Electrophoretically Deposited TiB2 Coatings in NaF-AlF3 Melt for Corrosion Resistance in Liquid Zinc
Abstract
1. Introduction
2. Materials and Experimental Procedure
- Synthesis of nanoscale TiB2 via borothermal reduction in NaF-AlF3 molten salts
- 2.
- EPD of TiB2 coatings in NaF-AlF3 molten salts
- 3.
- Corrosion resistance test of the TiB2 coating in molten zinc
- 4.
- Characterization of nano-TiB2 and TiB2 coatings
3. Results and Discussion
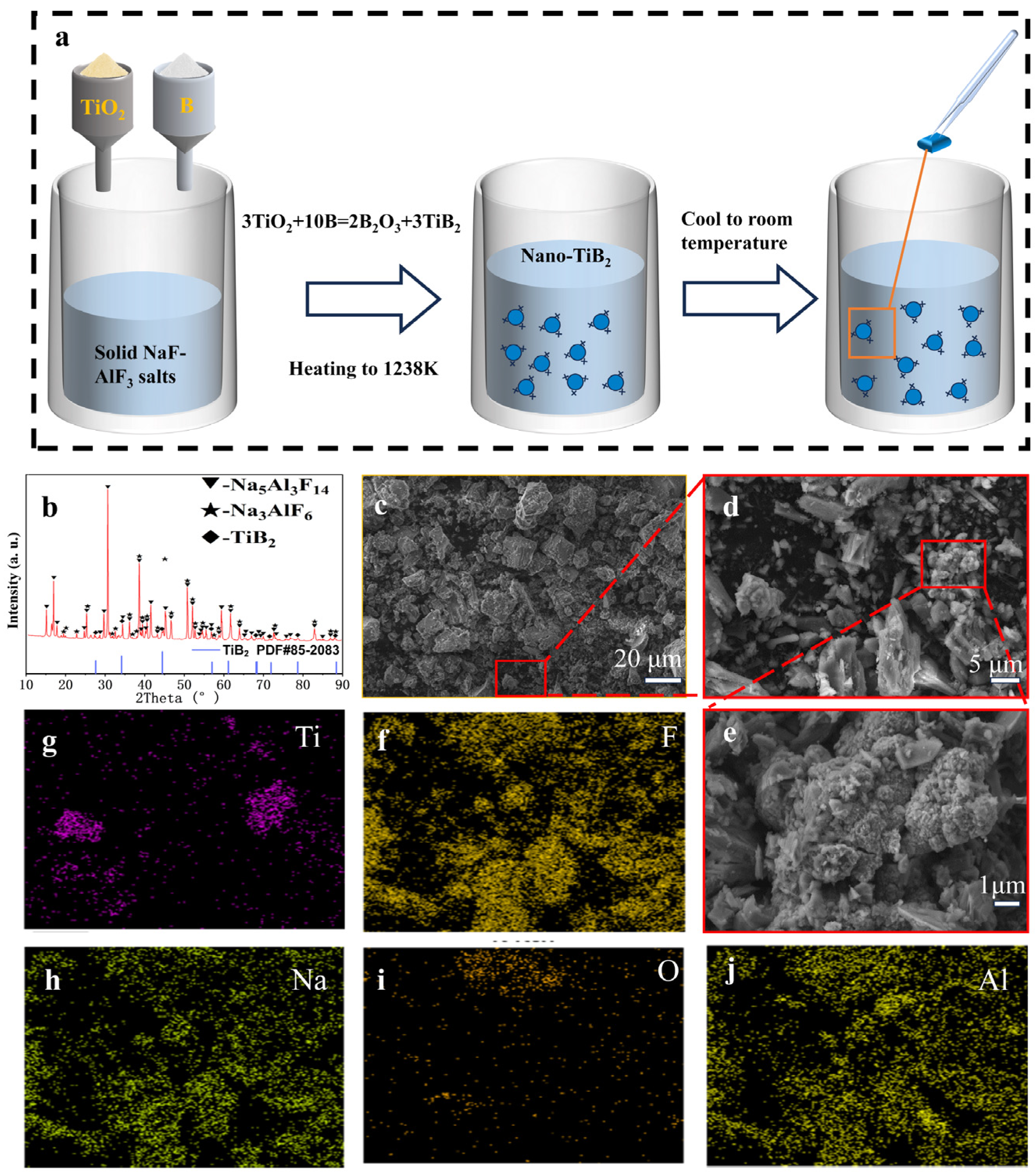
