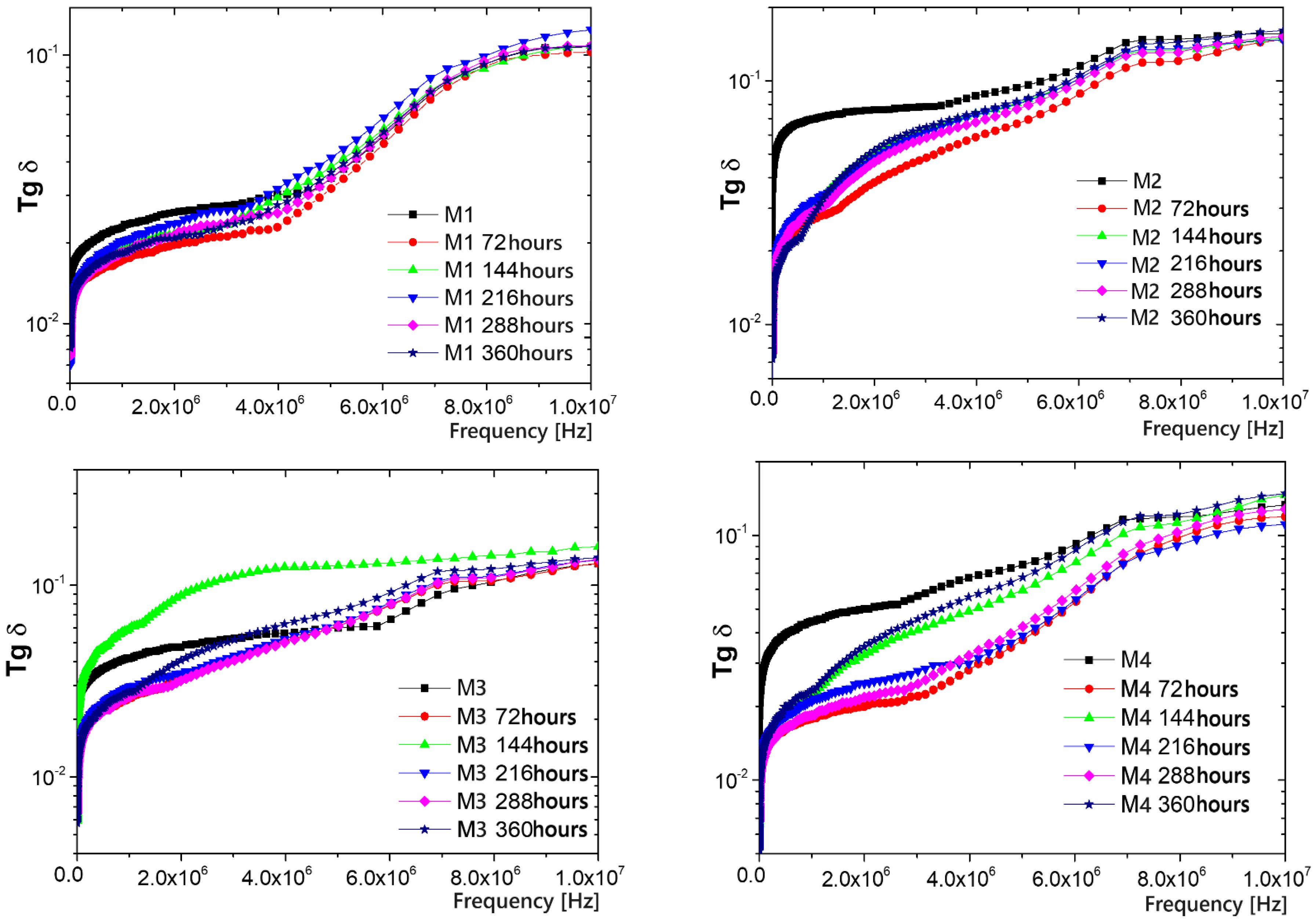
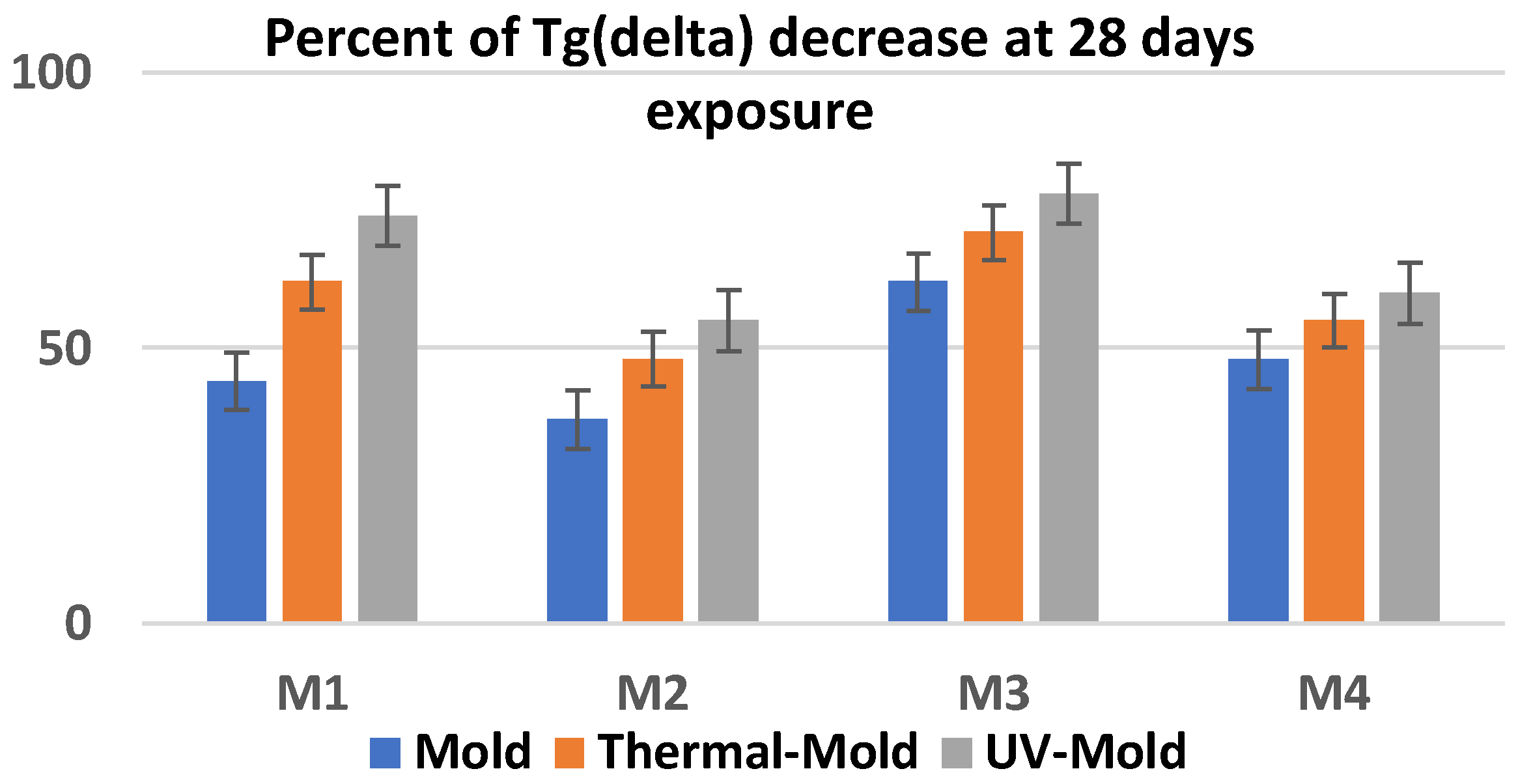
3.6. Resistance to the Action of UV Radiation and Lifetime Evaluation
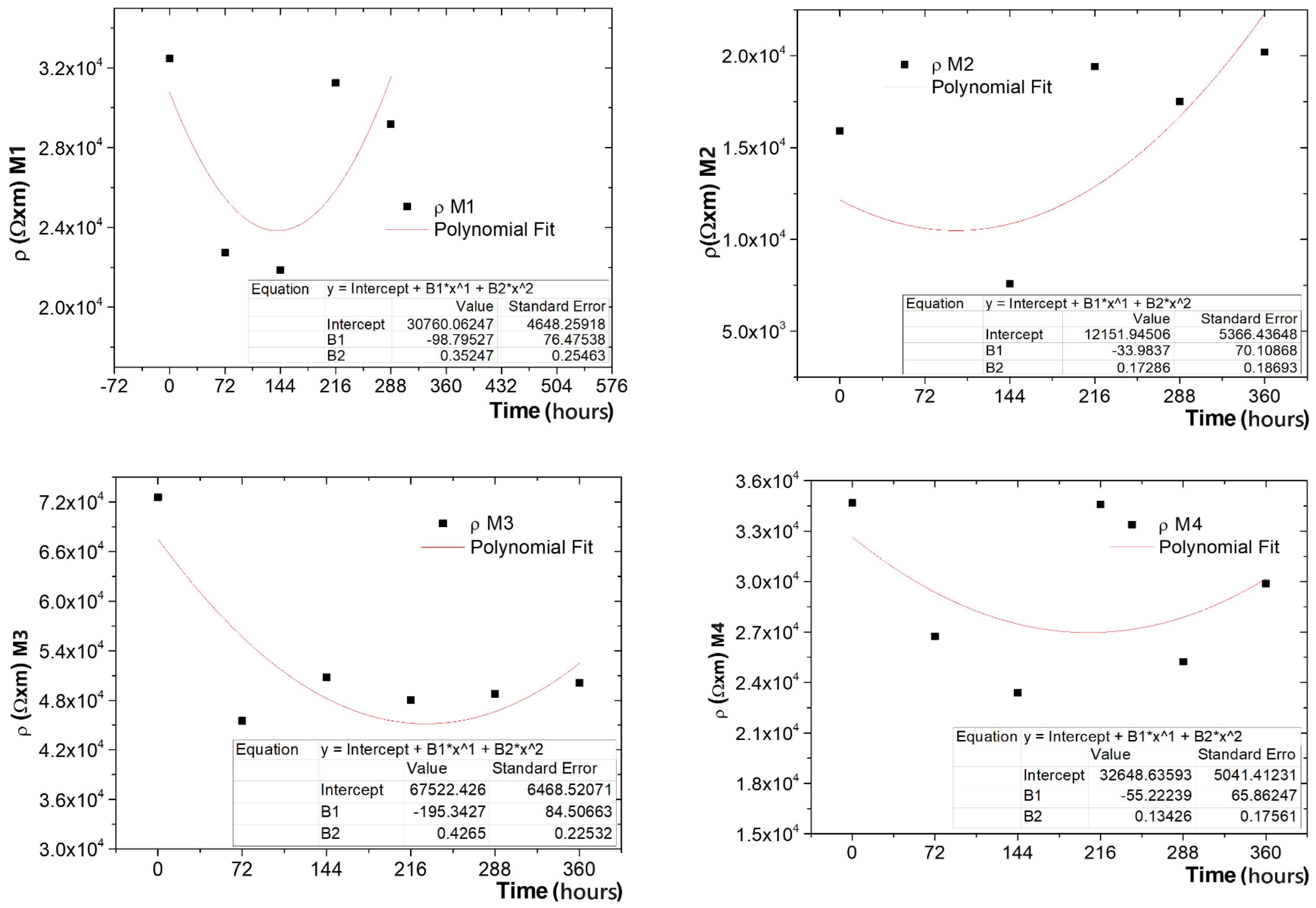
3.7. Resistance to the Action of Temperature and Lifetime Evaluation
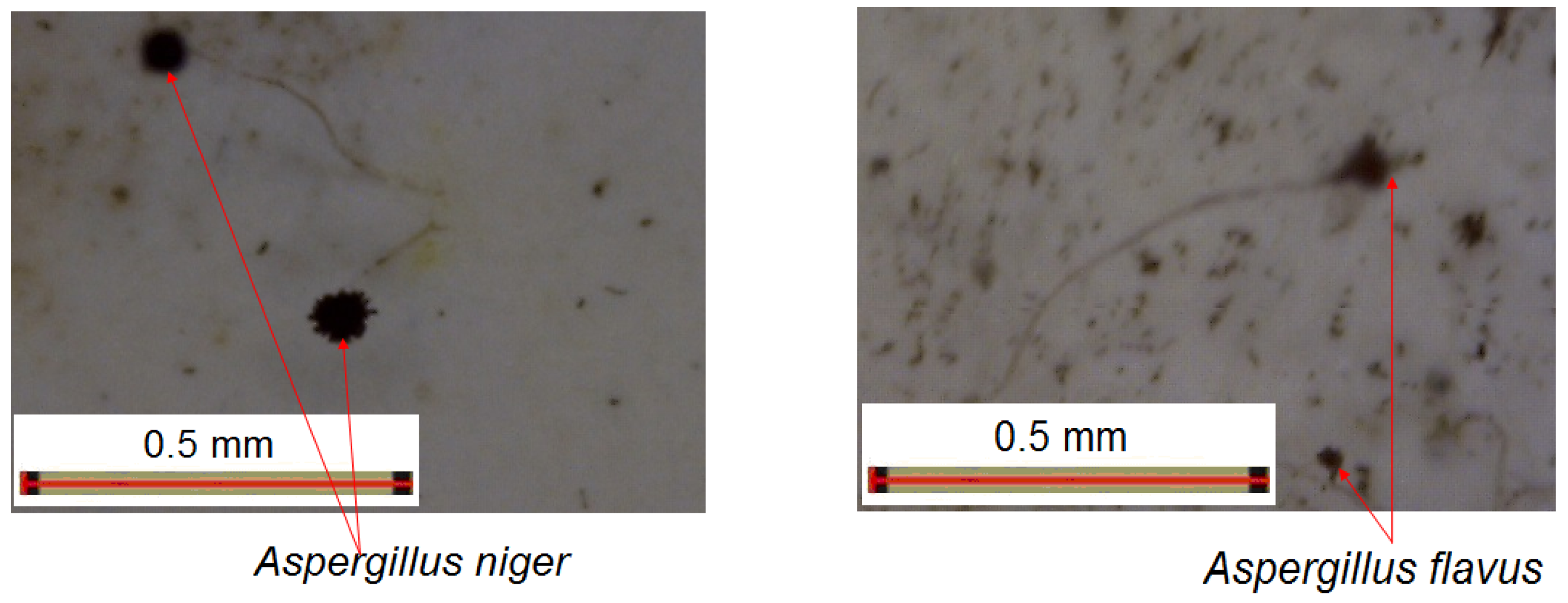
3.8. Resistance to the Action of Molds
- -
- After 3 days of exposure, no zone of mold growth was observed on the paint samples, but a very dense growth of mold could be observed around the paint on the plastic sample support, and there were very few fungal colonies starting to penetrate the edge of the paint samples.
- -
