Abstract
There has been an increasing recognition of the efficacy of various clay mineral elements in absorbing heavy metallic ions, which can be attributed to their cost-effectiveness, widespread, precise floor area, and remarkable practical groups. A co-pyrolyzing calcite/biochar (CP-CAL/BC) composite was prepared in this study by co-pyrolyzing calcite (CP-CAL) and coconut shell (CS) at 650–750 °C. Several methods were employed to analyze the properties of the synthesized composite. The composite showed efficient adsorption of Pb2+ at a pH of 4.5, primarily by the process of monolayer chemisorption, which is influenced by the composite’s pore structure and boundary layer diffusion. After several repeated experiments, it was observed that all of the CP-CAL/BC composites possessed exceptional regeneration capabilities, consistently removing Pb2+ at high rates. The CP-CAL/BC composite produced at 750 °C showed the greatest extent of resistance to corrosion, surpassing all other composites with a decrease in corrosion of 7.298 × 10−6 A/cm2. The present study confirmed that the CP-CAL/BC composite material has efficient adsorption features for Pb2+ and strong regenerative capability. Furthermore, the material synthesized at high temperatures demonstrated superior corrosion resistance.
1. Introduction
With the rapid advancement of global industrialization, heavy metal pollution has become a major threat to water health. Specifically in mining, metallurgy, leather manufacturing, dye production, electroplating, and other industries, the direct discharge of industrial wastewater without proper treatment leads to the accumulation of heavy metals in the natural environment [1,2]. These heavy metal ions pose a serious challenge to the ecosystem due to their high toxicity, extremely difficult degradation, easy enrichment in organisms, and potential radioactivity [3]. Lead, as a heavy metal with particularly significant toxicity and carcinogenicity, once excessively accumulated in the human body, may not only damage the central nervous system but also cause irreversible damage to hematopoietic function, kidney health, bone strength, and even the reproductive system, posing a major risk to the growth and development of children and adolescents [4,5,6]. A variety of industries produce lead industrial effluent, which includes battery production, paint manufacturing, and smelting operations. Efficient treatment alternatives are essential due to the serious hazards they pose to human health and the environment [7,8,9]. The predominant approaches employed to treat lead-containing effluent include reverse osmosis, ion exchange, membrane filtration, adsorption, and chemical precipitation. Adsorption is considered the simplest and most efficient approach among these owing to its remarkable efficacy, low energy requirements, ease of operation, and capability to exclusively remove particular metals.
Biochar (BC) is a type of biomaterial that is produced from solid, porous carbon byproducts that are frequently generated in oxygen-deficient environments [10]. The factors that make it more appealing include its cost-effectiveness, ease of application, and eco-friendliness. In our pilot study, we screened and evaluated biochar (BC) derived from tropical crop waste, including coconut husks, cassava stalks, oil tea husks, banana stems, and rubberwood. It was found that the biochar prepared with coir as a raw material showed significant adsorption capacity for Pb2+ in solution, which indicated that coir biochar had potential application value in the field of heavy metal ion adsorption. It has been demonstrated that biochar produced from fruit peels, sugarcane residue, walnut shells, sludge, and other raw materials can absorb Pb2+ from wastewater. China develops a substantial quantity of coconuts due to their nutritional value and aesthetically pleasing characteristics. The high carbon content and rigid consistency of these materials render them well-suited for biochar production and contribute to the fulfillment of resource recycling objectives [11]. However, concerns have arisen regarding biochar materials due to their inadequate adsorption capacity and ensuing contamination. Various techniques, including chemical modification, metal/metal oxide modification, and hydrothermal production, have been employed to increase the absorption efficacy of biochar substances. Furthermore, concerns pertaining to secondary illnesses and increasing expenses are prevalent.
In recent years, clay mineral materials have become a popular material in the field of heavy metal ion adsorption because of their economy, high specific surface area, and abundant active sites. For example, the experimental results of Sdiri et al. [12] show that the use of treated limestone as an adsorbent can effectively reduce the concentrations of Pb2+ and Cd2+ in wastewater, which is better than siderite and dolomite. Merrikhpour et al. [13] found that calcite sludge performed well in removing Zn2+, Pb2+, Cu2+, and other heavy metal ions from wastewater, with higher efficiency than feldspar and mica. Recently, there has been an increasing recognition of the efficacy of various clay mineral elements in absorbing heavy metallic ions, which can be attributed to their cost-effectiveness, widespread precise floor area, and remarkable practical groups [14,15]. Conventional biochar materials, altered from solvent-based clay minerals, have the potential to cause similar contamination of ecosystems. The majority of research efforts require the pre-treatment of raw char, which is a laborious and energy-intensive process. In this study, the collaborative pyrolysis of coir and calcite at variable temperatures was innovatively used to prepare CP-CAL/BC composites. This method significantly reduces the amount of acid–base and organic solvent used in the preparation process, not only simplifies the production process of the adsorbent but also realizes energy savings and consumption reduction and effectively reduces the production cost.
