Effect of Organic–Inorganic Mixed Intumescent Flame Retardants on Fire-Retardant Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MEG
2.3. Preparation of Intumescent Fire-Retardant (IFR) Coatings
- (1)
- Add IFR, EG, and MEG to an iron container according to the formula in Table 1 and stir with a high-speed dispersing agent at a speed of 1000 r/min for 20 min to prepare the coating slurry;
- (2)
- Add the styrene-acrylic emulsion to the above mixture and stir at a speed of 400 rpm for 20 min to prepare the coating;
- (3)
- Measure the solid content of each coating, then use a brush to separate the coating on wood 90 mm × 90 mm × 3 mm and 70 mm × 70 mm × 3 mm in size for the cone calorimeter test and smoke density test;
- (4)
- Brush the paint onto an iron plate with a size of 90 mm × 90 mm × 0.5 mm, which is used for the thermal insulation test;
- (5)
- Dry the finished sample at room temperature for 24 h, and then place it in a blast drying oven at a temperature of 50 °C for 24 h to obtain the final paint sample.
2.4. Measurements
3. Results and Discussion
3.1. Characterization of MEG
3.2. Cone Calorimeter Test
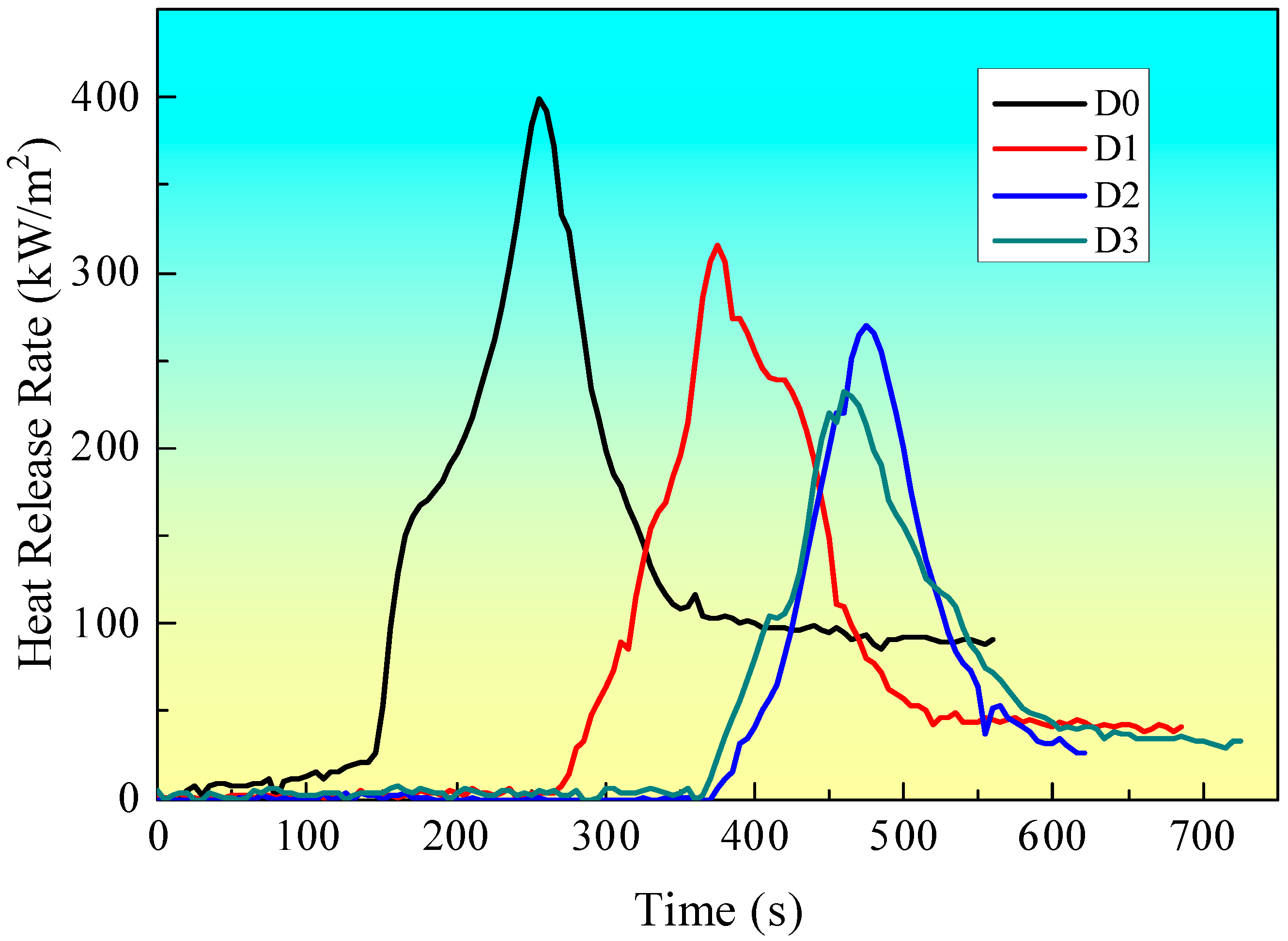
3.2.1. Heat Release Rate (HRR)
3.2.2. Mass
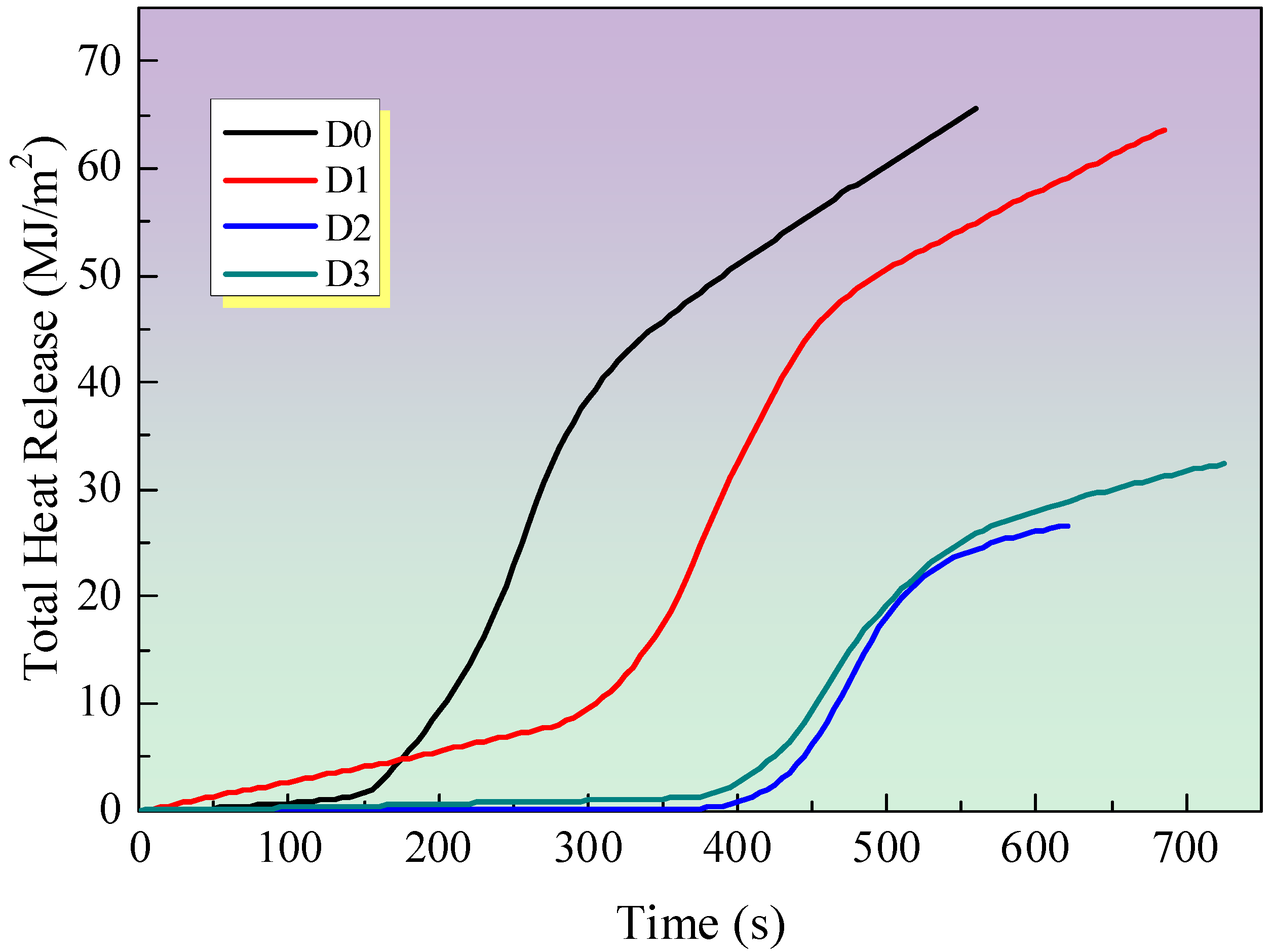