- After 7 days of exposure, mold colonies were noticed on the samples, but they did not cover more than 20% of the sample surface, under the circumstances that massive mold colonies were growing around the paint samples.
- -
- After 14, 21, and 28 days of exposure, the mold colonies persisted on the paint samples, but they were found at the same stage of development as for 7 days, i.e., the degree of coverage remained unchanged at about 25% of the paint sample surface.
- -
- After 3 days of exposure, no large zones of mold growth were observed on the paint samples, but some few fungal punctual colonies were noticed, along with a very dense growth of mold observed around the paint samples and on the paint edges.
- -
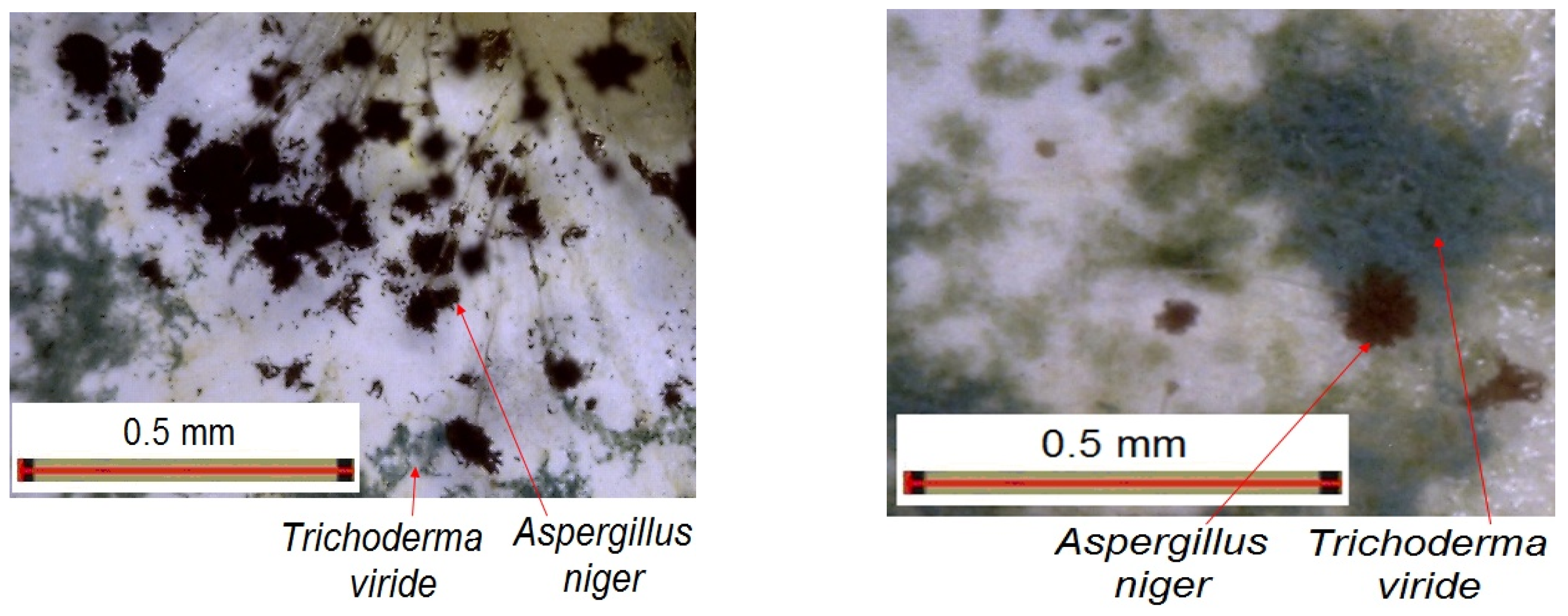