3.1. Test Analysis of Synthesized Nanoscale TiB2
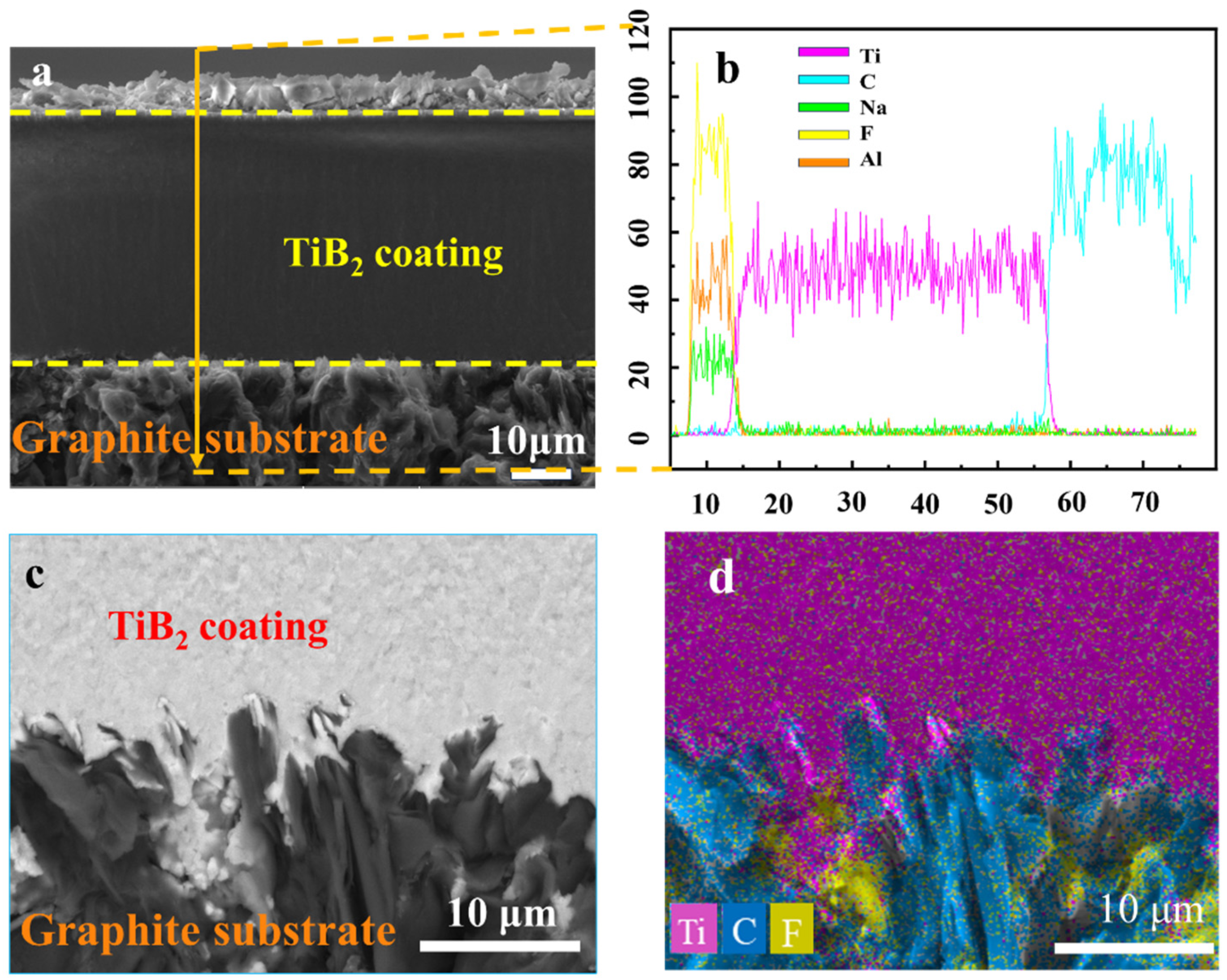
3.2. Test Analysis of TiB2 Coatings on Graphite Substrates
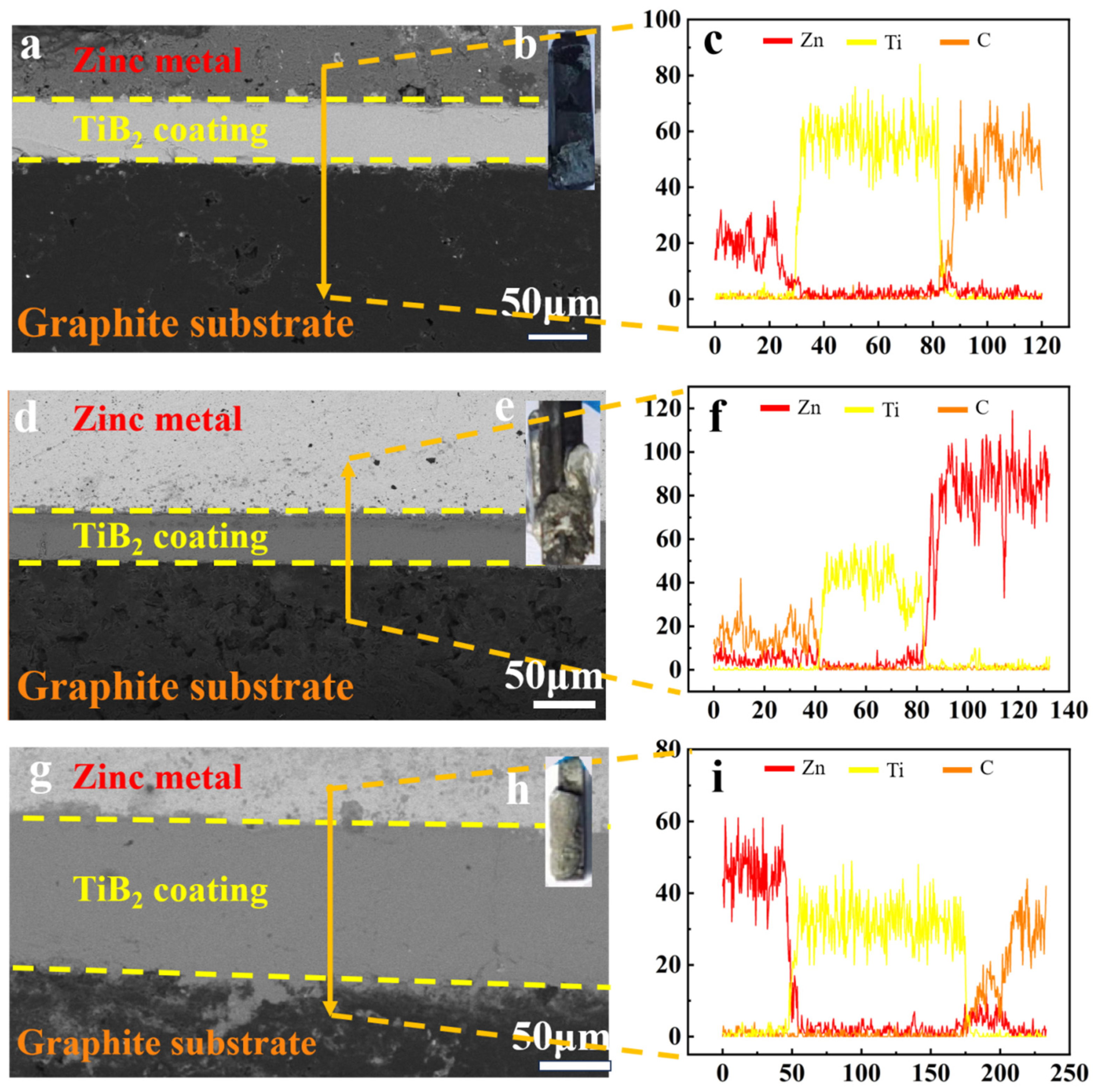
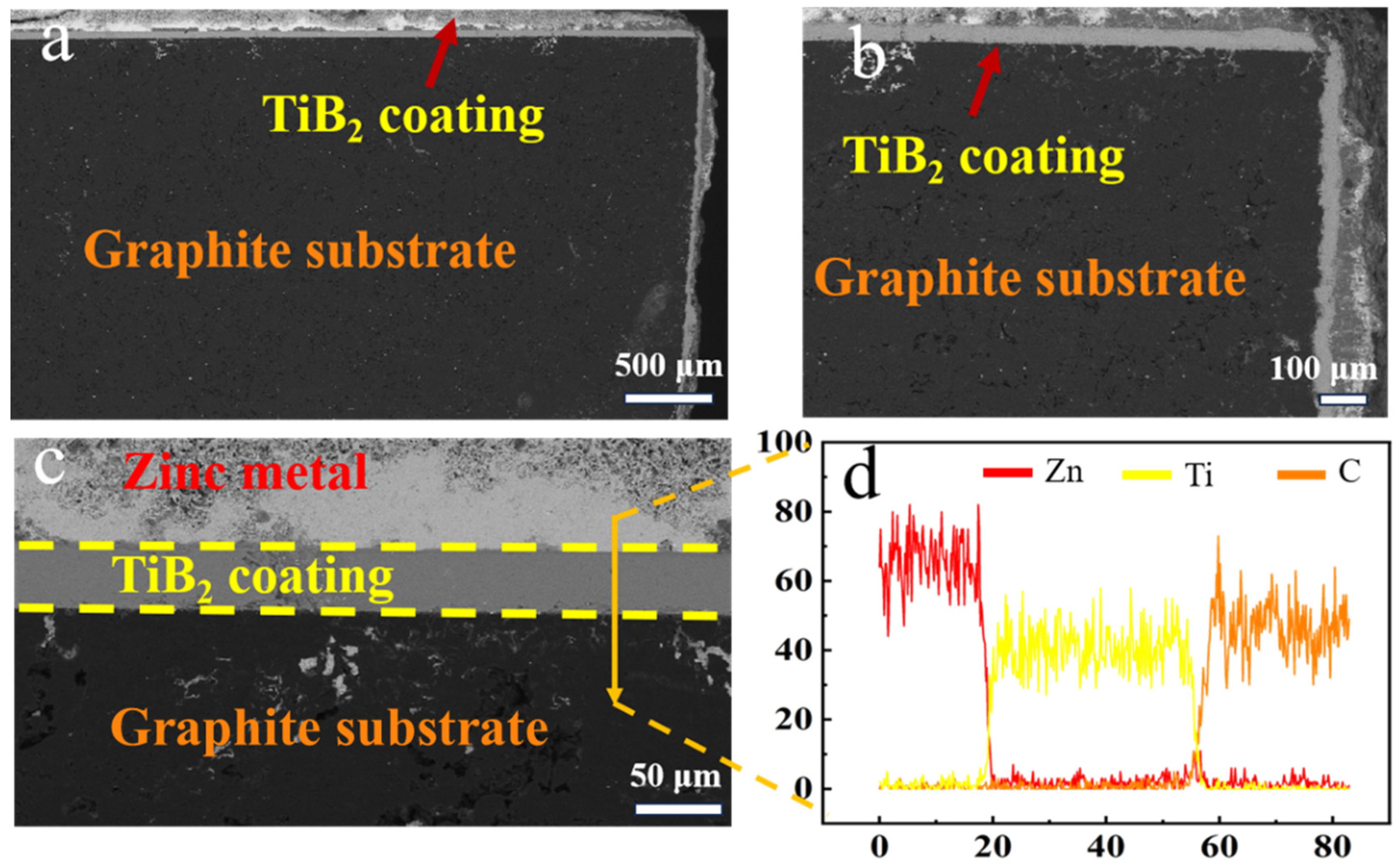