2. Methods and Processes
2.1. Carbonized Coconut Shell Composites
Chemical reagents such as Pb(NO3)2 are provided by Shanghai Yihui Biotechnology Co., Ltd. (Shanghai, China). Coconut shell and other experimental materials are provided by the Rongmao coconut shell activated carbon manufacturer (Haikou, China). The moisture content of coconut shells was maintained below 10% and subjected to fast processing followed by filtration via a 0.150 mm sieve, which was denoted as ‘CS.’ The CP-CAL and CS were mixed in the ratios of 1:1, 3:4, 1:2, and 1:4. The resulting combination was heated in a muffle furnace at a rate of 10 °C per minute, reaching temperatures of 650 °C, 700 °C, and 750 °C over an hour while being protected by a shield of nitrogen gas. The resulting fine powders were coded as CP-CAL/BC. The manipulated group used to produce BC fine powder, excluding CP-CAL, using a similar method and referred to it as BC.
2.2. Pb(NO3)2 Solution Simulation of Industrial Wastewater
The Pb(NO3)2 solution (1 g/L) was prepared by dissolving an appropriate amount of Pb(NO3)2 powder in 1 vol.% nitric acid solution. After that, the solution was diluted until it reached concentrations typical of industrial effluent. The pH of the solution was adjusted using the corresponding acid–base solution. All the reagents are analytically pure.
2.3. Adsorption Capacity Test
The objective of this analysis was to determine whether the adsorption properties of CP-CAL/BC changed before and after the composite was incorporated into various elements [16,17]. BC served as the standard group; for each of the 50 mL flasks containing adsorbent concentrations ranging from 0.25 to 2 g/L, 20 mL of a 0.1 g/L Pb2+ solution was added. The starting pH levels were adjusted to 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0 to examine the effect of initial pH on the ability of CP-CAL/BC to adsorb Pb2+. A variety of adsorbent quantities were added to the primary solution to ascertain the optimal solid-to-liquid ratio. The mixtures were vigorously agitated while being submerged separately in thermostatic water baths maintained at temperatures of 20, 30, and 40 °C. The reaction mixtures were filtered using 0.45 μm filters after reaction periods ranging from 0 to 1440 min. The Pb2+ concentrations in the filtrates were determined using the BION1771 portable Pb2+ ion analyzer (Bell Analytical Instruments Co., Ltd., Dalian, China). The control equation is as follows:
The equation represents the adsorption capacity at Qe equilibrium (mg/g) and the initial and final concentrations (mg/L) of C0 and Ce solutions. Furthermore, V in the equation represents the volume of solution (L), m denotes the adsorbent mass (g), and η denotes the removal efficiency.
2.4. Characterization
Scanning electron microscopy (Quanta FEG450, produced by FEI Company, Portland, OR, USA) was used to examine the surface morphology of materials. Inductively coupled plasma mass spectrometry was formerly used to verify the elemental composition of the surface of each substance. Fourier transform near-infrared spectroscopy was employed to examine the chemical characteristics of substances and their types. An X-ray diffractometer (XRDynamic 500, Vienna, Austria) was used to represent and depict the crystalline structure and geometry of materials. Using CuKα (λ = 1.5406 Å) as the X-ray source, the tube voltage is set to 40 kV, the tube current is 40 mA, the 2θ scanning range is 5° to 80°, the step size is 0.02°, the scanning speed is 2°/min, and the exposure time is 10 s/step.
A CS350 electrochemical notebook manufactured by Wuhan Coster Instruments, Wuhan, China, was used to evaluate the electrochemical conductivity of the CP-CAL/BC composite. Initially, 4.0 wt.% NaCl was utilized, accompanied by a scan rate of 0.8 mV/s and a viable sweep range of −0.8 V to 0.3 V. From Tafel plots, essential electrochemical metrics, including corrosion cutting-edge (Icorr) and corrosion workable (Ecorr), were derived.