- After 7 days of exposure, a consistent growth of fungal colonies on the paint samples were noticed, this time especially Trichoderma viride, mainly along with Aspergillus niger, exceeding 25% of the paint.
- -
- After 14, 21, and 28 days of exposure, the mold colonies were constantly developing upon the paint samples, but much more slowly, the degree of coverage remaining under 35% of the paint sample surface after 28 days, an example being indicated in Figure 15.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMC Electrically Conductive Paints. Available online: https://www.solianiemc.com/en/cp/emc-electrically-conductive-paints/?gad_source=1&gclid=Cj0KCQjwjLGyBhCYARIsAPqTz1-DOF1Sw7J8wFzhbDIZLBd4b8Z5UxsiopHr2oeQ_mM4k1MuQkk-btgaAsFvEALw_wcB (accessed on 30 May 2024).
- EMF and RFI Shielding with Conductive Paints. Available online: https://www.antala.uk/emf-and-rfi-shielding-with-conductive-paints/ (accessed on 30 May 2024).
- Conductive Paints and Coatings. Available online: https://limitless-shielding.com/conductive-paints-and-coatings/ (accessed on 30 May 2024).
- ECOS Paints. Available online: https://ecospaints.net/blog/what-to-know-about-electromagnetic-shielding-materials (accessed on 30 May 2024).
- Conductive Translucent Paint. Available online: https://hollandshielding.com/en/conductive-translucent-paint?gad_source=1&gclid=CjwKCAjw9cCyBhBzEiwAJTUWNZC2A5l8HgsLs4KaJrIJbPz6e8rSoxmem-6Wv9nuq7kMi8Koz6ZhDhoCCHAQAvD_BwE (accessed on 30 May 2024).
- Conductive Paints. Available online: https://mgchemicals.com/category/conductive-paint/ (accessed on 30 May 2024).
- Electric Paint. Available online: https://www.bareconductive.com/collections/electric-paint (accessed on 30 May 2024).
- Electrically Conductive Paints. Available online: https://www.euro-technologies.eu/en/product/electrically-conductive-paints/ (accessed on 30 May 2024).
- Reflective Paint: A Low-Tech Solution for Sustainable Cooling in Buildings. Available online: https://www.neto-innovation.com/post/reflective-paint-a-low-tech-solution-for-sustainable-cooling-in-buildings (accessed on 30 May 2024).
