Innovative Paper Coatings: Regenerative Superhydrophobicity through Self-Structuring Aqueous Wax-Polymer Dispersions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Water-Based Dispersions for Superhydrophobic Coatings on Model Papers
- Is it possible to disperse both substances with the determined composition (e.g., 5% CSE/95% EGDS) in a single aqueous dispersion at high consistency (ideally on or about 20 wt% solid) to enable a one-step coating process?
- If necessary, could natural additives with a focus on keeping additive quantities to a minimum be used to stabilize the dispersion and ensure their ability to run with common coating techniques (e.g., doctor-blading)? And to what extent is the coating effectiveness influenced by such additives:
- i.
- The dispersion must be capable of being applied to the paper with standard equipment, whereby viscosity plays a particularly important role as the coating should spread homogenously without penetrating directly into the paper.
- ii.
- It must be ensured that the dispersion additives do not prevent thermal regeneration by inhibiting the melting of the waxy substances.
- iii.
- The used surfactants should not increase wettability in a manner that suspends superhydrophobicity.
- iv.
- For the regeneration capability, the influence of the cationic starch component on the penetration of the coating waxes into the paper structure during several regeneration cycles is investigated.
- Does the dried coating gained from deposition of the aqueous dispersion on model paper substrates result in superhydrophobic paper properties with static contact angles above 150°, similar to those coatings of pure substances derived from organic solutions [44] or from melt (Supplementary Materials S2)?
- Is it finally possible to upscale the dispersion in the lab with regard to potential applications in the paper industry?
- Identification of suitable dispersion parameters and additives
- Influence of dispersion formulation on dispersion properties
- Influence of dispersion formulation on coating properties
- Up-scaling of functional aqueous dispersion
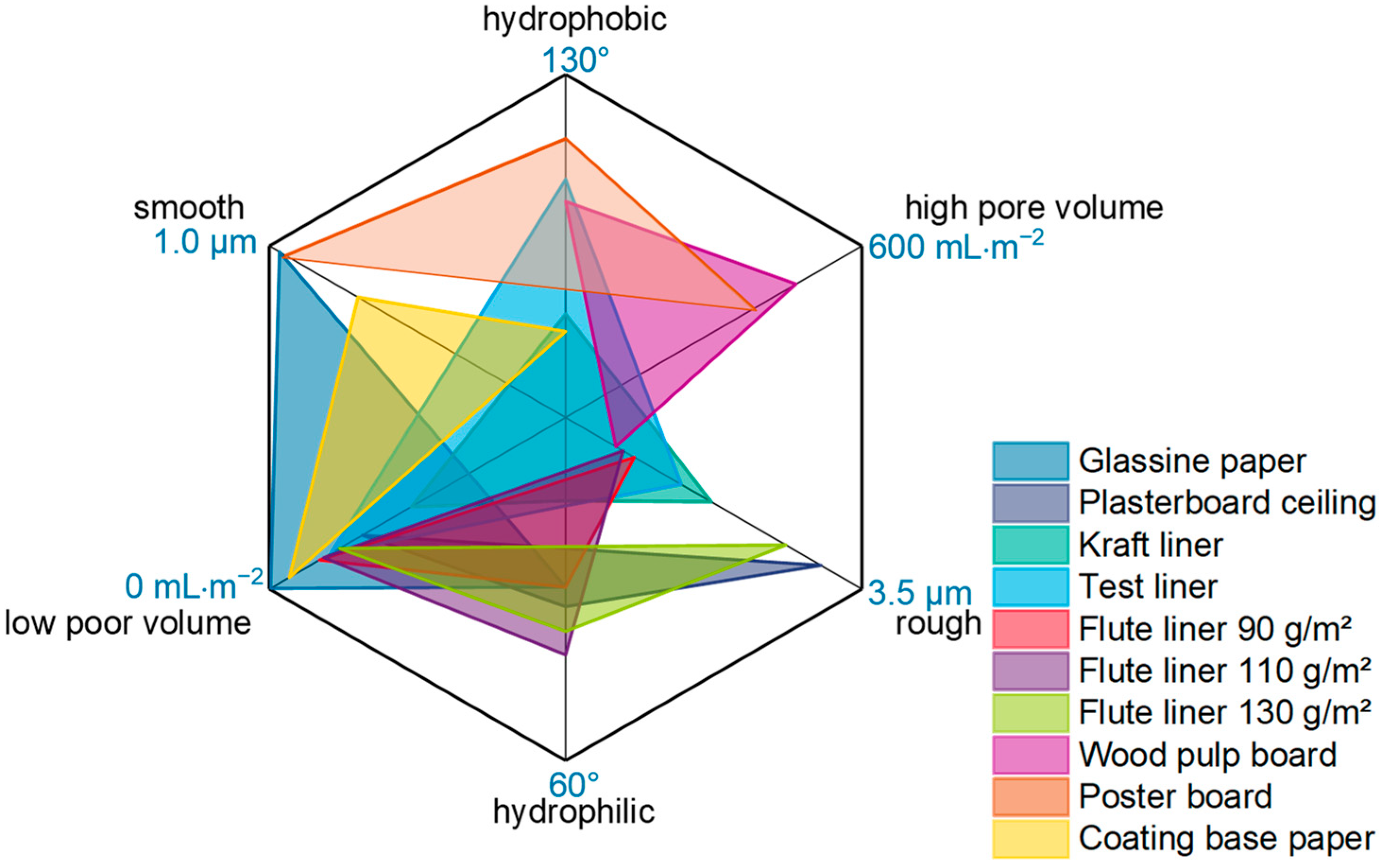
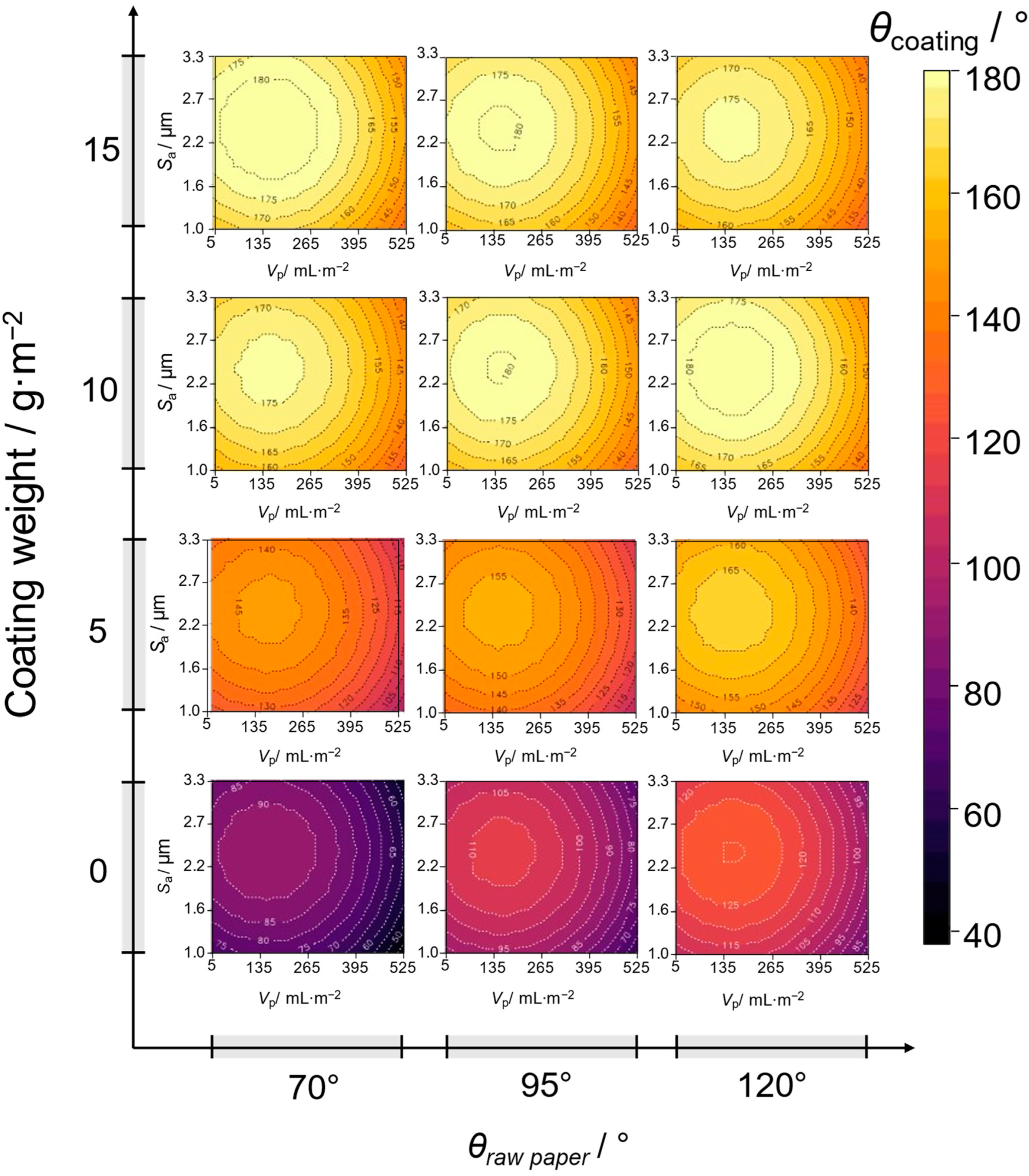
3.2. Transfer and Investigation of Commercial Paper Applications
- Which types of industrial papers are suited to generate a superhydrophobic surface (contact angles above 150°) with low coating weight?
- Are the paper’s intrinsic properties, such as surface roughness and porosity, affecting the coating performance?