3.2.3. Total Heat Release (THR)
3.2.4. Fire Performance Index (FPI) and Fire Growth Index (FGI)
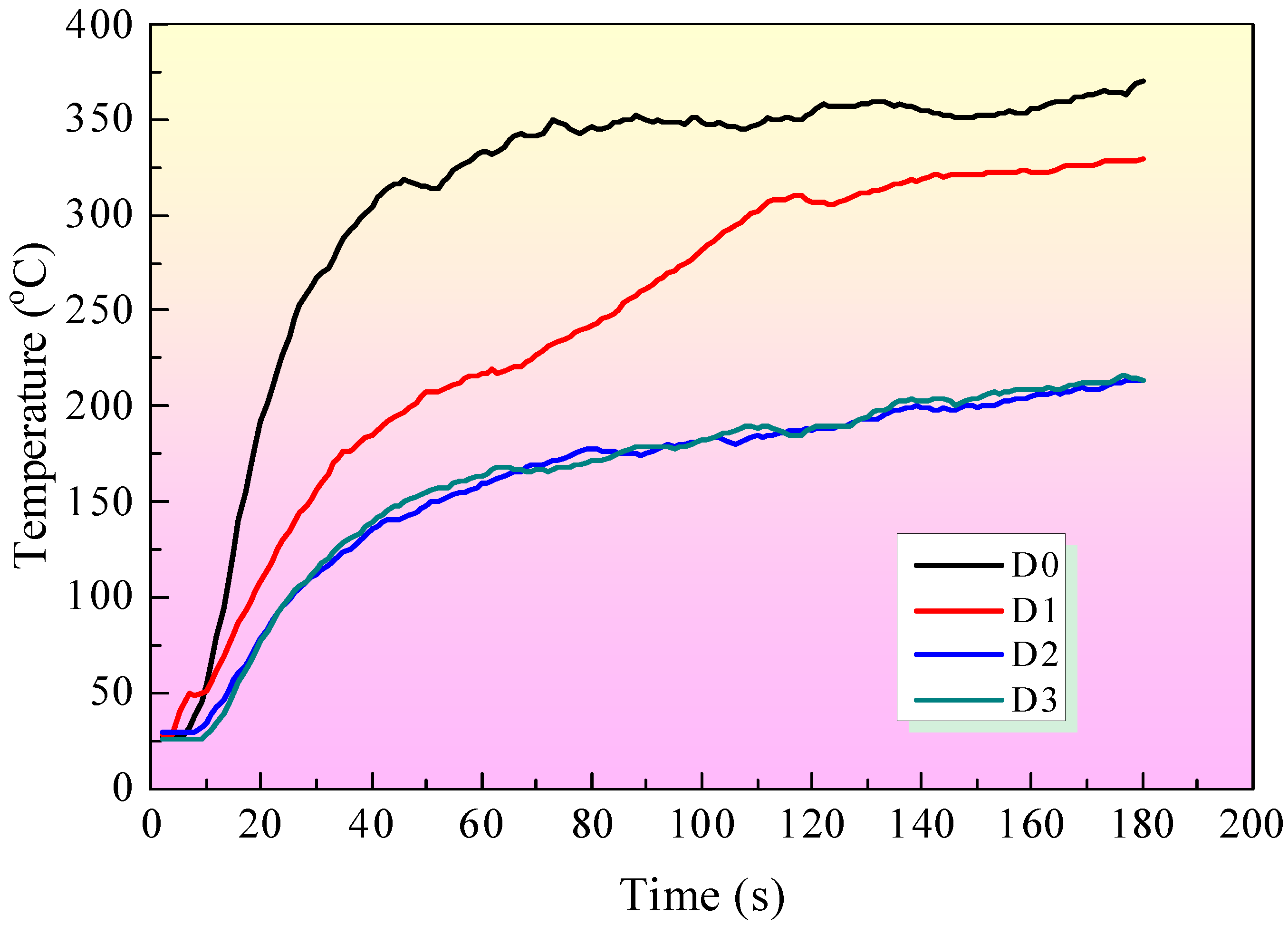
3.3. DaqPRO 5300 Radiation Heat Flow Meter Test
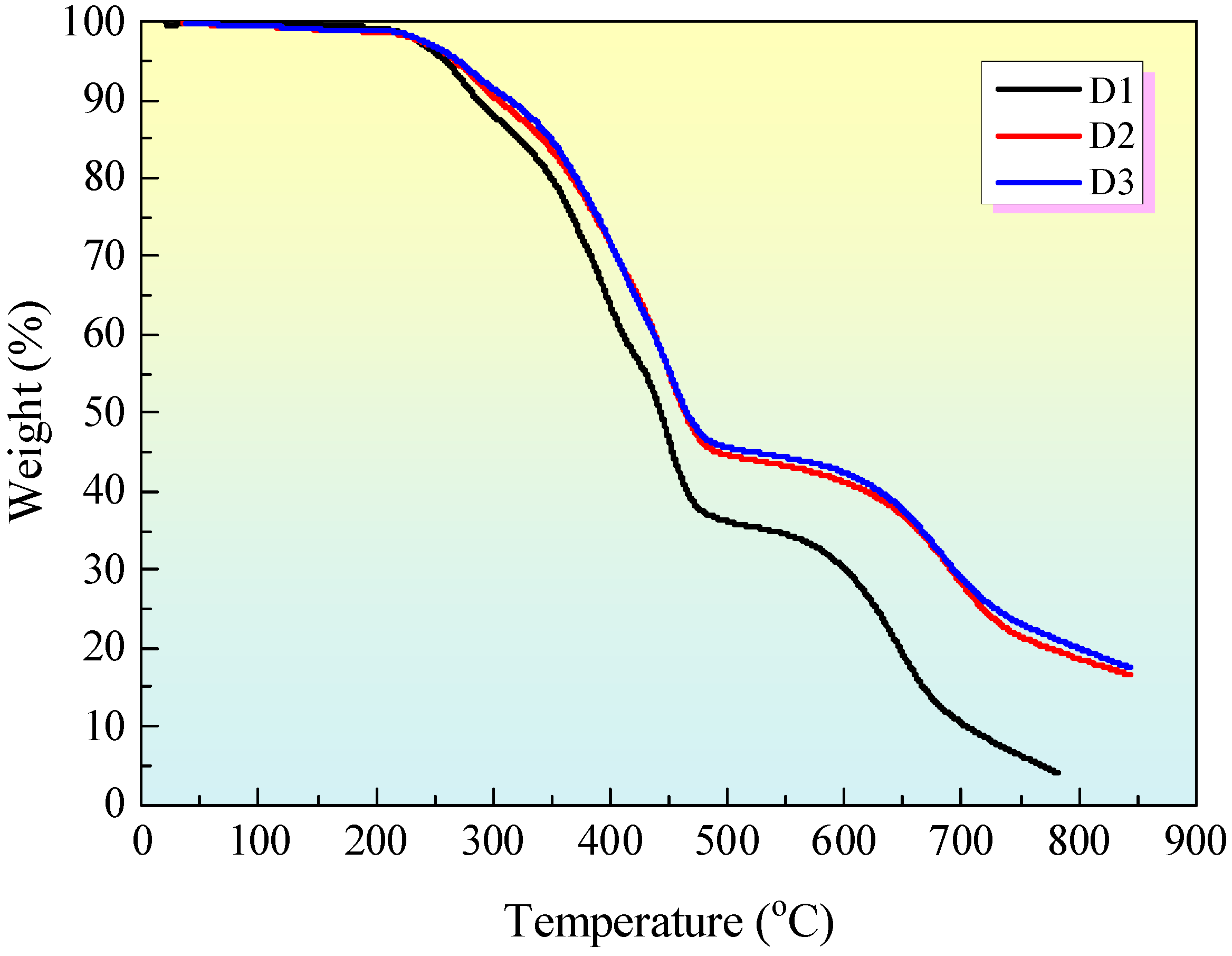
3.4. Thermal Gravimetric Analysis (TGA)
4. Conclusions
- (1)
- MEG can significantly reduce the HRR and THR of fire-resistant coating samples and improve the carbon formation of the coating.