- Merino, G.; Celeste, M. Synthesis and characterization of Co3O4 nanoparticles for use as pigments in solar absorbing paints. GSC Adv. Eng. Technol. 2021, 1, 7–20. [Google Scholar] [CrossRef]
- Gunde, M.K.; Orel, Z.C. Absorption and scattering of light by pigment particles in solar-absorbing paints. Appl. Opt. 2000, 39, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Coser, E. Development of paints with infrared radiation reflective properties. Polímeros 2015, 25, 305–310. [Google Scholar] [CrossRef]
- Joglekar-Athavale, A. Solar absorptive coating: A thermally stable spinel pigment based coating with inorganic binder for waterborne paint. Pigment. Resin Technol. 2021, 50, 302–308. [Google Scholar] [CrossRef]
- Kamjunk, N.; Spohn, U.; Morig, C.; Wagner, G. A Test Device for Microalgal Antifouling Using Fluctuating pH Values on Conductive Paints. Water 2020, 12, 1597. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Effect of environmental temperature variations on protective properties of organic coatings. Prog. Org. Coat. 2003, 46, 49–54. [Google Scholar] [CrossRef]
- Miszczyk, A.; Darowicki, K. Accelerated aging of organic coating systems by thermal treatment. Corros Sci. 2001, 43, 1337–1343. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Bergo, A.; Deflorian, F.; Valentinelli, L. Assessment of protective properties of organic coatings by thermal cycling. Prog. Org. Coat. 2003, 48, 271–280. [Google Scholar] [CrossRef]
- Ciobanu, R.; Spohn, U.; Morig, C.; Wagner, G.; Neu, T.R. Composite Paints with High Content of Metallic Microparticles for Electromagnetic Shielding Purposes. Coatings 2024, 14, 874. [Google Scholar] [CrossRef]
- Caramitu, A.R.; Mitrea, S.; Marinescu, V.; Ursan, G.A.; Aradoaie, M.; Lingvay, I. Dielectric Behavior and Morphostructural Characteristics of Some HDPE Composites /Metal Nanopowders. Mater. Plast. 2019, 56, 103–109. [Google Scholar] [CrossRef]
- Caramitu, A.; Ion, I.; Bors, A.M.; Ciobanu, C.R.; Schreiner, C.; Aradoaei, M.; Caramitu, A.M.D. Physico-chemical Characterization of Paint Films with Electromagnetic Properties. Mater. Plast. 2022, 59, 9–23. [Google Scholar] [CrossRef]
- Mancini, D.; Percot, A.; Bellot-Gurlet, L.; Colomban, P.; Carnazza, P. On-site contactless surface analysis of Modern paintings from Galleria Nazionale (Rome) by Reflectance FTIR and Raman spectroscopies. Talanta 2021, 227, 122159. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.A.; Vahur, S.; Leito, I. ATR-FTIR spectroscopy and quantitative multivariate analysis of paints and coating materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Herrero, L.; Cardaba, I.; Setien, S.; Bartolomé, L.; Alonso, M.L.; Maguregui, M.I. OPLS multivariate regression of FTIR-ATR spectra of acrylic paints for age estimation in contemporary artworks. Talanta 2019, 205, 120114. [Google Scholar] [CrossRef] [PubMed]
- Hamada, I.; Kumagai, T.; Shiotari, A.; Okuyama, H.; Hatta, S.; Aruga, T. Nature of hydrogen bonding in hydroxyl groups on a metal surface. Phys. Rev. B 2012, 86, 075432. [Google Scholar] [CrossRef]
- SR EN ISO 175/2011; Plastics—Methods of Test for the Determination of the Effects of Immersion in Liquid Chemicals. ISO: Geneva, Switzerland. Available online: https://e-standard.eu/en/standard/186982 (accessed on 30 May 2024).
- ISO 4892-3:2016; Plastics—Methods of Exposure to Laboratory Light Sources, Part 3: Fluorescent UV Lamps. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/67793.html (accessed on 30 May 2024).
