Study on Co-Pyrolysis and Characteristics of Calcite/Biochar Composites
Abstract
1. Introduction
2. Methods and Processes
2.1. Carbonized Coconut Shell Composites
2.2. Pb(NO3)2 Solution Simulation of Industrial Wastewater
2.3. Adsorption Capacity Test
2.4. Characterization
3. Results and Discussion
3.1. Analysis of Adsorption Capacity
3.2. Adsorption Mechanism of Composite Materials
3.3. Electrochemical Properties of CP-CAL/BC Composite Materials
3.4. Properties of CP-CAL/BC Composites in Recycling
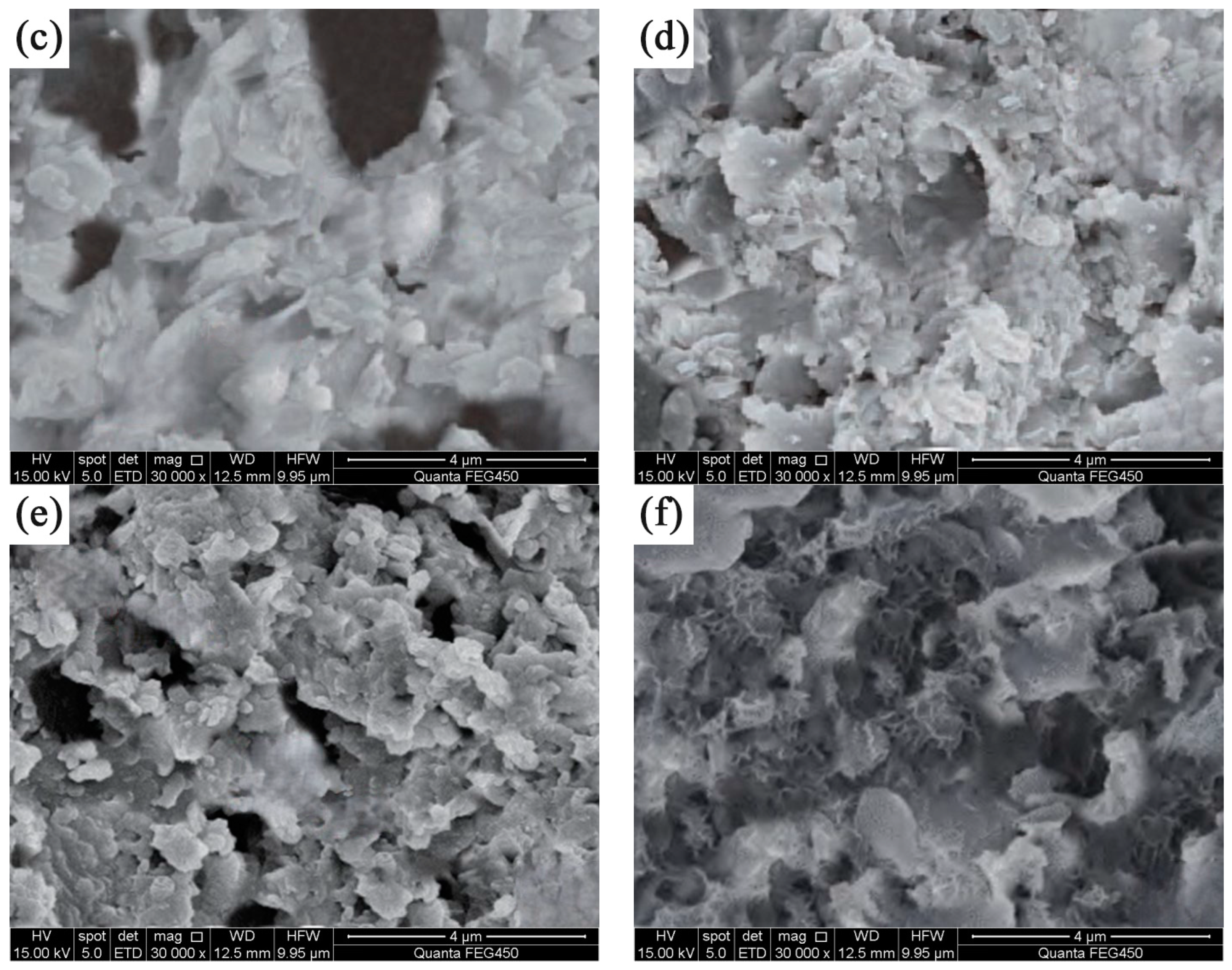
3.5. Characterization of CP-CAL/BC Composite Material
4. Conclusions
- (1)
- When embarking on the preparation process with an initial pH range of 4.0 to 5.0, optimal adsorption efficiency is achieved at a pH of 4.5. The extraction of lead ions from the CP-CAL/BC composite material is thought to unfold through a multifaceted mechanism. At the identified optimal pH, the surface charge of the composite and the solubility of lead ions are ideally balanced, facilitating the most effective adsorption. Further deviations from this pH could disrupt these interactions, leading to less efficient lead ion removal.