3.3. Corrosion Behavior of the TiB2 Coating in Molten Zinc
4. Conclusions
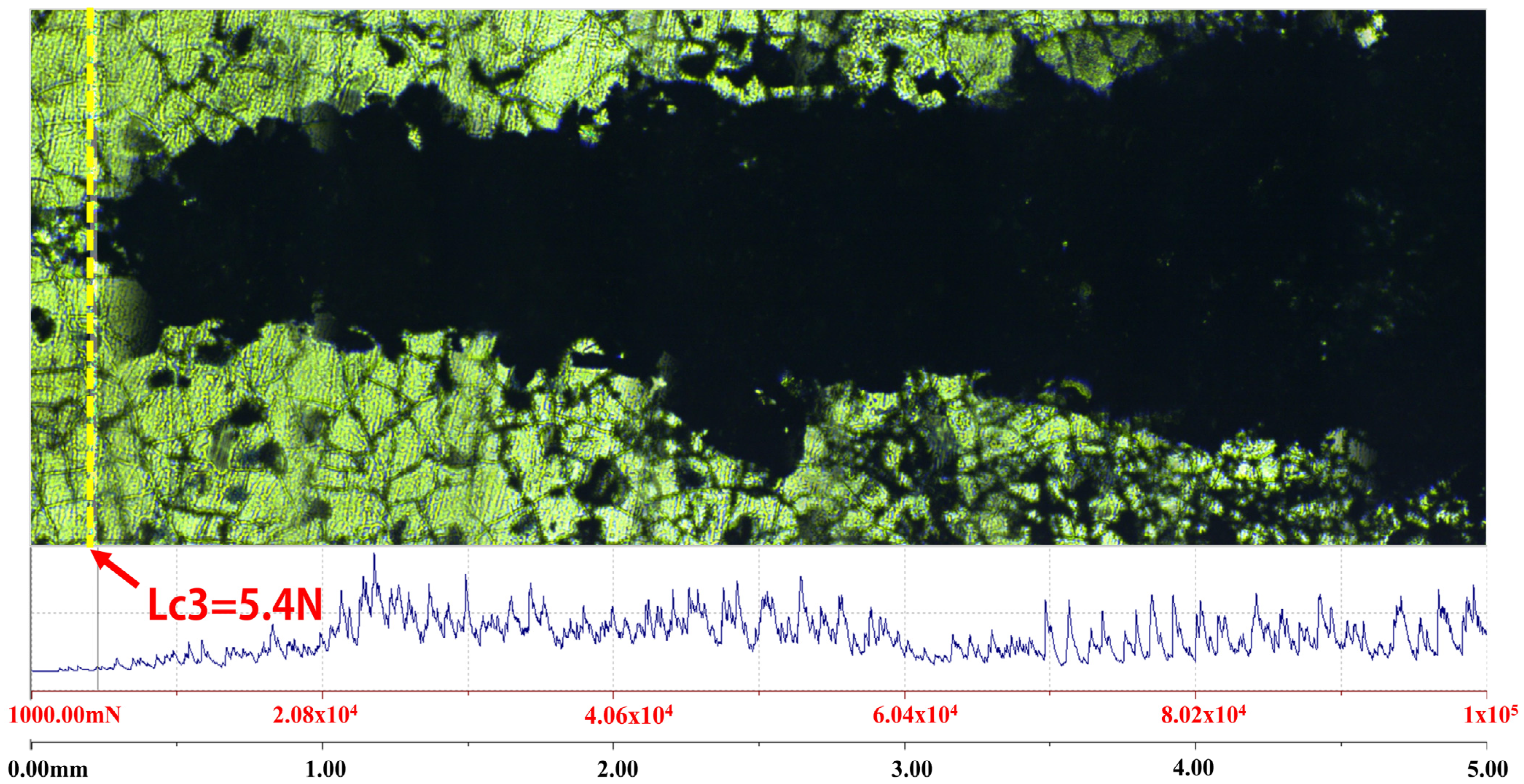
- In the NaF-AlF3 molten salt system at 1238 K, nanoscale TiB2 particles ranging from 50 to 100 nm were successfully synthesized via the borothermal reduction method. Applying an electrophoretic voltage of 1.2 V resulted in the deposition of these particles onto the graphite substrate, forming a TiB2 coating with good adhesion strength (critical load Lc3 = 5.4 N) and a fully dense structure.