- Aguilar-Rodríguez, P.; Mejía-González, A.; Zetina, S.; Colin-Molina, A.; Rodríguez-Molina, B.; Esturau-Escofet, N. Unexpected behavior of commercial artists’ acrylic paints under UVA artificial aging. Microchem. J. 2021, 160, 105743. [Google Scholar] [CrossRef]
- van der Weijde, D.H.; van Westing, E.P.M.; Ferrari, G.M.; de Wit, J.H.W. Lifetime Prediction of Organic Coatings with Impedance Spectroscopy. Polym. Mater. Sci. Eng. 1996, 74, 12–16. [Google Scholar]
- SR EN 60216-3:2007; Electroinsulating Materials. Thermal Endurance Properties. Part 3: INSTRUCTIONS for the Calculation of Thermal Endurance Characteristics. ISO: Geneva, Switzerland. Available online: https://magazin.asro.ro/ro/standard/117649 (accessed on 30 May 2024).
- IEC 60068-2-10:2005; Environmental Testing—Part 2-10: Tests—Test J and Guidance: Mould Growth. IEC: London, UK. Available online: https://standards.iteh.ai/catalog/standards/clc/d098bcec-f96d-46df-9495-300001cf8218/en-60068-2-10-2005 (accessed on 30 May 2024).
- Caramitu, A.; Butoi, N.; Rus, T.; Luchian, A.M.; Mitrea, S. The Resistance to the Action of Molds of Some Painting Materials Aged by Thermal Cycling and Exposed to an Electrical Field of 50 Hz. Mat. Plast. 2017, 54, 331–337. [Google Scholar] [CrossRef]
- Iscen, A.; Forero-Martinez, N.; Valsson, O.; Kremer, K. Acrylic Paints: An Atomistic View of Polymer Structure and Effects of Environmental Pollutants. J. Phys. Chem. B 2021, 125, 10854–10865. [Google Scholar] [CrossRef] [PubMed]
- Cogulet, A.; Blanchet, P.; Landry, V. Evaluation of the Impacts of Four Weathering Methods on Two Acrylic Paints: Showcasing Distinctions and Particularities. Coatings 2019, 9, 121. [Google Scholar] [CrossRef]
- Available online: https://safeguardstore.co.uk/dryzone-acs-anti-mould-paint-additive/ (accessed on 30 July 2024).





| Sample | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | MSD by Volume | ||
|---|---|---|---|---|---|
| Mean Diameter (nm) | Relative Variance | Skew | |||
| Al particles | 896.4 ± 35.6 | 0.113 ± 0.058 | 951.4 | 0.196 | 0.999 |
| Fe particles | 1317.3 ± 71.4 | 0.126 ± 0.022 | 1580.5 | 0.133 | −0.484 |
| Roughness (µm) | Thickness of Paint Layer (µm) | Ra | Rq | Rt |
|---|---|---|---|---|
| M1 | 19.96 | 2.95 | 3.73 | 25.65 |
| M2 | 25.87 | 3.78 | 4.82 | 30.16 |
| M3 | 20.49 | 1.01 | 1.25 | 8.39 |
| M4 | 26.20 | 1.34 | 1.66 | 10.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramitu, A.R.; Ciobanu, R.C.; Aradoaei, M.; Lungu, M.V.; Nicula, N.O.; Lungulescu, E.M. Endurance to Multiple Factors of Water-Based Electrically Conductive Paints with Metallic Microparticles. Coatings 2024, 14, 1016. https://doi.org/10.3390/coatings14081016
Caramitu AR, Ciobanu RC, Aradoaei M, Lungu MV, Nicula NO, Lungulescu EM. Endurance to Multiple Factors of Water-Based Electrically Conductive Paints with Metallic Microparticles. Coatings. 2024; 14(8):1016. https://doi.org/10.3390/coatings14081016
Chicago/Turabian StyleCaramitu, Alina Ruxandra, Romeo Cristian Ciobanu, Mihaela Aradoaei, Magdalena Valentina Lungu, Nicoleta Oana Nicula, and Eduard Marius Lungulescu. 2024. "Endurance to Multiple Factors of Water-Based Electrically Conductive Paints with Metallic Microparticles" Coatings 14, no. 8: 1016. https://doi.org/10.3390/coatings14081016
APA StyleCaramitu, A. R., Ciobanu, R. C., Aradoaei, M., Lungu, M. V., Nicula, N. O., & Lungulescu, E. M. (2024). Endurance to Multiple Factors of Water-Based Electrically Conductive Paints with Metallic Microparticles. Coatings, 14(8), 1016. https://doi.org/10.3390/coatings14081016