- (2)
- MEG can reduce the heat generated by the sample combustion and extend the combustion time, improving the high-temperature thermal stability of the sample and the sample carbon formation.
- (3)
- MEG exhibits a synergistic flame-retardant effect when combined with intumescent fire-retardant (IFR) systems.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huo, S.; Guo, Y.; Yang, Q.; Wang, H.; Song, P. Two-dimensional nanomaterials for flame-retardant polymer composites: A mini review. Adv. Nanocompos. 2024, 1, 240–247. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Shao, W.; Xiao, F. Benign design of intumescent fire protection coatings for steel structures containing biomass humic acid as carbon source. Constr. Build. Mater. 2023, 409, 134001. [Google Scholar] [CrossRef]
- Shin, J.; Moon, H.; Jee, S.; Lee, K.C.; Jung, H.W.; Paik, H.; Noh, S.M. Characteristics of phosphorus-nitrogen based flame-retardant monomers for UV-curable coatings on battery PET pouches. Prog. Org. Coat. 2024, 195, 108665. [Google Scholar] [CrossRef]
- Li, J.; Zhai, C.; Yang, R. Expanded charring flame retardant effect of caged polysilsesquioxane containing Schiff base structure on poly(ethylene terephthalate) combustion. Polym. Degrad. Stab. 2024, 227, 110891. [Google Scholar] [CrossRef]
- Attia, N.F.; Elashery, S.E.A.; El-Sayed, F.; Mohamed, M.; Osama, R.; Elmahdy, E.; Abd-Ellah, M.; El-Seedi, H.R.; Hawash, H.B.; Ameen, H. Recent advances in nanobased flame-retardant coatings for textile fabrics. Nano-Struct. Nano-Obj. 2024, 38, 101180. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R. Complex char formation in flame-retarded fibre-intumescent combinations—II. Thermal analytical studies. Polym. Degrad. Stab. 1996, 54, 289–303. [Google Scholar] [CrossRef]