3. Results and Discussion
3.1. Analysis of Adsorption Capacity
As illustrated in Figure 1, the CP-CAL/BC ratio displayed a significant influence on the adsorption of Pb2+. The adsorption capacity of lead ions was enhanced when CP-CAL was blended with BC; the resulting adsorption quantity was 45.3 mg/g, which was higher than that of BC produced at temperatures of 650 and 700 °C. The adsorption quantity was determined to be 85.47 mg/g at a 1:2 mixing ratio, which was higher than the 38.04 mg/g of pure BC. As the BC component in the CP-CAL/BC combination increased, the adsorption capacity of lead initially increased [18,19,20], then decreased, and eventually achieved a maximum value of 1:2. However, the adsorption capacity of lead decreased when the combination ratio exceeded 1:2. Furthermore, the CP-CAL/BC decreased significantly by 26.09 mg/g compared to a ratio of 1:2 when the combination ratio was changed to 1:4, possibly because excessive CP-CAL blocked the pore structure of BC. Hence, the ratio of 1:2 was considered the most appropriate [21,22]. The adsorption capacity of Pb2+ ions by CP-CAL/BC composites reaches an optimal high of 85.47 mg/g at a 1:2 CP-CAL to BC ratio, after which it declines, likely due to excess CP-CAL obstructing BC’s pore structure, underscoring 1:2 as the most effective ratio for maximizing lead ion adsorption.
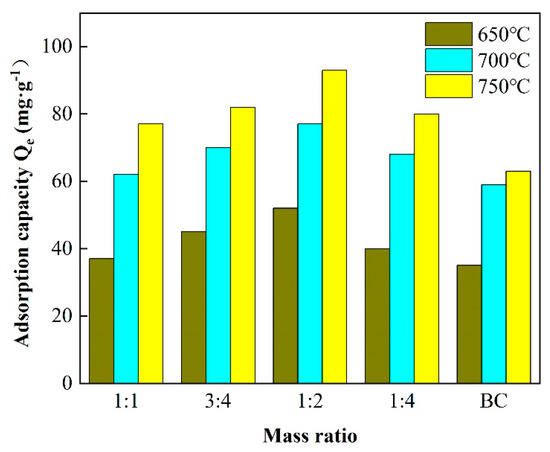
Figure 1.
The effect of CAL/BC ratio on composite materials.
Figure 2 shows the effect of the initial pH value on Pb(II) removal from CAL/BC composites and the zero charge point (pHpzc) of CAL/BC composites prepared at different temperatures. Due to the different ion morphology, surface charge, and chemical properties of functional groups on the surfaces of different materials, the solution pH has a significant effect on the adsorption capacity of heavy metal ions [23]. A slight decrease in lead adsorption was observed when the pH was 2.0 to 5.0, potentially attributable to the competitive interaction between H+ ions and lead ions [24,25,26]. An increase in pH resulted in a reduction in the concentration of H+, thereby decreasing the competitive pressure on CP-CAL/BC. This phenomenon is advantageous for the adsorption of lead ions. Pb2+ concentrations can be decreased through precipitation when the pH value is increased. Therefore, it is essential to control the initial pH within an appropriate range to optimize the absorption of Pb2+ by the CP-CAL/BC composite.
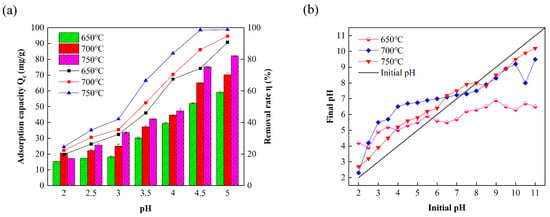
Figure 2.
(a) Different pH levels affect the Pb2+ extraction efficiency; (b) determination of the isoelectric point of CAL/BC composites produced at various temperatures.
The maximum Pb2+ adsorption capacity was observed at pH 4.5, with corresponding values of 49.36, 59.27, and 66.30 mg/g. Furthermore, the removal rates at this pH were determined to be 74.24, 86.12, and 98.56%. Therefore, a pH value of 4.5 was selected.
As shown in Figure 2b, pHpzc at 650, 700, and 750 °C corresponds to 5.73, 6.02, and 7.23, respectively. When the pH of the solution decreased below the pHpzc of the material, the compounds on the fabric surface received protons, resulting in a positively charged surface. Therefore, composite materials showed electrical neutrality.