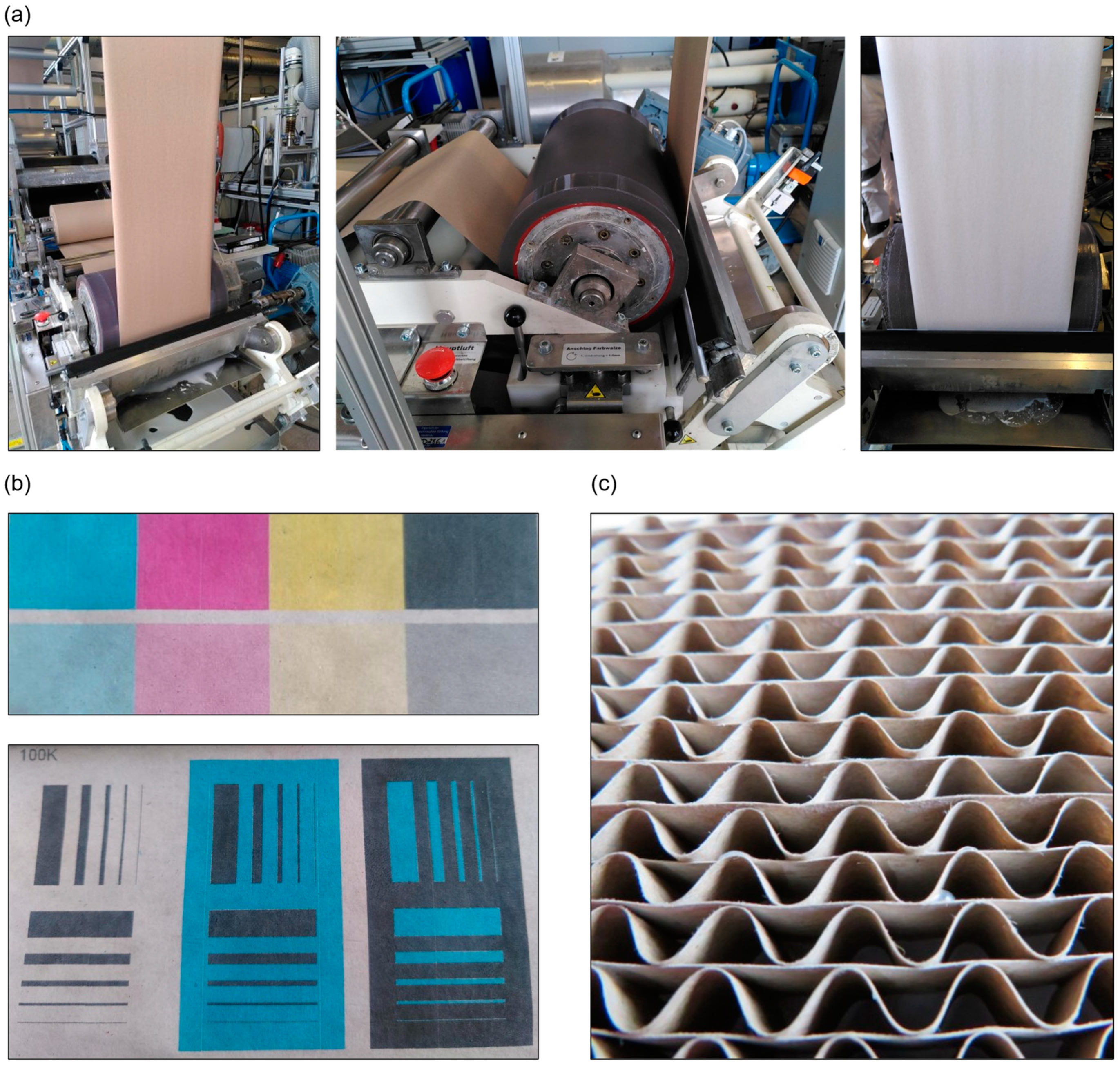
- Is it possible to apply the coating using continuous paper finishing techniques?
- Does the coating affect the recyclability of the paper materials according to “CEPI recyclability laboratory test method version 2—Standard mill”?
- Is it possible to process the coated paper further (e.g., print or glue it)? Is it possible to use the heat of processing (e.g., corrugation) in situ to regenerate the superhydrophobic properties?
- Selection and characterization of commercial paper grades
- Correlation of coating properties with paper intrinsic properties
- Application-related paper characterization, coating, and processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanli, E.; Bach, R.; Götzinger, R.; Kiziltoprak, N.; Knaack, U.; Schabel, S.; Schneider, J. Case study: Development and Evaluation methods for bio-based construction realized with paper-based building materials. AJCE 2019, 37, 664–671. [Google Scholar]
- Knaack, U.; Bach, R.; Schabel, S. (Eds.) Bauen mit Papier: Architektur und Konstruktion; Birkhäuser: Basel, Switzerland, 2023; ISBN 9783035621402. [Google Scholar]
- Samyn, P. Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils. Molecules 2021, 26, 3561. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emergent Mater. 2022, 5, 703–718. [Google Scholar] [CrossRef]
- Samyn, P. Wetting and hydrophobic modification of cellulose surfaces for paper applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Li, A.; Wang, G.; Zhang, Y.; Zhang, J.; He, W.; Ren, S.; Xu, Z.; Wang, J.; Ma, Y. Preparation methods and research progress of superhydrophobic paper. Coord. Chem. Rev. 2021, 449, 214207. [Google Scholar] [CrossRef]
- Yun, T.; Tao, Y.; Li, Q.; Cheng, Y.; Lu, J.; Lv, Y.; Du, J.; Wang, H. Superhydrophobic modification of cellulosic paper-based materials: Fabrication, properties, and versatile applications. Carbohydr. Polym. 2023, 305, 120570. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Phil. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Ensikat, H.-J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Getaneh, S.A.; Temam, A.G.; Nwanya, A.C.; Ejikeme, P.M.; Ezema, F.I. Advances in bioinspired superhydrophobic surface materials: A review on preparation, characterization and applications. Hybrid Adv. 2023, 3, 100077. [Google Scholar] [CrossRef]
- Sotoudeh, F.; Mousavi, S.M.; Karimi, N.; Lee, B.J.; Abolfazli-Esfahani, J.; Manshadi, M.K. Natural and synthetic superhydrophobic surfaces: A review of the fundamentals, structures, and applications. Alex. Eng. J. 2023, 68, 587–609. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Li, P.; Zhou, M.; Jian, B.; Lei, H.; Liu, R.; Zhou, X.; Li, X.; Wang, Y.; Zhou, B. Paper material coated with soybean residue nanocellulose waterproof agent and its application in food packaging. Ind. Crops Prod. 2023, 199, 116749. [Google Scholar] [CrossRef]
- Wu, X.; Yang, F.; Gan, J.; Zhao, W.; Wu, Y. A flower-like waterborne coating with self-cleaning, self-repairing properties for superhydrophobic applications. J. Mater. Res. Technol. 2021, 14, 1820–1829. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F.; Arenas, M.A. Super-hydrophobic nickel–cobalt alloy coating with micro-nano flower-like structure. Chem. Eng. J. 2015, 273, 638–646. [Google Scholar] [CrossRef]
- Pakdel, E.; Zhao, H.; Wang, J.; Tang, B.; Varley, R.J.; Wang, X. Superhydrophobic and photocatalytic self-cleaning cotton fabric using flower-like N-doped TiO2/PDMS coating. Cellulose 2021, 28, 8807–8820. [Google Scholar] [CrossRef]
- Peng, H.; Yang, H.; Ma, X.; Shi, T.; Li, Z.; Xue, S.; Wang, Q. In situ fabrication of flower-like ZnO on aluminum alloy surface with superhydrophobicity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128800. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Fiestas-Paradela, S.; Llorca-Isern, N. Non-Fluorinated, Sustainable, and Durable Superhydrophobic Microarrayed Surface for Water-Harvesting. Coatings 2020, 10, 314. [Google Scholar] [CrossRef]
- Weng, R.; Zhang, H.; Yin, L.; Rong, W.; Wu, Z.; Liu, X. Fabrication of superhydrophobic surface by oxidation growth of flower-like nanostructure on a steel foil. RSC Adv. 2017, 7, 25341–25346. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Liu, X.; Zhu, J. Synthesis of hierarchical flower-like particles and its application as super-hydrophobic coating. Powder Technol. 2017, 319, 408–414. [Google Scholar] [CrossRef]