- In the molten zinc at 823 K, no chemical reaction occurred between the TiB2 coating and the molten zinc; the coating surface and interface remained stable. During a prolonged immersion test period of up to 120 h, excellent corrosion resistance to liquid zinc was demonstrated, which is ascribed to the corrosion-resistant TiB2 coating, which was not only fully dense but also exhibited a low oxygen content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Golla, R.B.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Kirnbauer, A.; Ertelthaler, P.; Koller, C.M. High-entropy ceramic thin films; A case study on transition metal diborides. Scr. Mater. 2018, 149, 93–97. [Google Scholar] [CrossRef]
- Brzezinka, T.L.; Rao, J.; Paiva, J.M.; Azkona, I.; Kohlscheen, J.; Fox Rabinovich, G.S.; Veldhuis, S.C.; Endrino, J.L. Facilitating TiB2 for filtered vacuum cathodic arc evaporation. Coatings 2020, 10, 244. [Google Scholar] [CrossRef]
- Dorri, S.; Palisaitis, J.; Greczynski, G.; Petrov, I.; Birch, J.; Hultman, L.; Bakhit, B. Oxidation kinetics of overstoichiometric TiB2 thin films grown by dc magnetron sputtering. Corros. Sci. 2022, 206, 110493. [Google Scholar] [CrossRef]
- He, R.; Jiang, J.; Wang, R.; Yue, Y.; Chen, Y.; Pan, T. Anti-corrosion and conductivity of titanium diboride coating on metallic bipolar plates. Corros. Sci. 2020, 170, 108646. [Google Scholar] [CrossRef]
- Huang, X.; Tu, G.; Wang, S.; Song, J.; Liu, Y.; Wang, Z. Research progress in preparation and application of TiB2 coating. Rare Metal. Mater. Eng. 2022, 51, 1087–1099. [Google Scholar]
- Agarwal, A.; Dahotre, N.B. Laser deposited titanium diboride coating for protection of molten aluminum handling tools and molds. Laser Eng. 1999, 9, 169–193. [Google Scholar]
- Jensen, M.S.; Pezzotta, M.; Zhang, Z.; Einarsrud, M.A.; Grande, T. Degradation of TiB2 ceramics in liquid aluminum. J. Eur. Ceram. Soc. 2008, 28, 3155–3164. [Google Scholar] [CrossRef]
- Berger, M.; Hogmark, S. Evaluation of TiB2 coatings in sliding contact against aluminium. Surf. Coat. Technol. 2002, 149, 14–20. [Google Scholar] [CrossRef]
- Ramos-Masana, A.; Colominas, C. Evaluation of dc-ms and hipims TiB2 and TaN coatings as diffusion barriers against molten aluminum: An insight into the wetting mechanism. Surf. Coat. Technol. 2019, 375, 171–181. [Google Scholar] [CrossRef]
- Salyi, Z.; Kaptay, G.; Koncz-Horvath, D.; Somlyai-Sipos, L.; Kovacs, P.Z.; Lukacs, A.; Benke, M. Boride coatings on steel protecting it against corrosion by a liquid lead-Free solder alloy. Metall. Mater. Trans. B 2022, 53, 730–743. [Google Scholar] [CrossRef]
- Benke, M.; Salyi, Z.; Takats, V.; Csik, A.; Rugoczky, P.; Kaptay, G. The behaviour of steel coated with TiB2 in Sn-Ag-Cu melt. Mater. Sci. Technol. 2019, 35, 680–686. [Google Scholar] [CrossRef]
- Bulbul, F.; Efeoglu, I. Pulsed-dc bias magnetron sputtered TiB2 ceramic coating. Int. J. Refract. Met. Hard Mater. 2024, 120, 106624. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, P.; Ying, P.; Lin, C.; Yang, T.; Wu, J.; Li, C.; Huang, M.; Levchenko, V. Tribological properties of hard TiB2 thin films prepared at low temperatures using HIPIMS. Coatings 2024, 14, 492. [Google Scholar] [CrossRef]
- Gruber, D.P.; Zalesak, J.; Todt, J.; Tkadletz, M.; Sartory, B.; Suuronen, J.P.; Ziegelwanger, T.; Czettl, C.; Mitterer, C.; Keckes, J. Surface oxidation of nanocrystalline CVD TiB2 hard coatings revealed by cross-sectional nano-analytics and in-situ micro-cantilever testing. Surf. Coat. Technol. 2020, 399, 126181. [Google Scholar] [CrossRef]
- Yvenou, E.; Davis, B.; Guay, D.; Roue, L. Electrodeposited TiB2 on graphite as wettable cathode for al production. J. Am. Ceram. Soc. 2021, 104, 1247–1254. [Google Scholar] [CrossRef]