The adsorption capacity of composite materials for Pb2+ was comparatively low at a pH of 2.0, as demonstrated in Figure 2a,b. This is due to the advantageous cost on its floor at pH < pHpzc of the CP-CAL/BC composite, which repels the cationic Pb2+ in the solution, thereby rendering adsorption reactions challenging [27]. The adsorption process remains unaffected by the CaCO3 reaction, which showed a decrease with an increase in pH, thereby protecting the composite material. At a pH of 4.5, the CP-CAL/BC-based ion can readily absorb Pb2+. However, the diversity of protonated species decreased with increasing pH, limiting both the quantity of Pb2+ adsorption and the rate of elimination. The optimal pH for Pb2+ adsorption by the CP-CAL/BC composite is 4.5, where the adsorption capacity reaches a maximum of 66.30 mg/g and removal rates up to 98.56%, as lower pH values result in surface protonation and repulsion of Pb2+ ions, whereas higher pH values decrease the diversity of protonated species, hindering adsorption efficiency; this optimal point balances the competitive effects of H+ ions and the precipitation of Pb2+ ions for maximal adsorption.
In Figure 3, it can be seen that the addition of composite material contributed to an initial increase in the removal efficiency of Pb2+. However, this increase eventually became constant after reaching a specific threshold. The initial addition of 0.25 g/L led to a removal rate of 64.2%; increasing the addition to 2.0 g/L significantly improved the rate to 97.3%. However, the rate of increase in the removal rate decreased with a further increase in the addition of material. Simultaneously, the adsorption capacity per unit decreased with an increase in the amount of material added, showing a decrease from 140.67 to 43.15 mg/g. By considering equilibrium and economic efficiency, the optimal dosage for CP-CAL/BC composites was determined to be 1.75 g/L, which ensures both the high removal rates of Pb2+ and cost-effectiveness.
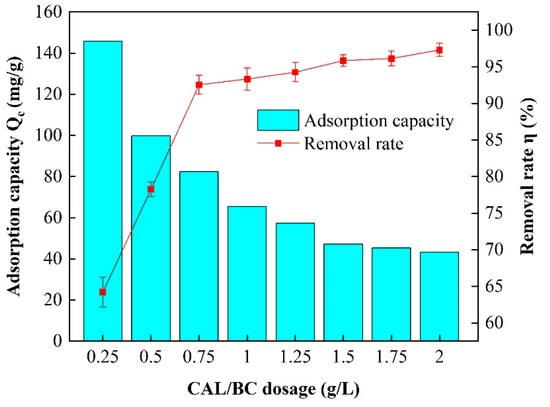
Figure 3.
Effect of integrating CP-CAL/BC composites on the Pb2+ adsorption capacity (Qe).
3.2. Adsorption Mechanism of Composite Materials
FTIR showed significant changes in the composite material during the adsorption process at 750 °C, as illustrated in Figure 4. The disappearance of the prominent peak at 1532 cm−1, which was attributed to carbonate (CO32−) ions, suggested the chemical reaction between Pb2+ and carbonate groups, resulting in the formation of insoluble lead carbonate (PbCO3). This indicates that the composite can remove Pb2+ through precipitation [28]. The initial broad hydroxyl (-OH) stretching, which was detected at 3513 cm−1, shifted to 3524 cm−1 in the presence of Pb2+, indicating the disruptions in hydrogen bonding and Pb-induced rearrangement of these groups. Furthermore, after adsorption, the absorbance of the carbonyl group at 1820 cm−1 decreased, indicating a potential exchange of Pb2+ and protons (H+) from the carboxylate (-COO-) groups. This may be attributed to the formation of lead carboxylates [29]. The observed spectral changes indicate the participation of particular functional groups in the binding of Pb2+ and provide insight into the mechanisms that enable its adsorption onto the CP-CAL/BC composite, including precipitation and surface complexation.
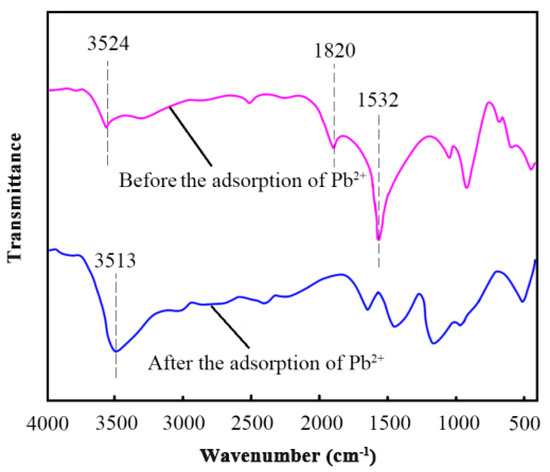
Figure 4.
FTIR spectroscopic analysis of CP-CAL/BC composite before and after the adsorption of Pb2+.