- Yang, C.; Yang, X.; Li, F.; Li, T.; Cao, W. Controlled synthesis of hierarchical flower-like Sb 2 WO 6 microspheres: Photocatalytic and superhydrophobic property. J. Ind. Eng. Chem. 2016, 39, 93–100. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Al Kobaisi, M.; Bhosale, S.V.; Bhargava, S.; Bhosale, S.V. Flower-like supramolecular self-assembly of phosphonic acid appended naphthalene diimide and melamine. Sci. Rep. 2015, 5, 14609. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D. Supramolecular self-assembly of amphiphilic hyperbranched polymers at all scales and dimensions: Progress, characteristics and perspectives. Chem. Commun. 2009, 10, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Jones, L.A. Flower-Like Superstructures: Structural Features, Applications and Future Perspectives. Chem. Rec. 2021, 21, 257–283. [Google Scholar] [CrossRef]
- Boccalon, E.; Gorrasi, G.; Nocchetti, M. Layered double hydroxides are still out in the bloom: Syntheses, applications and advantages of three-dimensional flower-like structures. Adv. Colloid Interface Sci. 2020, 285, 102284. [Google Scholar] [CrossRef]
- Zhang, K.; Geissler, A.; Chen, X.; Rosenfeldt, S.; Yang, Y.; Förster, S.; Müller-Plathe, F. Polymeric Flower-Like Microparticles from Self-Assembled Cellulose Stearoyl Esters. ACS Macro Lett. 2015, 4, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Tian, J.; Xie, Y.; Zhang, K. A Comparative Study of Self-Assembled Superstructures from Cellulose Stearoyl Ester and Poly(Vinyl Stearate). Macromol. Chem. Phys. 2018, 219, 1800229. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Deng, X.; Chen, L.; Rosenfeldt, S.; Förster, S.; Vana, P.; Zhang, K. Polymeric Flaky Nanostructures from Cellulose Stearoyl Esters for Functional Surfaces. Adv. Mater. Interfaces 2016, 3, 1600636. [Google Scholar] [CrossRef]
- Vierheller, T.R.; Foster, M.D.; Schmidt, A.; Mathauer, K.; Knoll, W.; Wegner, G.; Satija, S.; Majkrzak, C.F. Structure and Thermal Stability of Langmuir-Blodgett-Kuhn Layers of Hairy-Rod Polymers Probed with Neutron and X-ray Reflectometry. Macromolecules 1994, 1994, 6893–6902. [Google Scholar] [CrossRef]
- Vitale, S.A.; Katz, J.L. Liquid Droplet Dispersions Formed by Homogeneous Liquid−Liquid Nucleation: “The Ouzo Effect”. Langmuir 2003, 19, 4105–4110. [Google Scholar] [CrossRef]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives-Formation Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, e1900415. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Delaney, J.J.T.; Schubert, U.S. Nanoprecipitation and nanoformulation of polymers: From history to powerful possibilities beyond poly(lactic acid). Soft Matter 2011, 7, 1581–1588. [Google Scholar] [CrossRef]
- Gavory, C.; Durand, A.; Six, J.-L.; Nouvel, C.; Marie, E.; Leonard, M. Polysaccharide-covered nanoparticles prepared by nanoprecipitation. Carbohydr. Polym. 2011, 84, 133–140. [Google Scholar] [CrossRef]
- Geissler, A.; Biesalski, M.; Heinze, T.; Zhang, K. Formation of nanostructured cellulose stearoyl esters via nanoprecipitation. J. Mater. Chem. A 2014, 2, 1107–1116. [Google Scholar] [CrossRef]
- Geissler, A.; Chen, L.; Zhang, K.; Bonaccurso, E.; Biesalski, M. Superhydrophobic surfaces fabricated from nano- and microstructured cellulose stearoyl esters. Chem. Commun. 2013, 49, 4962–4964. [Google Scholar] [CrossRef]
- Li, L.; Wei, J.; Zhang, J.; Li, B.; Yang, Y.; Zhang, J. Challenges and strategies for commercialization and widespread practical applications of superhydrophobic surfaces. Sci. Adv. 2023, 2023, 1554. [Google Scholar] [CrossRef] [PubMed]
- Loesch-Zhang, A.; Meckel, T.; Biesalski, M.; Geissler, A. Enhancing Hydrophobic Properties in Olive Oil-Coated Papers through Thermal Treatment. Coatings 2024, 14, 364. [Google Scholar] [CrossRef]
- Cordt, C.; Geissler, A.; Biesalski, M. Regenerative Superhydrophobic Paper Coatings by In Situ Formation of Waxy Nanostructures. Adv. Mater. Interfaces 2020, 8, 2001265. [Google Scholar] [CrossRef]
- ISO 5269-2:2004(E); Pulps–Preparation of Laboratory Sheets for Physical Testing–Part 2: Rapid-Köthen Method. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 536:2019; Paper and Board–Determination of Grammage. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 534:2011; Paper and Board–Determination of Thickness, Density and Specific Volume. International Organization for Standardization: Geneva, Switzerland, 2011.