- Mohammadkhani, S.; Bily, A.; Davis, B.; Dolatabadi, A.; Moreau, C.; Roué, L.; Guay, D. Impact of density on the behavior of suspension plasma-sprayed TiB2 coatings in the presence of molten aluminum. J. Therm. Spray Technol. 2022, 31, 1499–1507. [Google Scholar] [CrossRef]
- Magnuson, M.; Hultman, L.; Högberg, H. Review of transition-metal diboride thin films. Vacuum 2022, 196, 110567. [Google Scholar] [CrossRef]
- Jin, W.; Xiao, S.; Kou, Q.; Ding, D.; Zhang, J.; Fang, X.; Ge, C.; Zhong, C.; Zhu, H.; Haarberg, G.Z. Preparation of diboride coatings by electrophoretic deposition in nanoparticle-containing molten inorganic salts. Mater. Lett. 2022, 306, 130908. [Google Scholar] [CrossRef]
- Jin, W.; Pang, J.; Kou, Q.; Ge, C.; Zhu, H.; Haarberg, G.M.; Xiao, S.; Zhang, J. Synthesis of a (Ti,Mo)B2 coating by electro-codeposition in NaCl-KCl-AlCl3-MoO3 melt containing TiB2 nanoparticles. J. Am. Ceram. Soc. 2023, 106, 860–866. [Google Scholar] [CrossRef]
- Pang, J.; Kou, Q.; Ge, C.; Xie, L.; Jin, W.; Qi, W.; Zhang, J.; Zhu, H.; Xiao, S. Electrophoretic deposition of ZrB2 coatings in NaCl-KCl-AlF3 melt containing synthesized ZrB2 nanoparticles. J. Am. Ceram. Soc. 2023, 106, 5147–5156. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, J.; Jin, W.; Chu, S.; Haarberg, G.M.; Xiao, S. Production of TiB2 coatings on graphite substrates by electrophoretic deposition in NaF-AlF3 melt. Process. Appl. Ceram. 2023, 17, 9–13. [Google Scholar] [CrossRef]
- Ge, C.; Kou, Q.; Pang, J.; Zhang, J.; Jin, W.; Zhu, H.; Haarberg, G.M.; Xiao, S. Preparation of ZrB2 coatings by electrophoretic deposition in NaCl-KCl-AlCl3 molten salts. J. Mater. Res. Technol. 2022, 20, 772–780. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, S.; Jin, W.; Cai, F.; Zhu, H.; Xiao, S. Fabrication of TiB2 coatings by electrophoretic deposition of synthesized TiB2 nanoparticles in molten salts. J. Mater. Res. Technol. 2022, 18, 2451–2457. [Google Scholar] [CrossRef]
- Yvenou, É.; Bily, A.; Ettouil, F.B.; Dolatabadi, A.; Davis, B.; Guay, D.; Moreau, C.; Roue, L. TiB2 deposited on graphite by suspension plasma spray as Al wettable cathode. J. Therm. Spray Technol. 2021, 30, 1535–1543. [Google Scholar] [CrossRef]
- Zhevtun, I.; Gordienko, P.; Kulchin, Y.; Nikitin, A.; Pivovarov, D.; Yarusova, S.; Golub, A.; Nikiforov, P.; Timchenko, V. Influence of titanium surface porosity on adhesive strength of coatings containing calcium silicate. Materials 2020, 13, 4493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, Z.; Jing, X. Pinning growth of tin films toward porous Ti matrix. J. Mater. Sci. 2022, 57, 18949–18968. [Google Scholar] [CrossRef]
- Pierson, H.O.; Randich, E. Titanium diboride coatings and their interaction with the substrates. Thin Solid Film. 1978, 54, 119–128. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Xu, J.; Ge, C.; Pang, J.; Zhang, J.; Haarberg, G.M.; Xiao, S. Electrophoretically Deposited TiB2 Coatings in NaF-AlF3 Melt for Corrosion Resistance in Liquid Zinc. Coatings 2024, 14, 1021. https://doi.org/10.3390/coatings14081021
Jiang T, Xu J, Ge C, Pang J, Zhang J, Haarberg GM, Xiao S. Electrophoretically Deposited TiB2 Coatings in NaF-AlF3 Melt for Corrosion Resistance in Liquid Zinc. Coatings. 2024; 14(8):1021. https://doi.org/10.3390/coatings14081021
Chicago/Turabian StyleJiang, Tao, Junjie Xu, Chuntao Ge, Jie Pang, Jun Zhang, Geir Martin Haarberg, and Saijun Xiao. 2024. "Electrophoretically Deposited TiB2 Coatings in NaF-AlF3 Melt for Corrosion Resistance in Liquid Zinc" Coatings 14, no. 8: 1021. https://doi.org/10.3390/coatings14081021
APA StyleJiang, T., Xu, J., Ge, C., Pang, J., Zhang, J., Haarberg, G. M., & Xiao, S. (2024). Electrophoretically Deposited TiB2 Coatings in NaF-AlF3 Melt for Corrosion Resistance in Liquid Zinc. Coatings, 14(8), 1021. https://doi.org/10.3390/coatings14081021