Calcium carbonate (CaCO3) ionizes in water to produce calcium ions (Ca2+) and CO32−, which then react with Pb2+ to form PbCO3 as a precipitate. Alcoholic compounds showed the displacement of hydroxyl groups (-OH) by lead ions (Pb2+), resulting in the formation of negative ions (R-O−-Pb2+) and water (H2O) while simultaneously emitting positive ions (H+). Carboxylic acid compounds form negative ions (R-COO−-Pb2+) and water (H2O) through the bonding of their carboxyl groups (-COOH) with lead ions (Pb2+), thereby releasing positive ions (H+). Both reactions involve the liberation of protons (H+), resulting in changes to the pH value of the solution.
The π-electrons in the C=C bonds of CP-CAL/BC form bonds with Pb2+, while the porous structure of the composite offers sites for adsorption. An initial pH greater than pHpzc induces electrostatic attraction towards Pb2+, which facilitates their elimination from water. FTIR analysis reveals that the CP-CAL/BC composite removes Pb2+ ions through various mechanisms, including the formation of insoluble lead carbonate (PbCO3) via reaction with carbonate groups, alterations in hydroxyl (-OH) and carbonyl group interactions suggesting lead-induced rearrangement and potential proton exchange, and the involvement of π-electrons in C=C bonds for bonding with Pb2+, all facilitated by an initial pH above the pHpzc that induces electrostatic attraction and aids in the precipitation and surface complexation of Pb2+ ions.
3.3. Electrochemical Properties of CP-CAL/BC Composite Materials
The results of the composite material following the adsorption are shown in Figure 5. The data were analyzed and summarized in Table 1, which presents the corrosion currents derived from the composite plots.
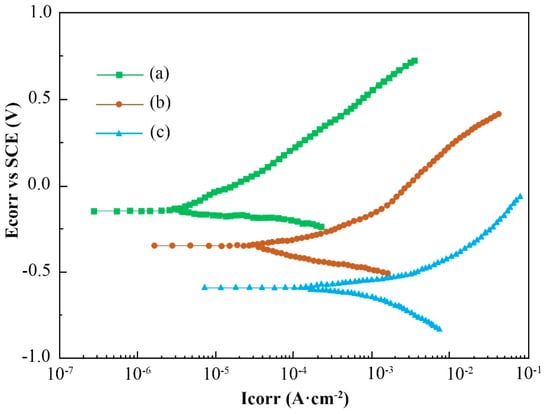
Figure 5.
Electrochemical polarization curves of CP-CAL/BC composite produced at (a) 650 °C, (b) 700 °C and (c) 750 °C.

Table 1.
Electrochemical performance data of composite materials.
The maximum corrosion current density of the sample prepared at 650 °C was determined to be 9.732 × 10−5 A/cm2. The observed high current density indicated that this particular composite possessed the lowest corrosion resistance in comparison to the other composites. The increased corrosion rate can be attributed to the porous structure of the composite, which facilitates the penetration of NaCl solution, thereby accelerating the corrosion process. On the other hand, the corrosion current at 700 °C was found to be 4.581 × 10−5 A/cm2. Interestingly, the composite material prepared at 750 °C showed the lowest corrosion current density among the three samples, with a value of 7.298 × 10−6 A/cm2. The observation suggested that the superior corrosion resistance can be potentially attributed to a higher concentration of CP-CAL in this particular composite. The enhanced density and uniformity of the structure, potentially attributed to the increased concentration of CP-CAL, hinder the penetration and attack of the material via the corrosive NaCl solution. The composite material prepared at 750 °C exhibits the lowest corrosion current density of 7.298 × 10−6 A/cm2 among the three samples, indicating superior corrosion resistance, possibly due to a higher concentration of CP-CAL, which enhances structural density and uniformity, thereby inhibiting the penetration and corrosive action of NaCl solution compared to the composites prepared at lower temperatures.
3.4. Properties of CP-CAL/BC Composites in Recycling
Figure 6 indicates the ability of the material to adsorb Pb2+ after a couple of cycles. Following the initial adsorption-desorption cycle, the CP-CAL/BC prepared at temperatures of 650, 700, and 750 °C showed elimination rates of 70.58, 80.34, and 95.24%, respectively, when subjected to an initial concentration of 8 mg/L of Pb2+. After four cycles, the removal rate decreased by 9.42, 7.25, and 3.21%, respectively. The decrease in removal rate could potentially be attributed to the elimination of voids and the reduction in active sites within the composite material.