- DIN 53122-1:2001-08; Testing of Plastics and Elastomer Films, Paper, Board and Other Sheet Materials–Determination of Water Vapour Transmission–Part 1: Gravimetric Method. Deutsches Institut für Normung e.V.: Berlin, Germany, 2001.
- ISO 535:2023; Paper and Board–Determination of Water Absorptiveness–Cobb Method. International Organization for Standardization: Geneva, Switzerland, 2023.
- Johansson, J.; Lindström, T. A study on AKD-size retention, reaction and sizing efficiency Part 1: The effects of pulp bleaching on AKD-sizing. Nord. Pulp Pap. Res. J. 2004, 19, 330–335. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; An, X.; Liu, H.; Nie, S.; Cao, H.; Xu, Q.; Lu, B. Improving sizing performance of middle layer of liquid packaging board containing high-yield pulp. Cellulose 2020, 27, 4707–4719. [Google Scholar] [CrossRef]
- Kumar, A.; Shaveta, K. Efficacy of ASA Sizinh with Agro-Residue and Recycled Pulps Using Different Fillers. Ippta 2012, 2012, 92–98. [Google Scholar]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; van den Abbeele, H. Modifications of paper and paperboard surfaces with a nanostructured polymer coating. Prog. Org. Coat. 2010, 69, 442–454. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Q.; Li, X.; Meng, Y.; Ling, Z.; Ji, Z.; Chen, F. Preparation and Characterization of Degradable Cellulose-Based Paper with Superhydrophobic, Antibacterial, and Barrier Properties for Food Packaging. Int. J. Mol. Sci 2022, 23, 1158. [Google Scholar] [CrossRef]
- Reverdy, C.; Belgacem, N.; Moghaddam, M.S.; Sundin, M.; Swerin, A.; Bras, J. One-step superhydrophobic coating using hydrophobized cellulose nanofibrils. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 152–158. [Google Scholar] [CrossRef]
- Ye, M.; Tian, Z.; Wang, S.; Ji, X.; Wang, D.; Ci, X. Simple preparation of environmentally friendly and durable superhydrophobic antibacterial paper. Cellulose 2023, 30, 2427–2440. [Google Scholar] [CrossRef]
- Werner, O.; Quan, C.; Turner, C.; Pettersson, B.; Wågberg, L. Properties of superhydrophobic paper treated with rapid expansion of supercritical CO2 containing a crystallizing wax. Cellulose 2010, 17, 187–198. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Haapanen, J.; Aromaa, M.; Stepien, M.; Mäkelä, J.M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. Switchable water absorption of paper via liquid flame spray nanoparticle coating. Cellulose 2014, 21, 2033–2043. [Google Scholar] [CrossRef]