- Xia, Y.; Chai, W.; Liu, Y.; Su, X.; Liao, C.; Gao, M.; Li, Y.; Zheng, Z. Facile fabrication of starch-based, synergistic intumescent and halogen-free flame retardant strategy with expandable graphite in enhancing the fire safety of polypropylene. Ind. Crops Prod. 2022, 184, 115002. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Dai, B.; Meng, C.; Guo, Z. Fabrication of cellulose-based halogen-free flame retardant and its synergistic effect with expandable graphite in polypropylene. Carbohydr. Polym. 2019, 213, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, X.; Zhu, G.; Gu, X.; Li, H.; Zhang, S.; Sun, J.; Jin, X. Preparation of a novel supramolecular intumescent flame retardants containing P/N/S/Fe/Zn and its application in polylactic acid. Fire Saf. J. 2022, 128, 103536. [Google Scholar] [CrossRef]
- Panczek, P.; Ostrysz, R.; Krassowski, D. Flame Retardants 2000. In Proceedings of the 9th Flame Retardants 2000 Conference, London, UK, 8–9 February 2000; pp. 105–111. [Google Scholar]
- Okisaki, F. FLAMECUT GREP series new non-halogenated flame-retardant systems. In Proceedings of the New Developments and Future Trends in Fire Safety on a Global Basis International Conference, San Francisco, CA, USA, 16–19 March 1997; pp. 11–24. [Google Scholar]
- Krassowski, D.W.; Hutchings, D.A.; Oureshi, S.P. Tomorrow’s Trends in Fire-retardant Regulations, Testing, Applications, and Current Technologies. In Proceedings of the Fire-Retardant Chemicals Association Fall Conference, Naples, FL, USA, 13–16 October 1996; pp. 137–146. [Google Scholar]