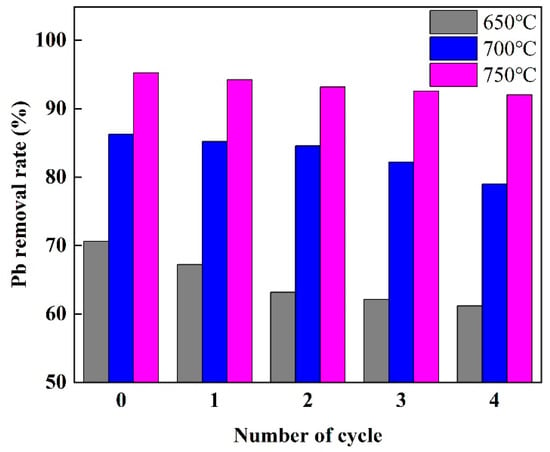
Figure 6.
Reusability of CP-CAL/BC composite for Pb2+ adsorption after multiple recycling cycles.
3.5. Characterization of CP-CAL/BC Composite Material
The surface morphology of CP-CAL displays a blocky structure and various pores, as shown in the SEM images presented in Figure 7. BC produced at 650 °C showed a reduced pore count and exhibited a smoother and more dense appearance, as shown in Figure 7a. However, BC prepared at a higher temperature of 750 °C showed higher porosity, suggesting that higher temperatures have a beneficial effect on gap formation (see Figure 7b). The formation of block-shaped particles by CP-CAL and CS at three distinct pyrolysis temperatures is indicative of their interaction. The composite material develops a rougher and more porous texture as CP-CAL accumulates continually on its surface. CP-CAL/BC had a relatively high porosity at 750 °C (see Figure 7e); therefore, the incorporation of CP-CAL has no discernible effect on the material pores. Furthermore, CP-CAL thoroughly covered the exterior of the material at the other two temperatures.
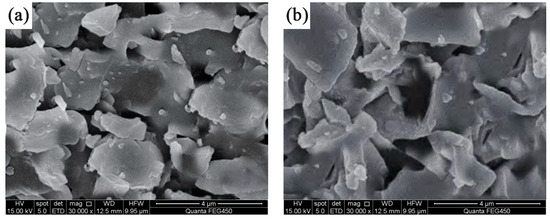
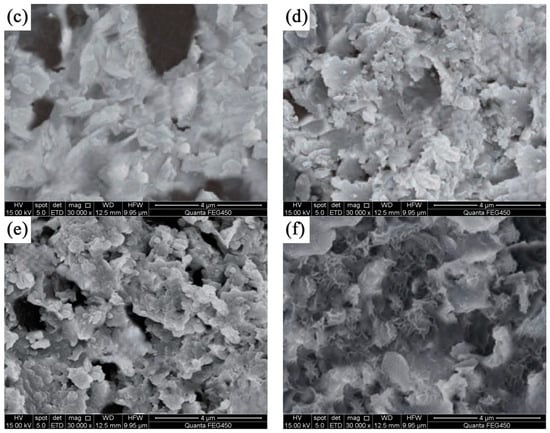
Figure 7.
SEM images of BC and CP-CAL/BC composites before and after the adsorption of Pb2+: (a) BC produced at 650 °C, (b) BC produced at 750 °C, (c) CP-CAL/BC composite produced at 650 °C, (d) CP-CAL/BC composite produced at 700 °C, (e) CP-CAL/BC composite produced at 750 °C, and (f) CP-CAL/BC composite after the adsorption of Pb2+.
The results indicated that increasing the pyrolysis temperature improved the combination of CP-CAL and BC, leading to a more efficient composite. The surface smoothness of the CP-CAL/BC composite was substantially enhanced in SEM images after Pb2+ adsorption compared to its previous rough texture. The presence of several filled pores suggests that Pb2+ predominantly adheres to spherical and cubic particles as well as empty spaces. The aforementioned enhanced interaction indicates that CP-CAL/BC composites can remove Pb2+ from solutions with higher durability and efficacy, thereby emphasizing their potential for environmental applications. SEM imaging shows that the integration of CP-CAL with BC leads to a more porous and textured surface, which is conducive to the adsorption of Pb2+ ions, as evidenced by the increased number of filled pores post-adsorption. The enhanced surface characteristics suggest that the CP-CAL/BC composite is effective in removing Pb2+ from solutions with high efficiency and durability.
Figure 8 presents spectroscopic analyses of biochar and calcite/biochar derived from different pyrolytic treatments. The broad peaks observed at 23° and 44° in the image of BC (Figure 8a) correspond to the graphitic crystalline planes (002) and (100), respectively. These findings indicate that CS may trigger graphitization when exposed to high-temperature pyrolysis.