- Mirvakili, M.N.; Hatzikiriakos, S.G.; Englezos, P. Superhydrophobic lignocellulosic wood fiber/mineral networks. ACS Appl. Mater. Interfaces 2013, 5, 9057–9066. [Google Scholar] [CrossRef] [PubMed]
- Vaca-Garcia, C.; Gozzelino, G.; Glasser, W.G.; Borredon, M.E. Dynamic mechanical thermal analysis transitions of partially and fully substituted cellulose fatty esters. J. Polym. Sci. B Polym. Phys. 2003, 41, 281–288. [Google Scholar] [CrossRef]
- Geissler, A.; Loyal, F.; Biesalski, M.; Zhang, K. Thermo-responsive superhydrophobic paper using nanostructured cellulose stearoyl ester. Cellulose 2014, 21, 357–366. [Google Scholar] [CrossRef]
- Zhang, K.; Geissler, A.; Standhardt, M.; Mehlhase, S.; Gallei, M.; Chen, L.; Marie Thiele, C. Moisture-responsive films of cellulose stearoyl esters showing reversible shape transitions. Sci. Rep. 2015, 5, 11011. [Google Scholar] [CrossRef]
- Sealey, J.E.; Samaranayake, G.; Todd, J.G.; Glasser, W.G. Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J. Polym. Sci. B Polym. Phys. 1996, 34, 1613–1620. [Google Scholar] [CrossRef]