- Yu, J.; Sun, L.; Ding, L.; Cao, Y.; Liu, X.; Ren, Y.; Li, Y. A UV-curable coating constructed from bio-based phytic acid, D-sorbitol and glycine for flame retardant modification of rigid polyurethane foam. Polym. Degrad. Stab. 2024, 227, 110892. [Google Scholar] [CrossRef]
- Lian, R.; Guan, H.; Zhang, Y.; Ou, M.; Jiang, Y.; Liu, L.; Jiao, C.; Chen, X. A green organic-inorganic PAbz@ZIF hybrid towards efficient flame-retardant and smoke-suppressive epoxy coatings with enhanced mechanical properties. Polym. Degrad. Stab. 2023, 217, 110534. [Google Scholar] [CrossRef]
- Zhou, Y.; Tawiah, B.; Noor, N.; Zhang, Z.; Sun, J.; Yuen, R.K.K.; Fei, B. A facile and sustainable approach for simultaneously flame retarded, UV protective and reinforced poly(lactic acid) composites using fully bio-based complexing couples. Compos. Part B Eng. 2021, 215, 108833. [Google Scholar] [CrossRef]
- Zhou, R.; Mu, J.; Sun, X.; Ding, Y.; Jiang, J. Application of intumescent flame retardant containing aluminum diethyphosphinate, neopentyl glycol, and melamine for polyethylene. Saf. Sci. 2020, 131, 104849. [Google Scholar] [CrossRef]
- Chang, M.; Hwang, S.; Liu, S. Flame retardancy and thermal stability of ethylene-vinyl acetate copolymer nanocomposites with alumina trihydrate and montmorillonite. J. Ind. Eng. Chem. 2014, 20, 1596–1601. [Google Scholar] [CrossRef]
- Liang, J.Z.; Feng, J.Q.; Tsui, C.P.; Tang, C.Y.; Liu, D.F.; Zhang, S.D.; Huang, W.F. Mechanical properties and flame-retardant of PP/MRP/Mg(OH)2/Al(OH)3 composites. Compos. Part B Eng. 2015, 71, 74–81. [Google Scholar] [CrossRef]