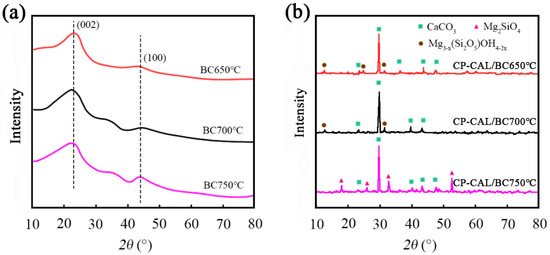
Figure 8.
XRD patterns of (a) BC and (b) CP-CAL/BC composite materials prepared under different temperature conditions.
The composite material in Figure 8b is primarily comprised of CaCO3 crystals. The presence of CaCO3 as the primary component in the composite matrix was confirmed by the prominent diffraction peaks observed at 23.1°, 29.4°, 36.0°, 39.4°, and 47.5°. Furthermore, the XRD spectra of CP-CAL/BC samples processed at 650 °C and 700 °C showed additional peaks at 2θ values of 12.0°, 24.4°, and 31.1°. These peaks corresponded to the presence of the hydroxy silicate impurity phase of Mg3−x(Si2O5)OH4−2x. However, the peak associated with Mg3−x(Si2O5) OH4−2x decreased in the image of the composite. Conversely, the emergence of new peaks at 2θ values of 17.5°, 25.4°, 32.6°, and 52.3° signified the presence of a crystalline form of magnesium silicate (Mg2SiO4). The observed alterations in the mineralogical composition of the composite indicate that the pyrolysis temperature triggered a structural change in the material.
In addition, the XRD patterns of different materials under distinct conditions revealed a variety of CaCO3 diffraction peaks, indicating that CaCO3 served as a primary component [30]. Magnesium silicate-containing water showed additional diffraction peaks in samples prepared at temperatures of 650 and 700 °C; these peaks were attributed to the presence of hydroxy silicate impurities. At 750 °C, the Mg3−x(Si2O5)OH4−2x-related diffraction peaks disappeared while new diffraction peaks emerged, indicating the presence of Mg2SiO4 crystals. Spectroscopic analysis reveals that BC exhibits graphitic crystalline planes, while CP-CAL/BC composites predominantly consist of CaCO3 with additional Mg3−x(Si2O5)OH4−2x impurities at lower pyrolysis temperatures, which transform into Mg2SiO4 crystals at 750 °C, indicating that the pyrolysis temperature induces structural changes in the composite material, affecting its mineralogical composition.
4. Conclusions
- (1)
- When embarking on the preparation process with an initial pH range of 4.0 to 5.0, optimal adsorption efficiency is achieved at a pH of 4.5. The extraction of lead ions from the CP-CAL/BC composite material is thought to unfold through a multifaceted mechanism. At the identified optimal pH, the surface charge of the composite and the solubility of lead ions are ideally balanced, facilitating the most effective adsorption. Further deviations from this pH could disrupt these interactions, leading to less efficient lead ion removal.
- (2)
- The material exhibited exceptional resilience against corrosion at an elevated temperature of 750 °C, markedly surpassing its performance at lower development conditions of 650 and 700 °C. This characteristic is critical for applications in high-temperature environments, where corrosion can significantly impact material integrity and operational efficiency. Moreover, the material’s ability to retain a high level of lead removal performance over multiple cycles, despite slight efficiency losses, indicates its suitability for cyclic processes. The slight decrease in removal rate can be attributed to the accumulation of lead or other contaminants on the material’s surface, which can be mitigated through effective regeneration techniques to maintain optimal performance over extended periods.
- (3)
- The material showed different surface morphologies and microstructural features under different conditions. Particularly, the material synthesized at 750 °C possessed a greater number of active sites, making them advantageous for the adsorption of Pb2+.