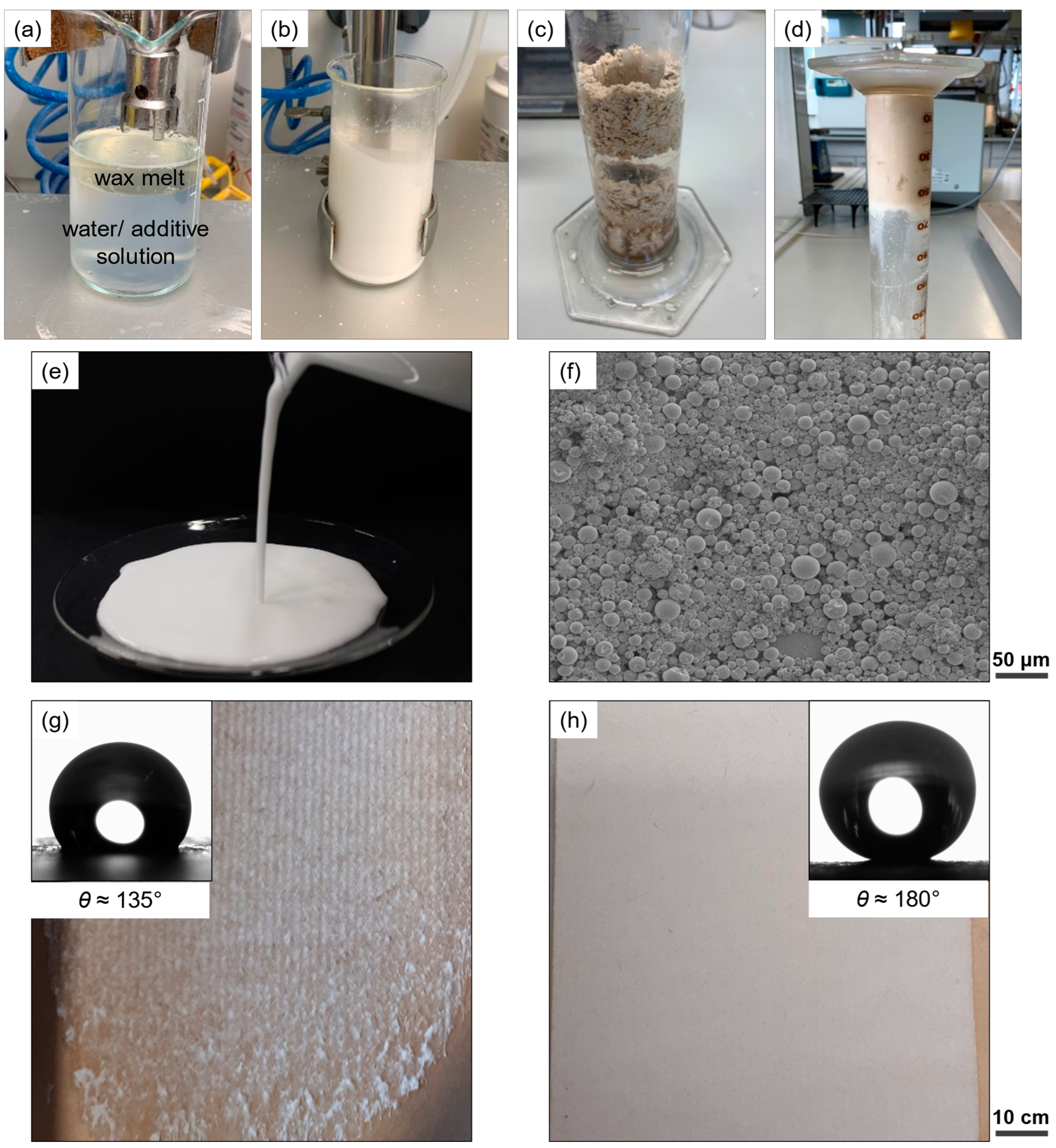

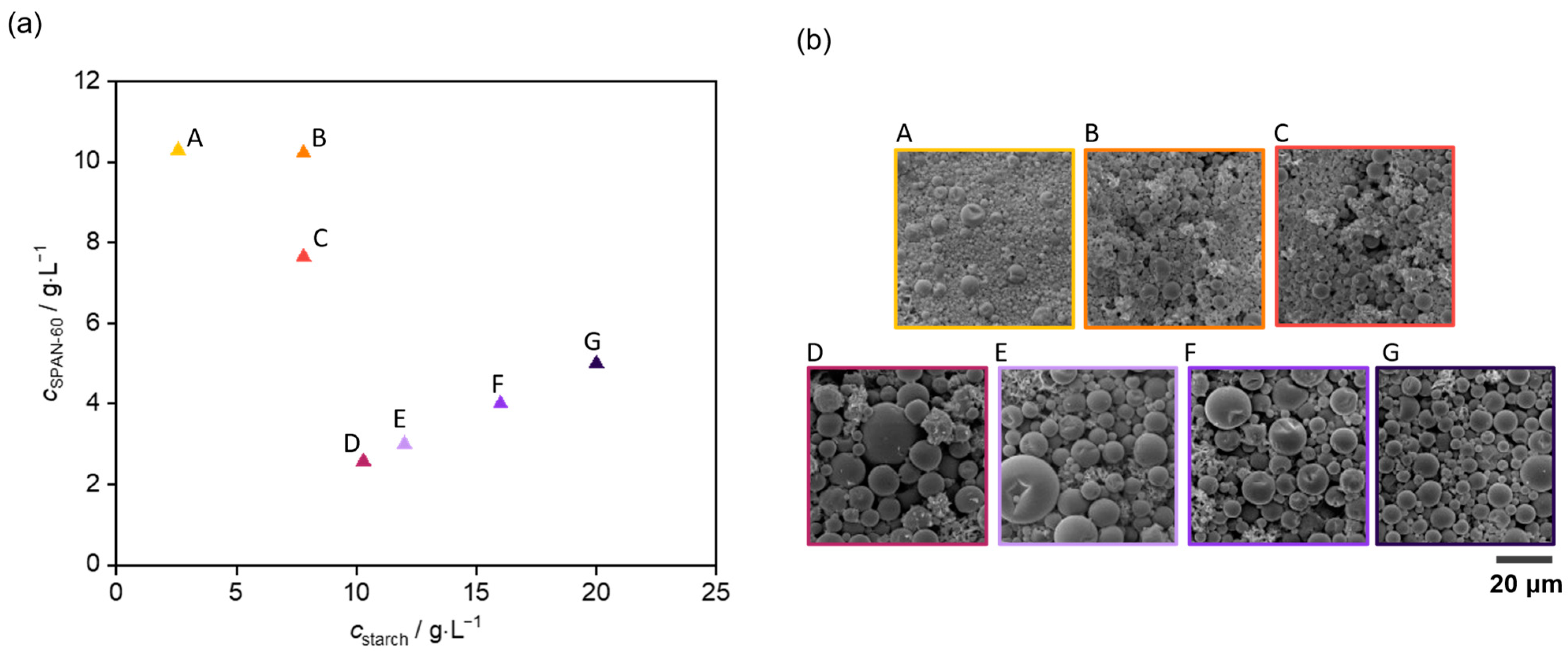
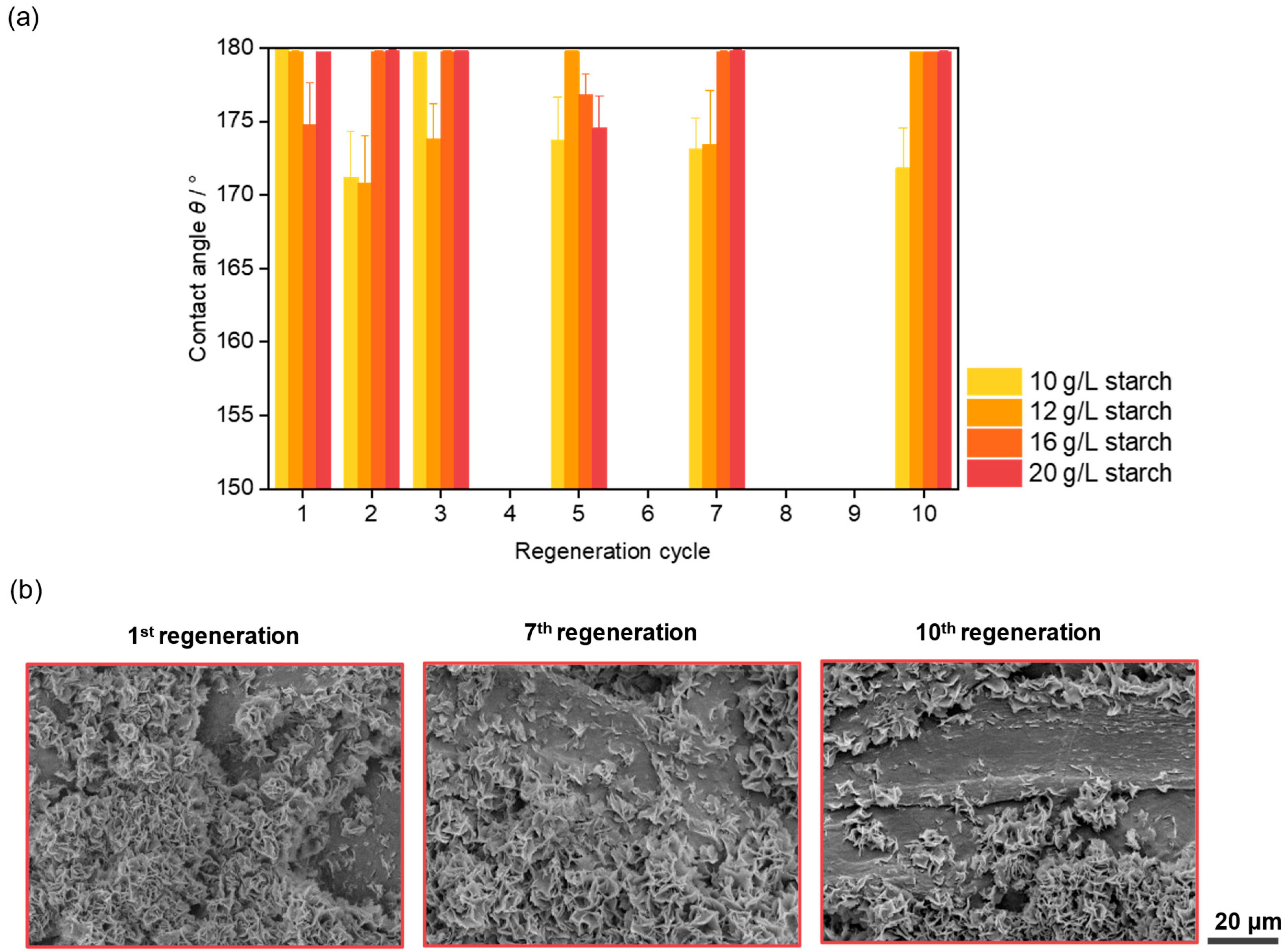
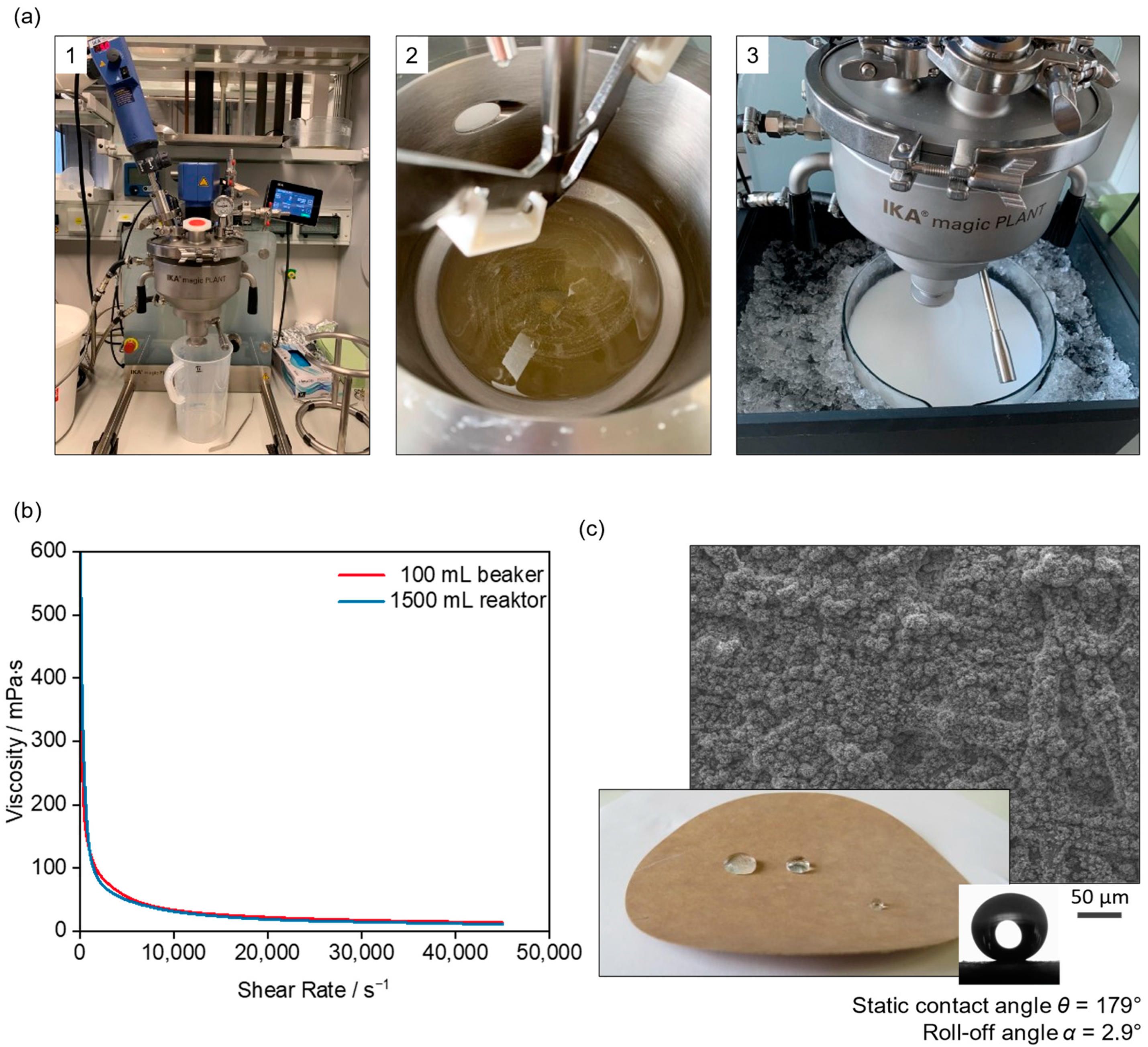


| Substance | Amount/g |
|---|---|
| Water | 100 |
| Cat. starch | 0.780 |
| Span® 60 | 0.503 |
| CSE | 1.250 |
| EGDS | 23.780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordt, C.; Daeg, J.; Elle, O.; Geissler, A.; Biesalski, M. Innovative Paper Coatings: Regenerative Superhydrophobicity through Self-Structuring Aqueous Wax-Polymer Dispersions. Coatings 2024, 14, 1028. https://doi.org/10.3390/coatings14081028
Cordt C, Daeg J, Elle O, Geissler A, Biesalski M. Innovative Paper Coatings: Regenerative Superhydrophobicity through Self-Structuring Aqueous Wax-Polymer Dispersions. Coatings. 2024; 14(8):1028. https://doi.org/10.3390/coatings14081028
Chicago/Turabian StyleCordt, Cynthia, Jennifer Daeg, Oliver Elle, Andreas Geissler, and Markus Biesalski. 2024. "Innovative Paper Coatings: Regenerative Superhydrophobicity through Self-Structuring Aqueous Wax-Polymer Dispersions" Coatings 14, no. 8: 1028. https://doi.org/10.3390/coatings14081028