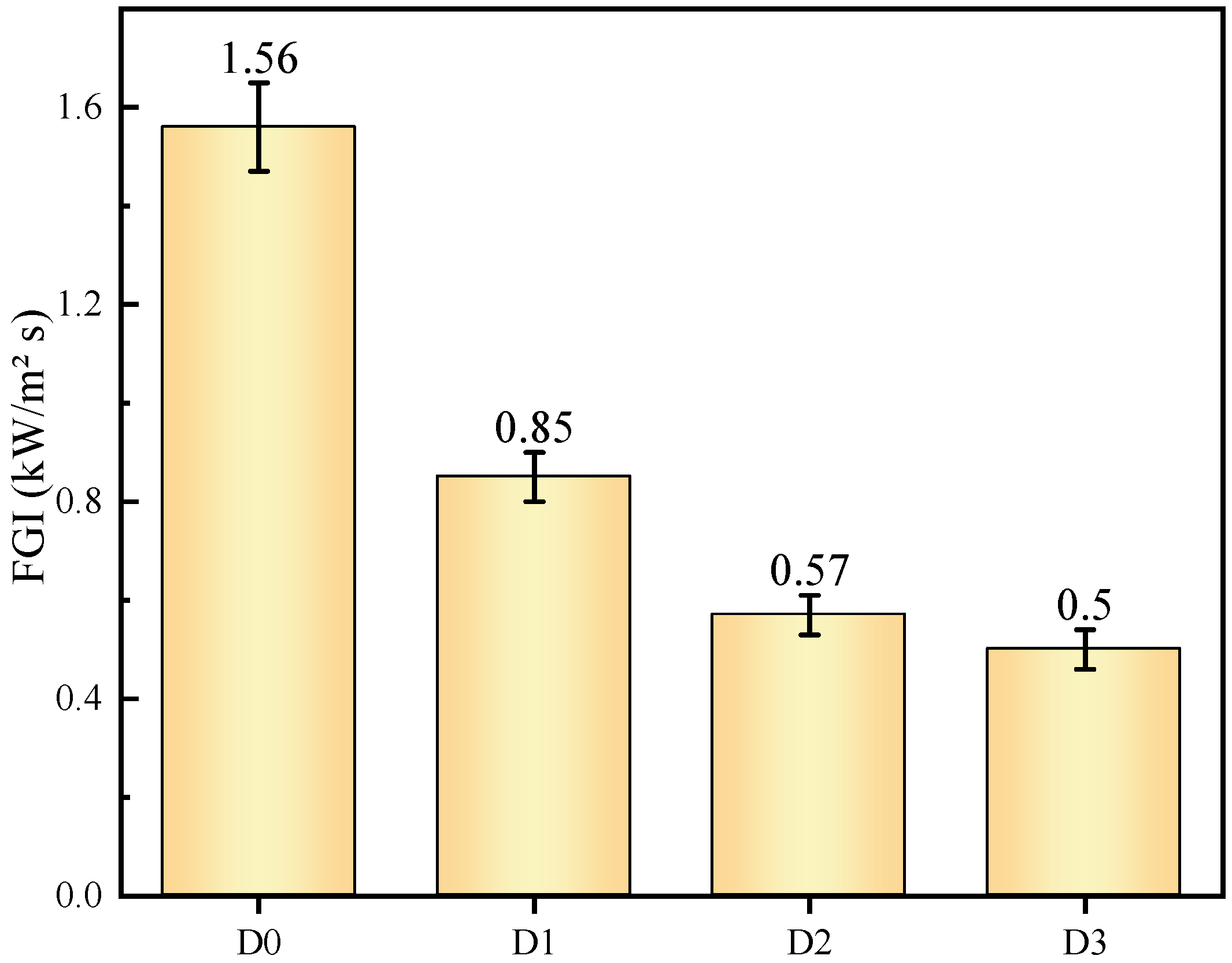
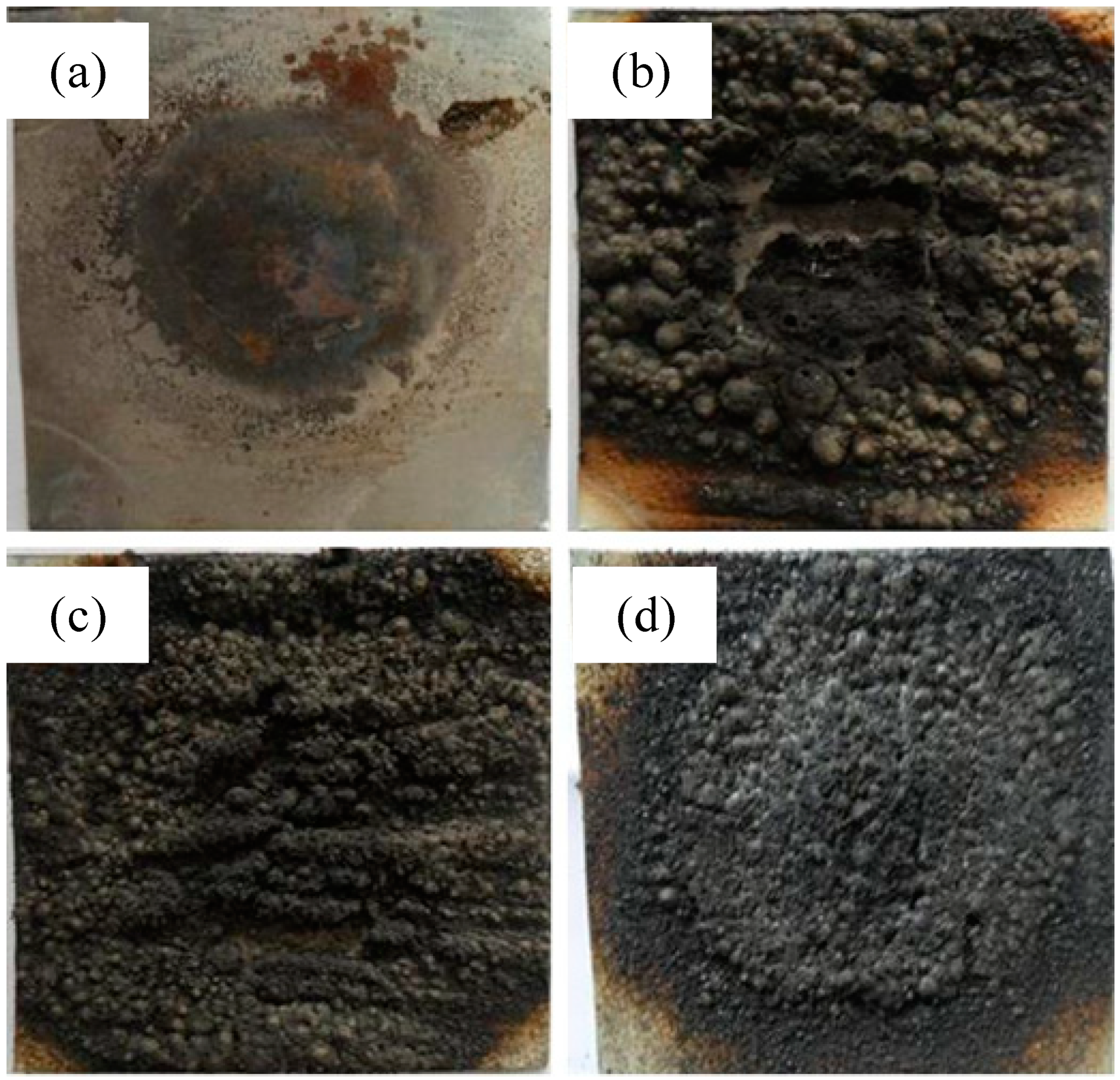

| Samples | D0 (Blank Board) | D1 | D2 | D3 |
|---|---|---|---|---|
| Styrene-acrylic emulsion/wt% | 0 | 22.0 | 22.0 | 22.0 |
| IFR/wt% | 0 | 49.0 | 47.6 | 47.6 |
| EG/wt% | 0 | 0 | 1.4 | 0 |
| MEG/wt% | 0 | 0 | 0 | 1.4 |
| Distilled water/wt% | 0 | 19.0 | 19.0 | 19.0 |
| Hydroxyethyl cellulose/wt% | 0 | 8.0 | 8.0 | 8.0 |
| Ethanediol/wt% | 0 | 1.6 | 1.6 | 1.6 |
| Antifoaming agent/wt% | 0 | 1.2 | 1.2 | 1.2 |
| Film-forming agent | 0 | 0.5 | 0.5 | 0.5 |
| Sample Code | PHRR/kW/m2 | TTP/s | TTI/s |
|---|---|---|---|
| D0 | 398.6 | 255 | 128 |
| D1 | 316.2 | 375 | 269 |
| D2 | 270.3 | 475 | 352 |
| D3 | 232.8 | 470 | 356 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Song, Q.; Dong, F.; Yang, H.; Xia, Z.; Liu, J. Effect of Organic–Inorganic Mixed Intumescent Flame Retardants on Fire-Retardant Coatings. Coatings 2024, 14, 1034. https://doi.org/10.3390/coatings14081034
Ma L, Song Q, Dong F, Yang H, Xia Z, Liu J. Effect of Organic–Inorganic Mixed Intumescent Flame Retardants on Fire-Retardant Coatings. Coatings. 2024; 14(8):1034. https://doi.org/10.3390/coatings14081034
Chicago/Turabian StyleMa, Liyong, Qingfeng Song, Fang Dong, Hongli Yang, Zihao Xia, and Jianlin Liu. 2024. "Effect of Organic–Inorganic Mixed Intumescent Flame Retardants on Fire-Retardant Coatings" Coatings 14, no. 8: 1034. https://doi.org/10.3390/coatings14081034
APA StyleMa, L., Song, Q., Dong, F., Yang, H., Xia, Z., & Liu, J. (2024). Effect of Organic–Inorganic Mixed Intumescent Flame Retardants on Fire-Retardant Coatings. Coatings, 14(8), 1034. https://doi.org/10.3390/coatings14081034