Author Contributions
Methodology, Y.L.; software, H.W.; formal analysis, T.P.; investigation, T.Z.; resources, J.J.; data curation, L.W.; writing—original draft preparation, P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Gansu Provincial Science and Technology Plan-Key R&D Program Project (No. 23YFGA0073), the Lanzhou City Science and Technology Plan Project (No. 2023-3-76), and the Fundamental Research Funds for the Central Universities (No. 31920230143).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy metal pollution in the environment and its impact on health: Exploring green technology for remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Singh, R.; Shekhar, A. Effects of Lead: Neurological and Cellular Perspective. In Lead Toxicity Mitigation: Sustainable Nexus Approaches; Springer Nature: Cham, Switzerland, 2024; pp. 17–33. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Q.; Liu, Y.; Zhu, J.; Deng, Y.; Fu, Q.; Hu, H. Co-Pyrolysis Biochar Derived from Rape Straw and Phosphate Rock: Carbon Retention, Aromaticity, and Pb Removal Capacity. Energy Fuels 2019, 33, 413–419. [Google Scholar] [CrossRef]
- Farrell, P.; Tsakalidou, K. Recent Trends in the Re-Integration of Pupils with Emotional and Behavioural Difficulties in the United Kingdom. Sch. Psychol. Int. 1999, 20, 323–337. [Google Scholar] [CrossRef]
- Hu, X.-F.; DU, Y.; Feng, J.-W.; Fang, S.-Q.; Gao, X.-J.; Xu, S.-Y. Spatial and Seasonal Variations of Heavy Metals in Wetland Soils of the Tidal Flats in the Yangtze Estuary, China: Environmental Implications. Pedosphere 2013, 23, 511–522. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Zeller, M.; Netsch, N.; Richter, F.; Leibold, H.; Stapf, D. Chemical Recycling of Mixed Plastic Wastes by Pyrolysis—Pilot Scale Investigations. Chem. Ing. Tech. 2021, 93, 1763–1770. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T.; Jamoussi, F.; Bouaziz, S. Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J. Environ. Manag. 2012, 93, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Merrikhpour, H.; Jalali, M. Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Technol. Environ. Policy 2012, 14, 845–855. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Cui, M.-H.; Ren, Y.-G.; Guo, J.-C.; Zheng, Z.-Y.; Liu, H. Towards Understanding the Mechanism of Heavy Metals Immobilization in Biochar Derived from Co-pyrolysis of Sawdust and Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.X.; Xia, M.W.; Yang, H.P.; Chen, Y.Q.; Chen, X.; Che, Q.F.; Chen, H.P. Catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae with biochar catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Phakedi, D.; Ude, A.U.; Oladijo, P.O. Co-pyrolysis of polymer waste and carbon-based matter as an alternative for waste management in the developing world. J. Anal. Appl. Pyrolysis 2021, 155, 105077. [Google Scholar] [CrossRef]
- Heitkemper, D.T.; Vela, N.P.; Stewart, K.R.; Westphal, C.S. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2001, 16, 299–306. [Google Scholar] [CrossRef]
- Kroslakova, I.; Günther, D. Elemental fractionation in laser ablation-inductively coupled plasma-mass spectrometry: Evidence for mass load induced matrix effects in the ICP during ablation of a silicate glass. J. Anal. At. Spectrom. 2007, 22, 51–62. [Google Scholar] [CrossRef]
- Aydinli, B.; Caglar, A. A degradation kinetic study on pyrolysis of three biomass samples and co-pyrolysis of hazelnut shell and ultra-high molecular weight polyethylene blends using a silver indicator. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 900–908. [Google Scholar] [CrossRef]
- Bernardo, M.S.; Lapa, N.; Barbosa, R.; Gonçalves, M.; Mendes, B.; Pinto, F.; Gulyurtlu, I. Chemical and ecotoxicological characterization of solid residues produced during the co-pyrolysis of plastics and pine biomass. J. Hazard. Mater. 2009, 166, 309–317. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.J.; Park, Y.J. Removal of Pb (II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]
- Armenise, S.; Wong, S.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Nyakuma, B.B.; Rams, J.; Muñoz, M. Application of computational approach in plastic pyrolysis kinetic modelling: A review. React. Kinet. Catal. Lett. 2021, 134, 591–614. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.; Zhang, C.; Feng, X.; Kong, X.; Wang, X.; Wang, S. High removal efficiency of tetracycline (TC) by biochar-supported zerovalent iron composite prepared by co-pyrolysis of hematite and pinewood. Environ. Pollut. Bioavailab. 2021, 33, 247–254. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process. Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Wang, B.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J. Mater. Res. Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Zhang, Y.; Li, H. Pulse-electrodeposited Ni/W-Al2O3 nanocomposites at different current densities. J. Nanoparticle Res. 2023, 25, 208. [Google Scholar] [CrossRef]
- Almezgagi, M.; Kaya, G.G.; Kar, Y.; Deveci, H. Biochar produced from co-pyrolysis of olive pomace & crude oil as an adsorbent for Cr (vi) removal from aqueous solutions. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2022, 41, 1199–1210. [Google Scholar]
- Ma, C.; He, H.; Xia, F.; Xiao, Z.; Liu, Y. Performance of Ni-SiC composites deposited using magnetic-field-assisted electrodeposition under different magnetic-field directions. Ceram. Int. 2023, 49, 35907–35916. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).