- (2)
- The material exhibited exceptional resilience against corrosion at an elevated temperature of 750 °C, markedly surpassing its performance at lower development conditions of 650 and 700 °C. This characteristic is critical for applications in high-temperature environments, where corrosion can significantly impact material integrity and operational efficiency. Moreover, the material’s ability to retain a high level of lead removal performance over multiple cycles, despite slight efficiency losses, indicates its suitability for cyclic processes. The slight decrease in removal rate can be attributed to the accumulation of lead or other contaminants on the material’s surface, which can be mitigated through effective regeneration techniques to maintain optimal performance over extended periods.
- (3)
- The material showed different surface morphologies and microstructural features under different conditions. Particularly, the material synthesized at 750 °C possessed a greater number of active sites, making them advantageous for the adsorption of Pb2+.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy metal pollution in the environment and its impact on health: Exploring green technology for remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Singh, R.; Shekhar, A. Effects of Lead: Neurological and Cellular Perspective. In Lead Toxicity Mitigation: Sustainable Nexus Approaches; Springer Nature: Cham, Switzerland, 2024; pp. 17–33. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Q.; Liu, Y.; Zhu, J.; Deng, Y.; Fu, Q.; Hu, H. Co-Pyrolysis Biochar Derived from Rape Straw and Phosphate Rock: Carbon Retention, Aromaticity, and Pb Removal Capacity. Energy Fuels 2019, 33, 413–419. [Google Scholar] [CrossRef]
- Farrell, P.; Tsakalidou, K. Recent Trends in the Re-Integration of Pupils with Emotional and Behavioural Difficulties in the United Kingdom. Sch. Psychol. Int. 1999, 20, 323–337. [Google Scholar] [CrossRef]
- Hu, X.-F.; DU, Y.; Feng, J.-W.; Fang, S.-Q.; Gao, X.-J.; Xu, S.-Y. Spatial and Seasonal Variations of Heavy Metals in Wetland Soils of the Tidal Flats in the Yangtze Estuary, China: Environmental Implications. Pedosphere 2013, 23, 511–522. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar] [CrossRef]
- Zeller, M.; Netsch, N.; Richter, F.; Leibold, H.; Stapf, D. Chemical Recycling of Mixed Plastic Wastes by Pyrolysis—Pilot Scale Investigations. Chem. Ing. Tech. 2021, 93, 1763–1770. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T.; Jamoussi, F.; Bouaziz, S. Effects of impurities on the removal of heavy metals by natural limestones in aqueous systems. J. Environ. Manag. 2012, 93, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Merrikhpour, H.; Jalali, M. Waste calcite sludge as an adsorbent for the removal of cadmium, copper, lead, and zinc from aqueous solutions. Clean Technol. Environ. Policy 2012, 14, 845–855. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Cui, M.-H.; Ren, Y.-G.; Guo, J.-C.; Zheng, Z.-Y.; Liu, H. Towards Understanding the Mechanism of Heavy Metals Immobilization in Biochar Derived from Co-pyrolysis of Sawdust and Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.X.; Xia, M.W.; Yang, H.P.; Chen, Y.Q.; Chen, X.; Che, Q.F.; Chen, H.P. Catalytic deoxygenation co-pyrolysis of bamboo wastes and microalgae with biochar catalyst. Energy 2018, 157, 472–482. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Phakedi, D.; Ude, A.U.; Oladijo, P.O. Co-pyrolysis of polymer waste and carbon-based matter as an alternative for waste management in the developing world. J. Anal. Appl. Pyrolysis 2021, 155, 105077. [Google Scholar] [CrossRef]
- Heitkemper, D.T.; Vela, N.P.; Stewart, K.R.; Westphal, C.S. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2001, 16, 299–306. [Google Scholar] [CrossRef]
- Kroslakova, I.; Günther, D. Elemental fractionation in laser ablation-inductively coupled plasma-mass spectrometry: Evidence for mass load induced matrix effects in the ICP during ablation of a silicate glass. J. Anal. At. Spectrom. 2007, 22, 51–62. [Google Scholar] [CrossRef]
- Aydinli, B.; Caglar, A. A degradation kinetic study on pyrolysis of three biomass samples and co-pyrolysis of hazelnut shell and ultra-high molecular weight polyethylene blends using a silver indicator. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 900–908. [Google Scholar] [CrossRef]
- Bernardo, M.S.; Lapa, N.; Barbosa, R.; Gonçalves, M.; Mendes, B.; Pinto, F.; Gulyurtlu, I. Chemical and ecotoxicological characterization of solid residues produced during the co-pyrolysis of plastics and pine biomass. J. Hazard. Mater. 2009, 166, 309–317. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Zhang, Z.; Sun, J.; Wang, Q.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Kamala-Kannan, S.; Lee, K.J.; Park, Y.J. Removal of Pb (II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem. Eng. J. 2013, 217, 54–60. [Google Scholar] [CrossRef]
- Armenise, S.; Wong, S.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Nyakuma, B.B.; Rams, J.; Muñoz, M. Application of computational approach in plastic pyrolysis kinetic modelling: A review. React. Kinet. Catal. Lett. 2021, 134, 591–614. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.; Zhang, C.; Feng, X.; Kong, X.; Wang, X.; Wang, S. High removal efficiency of tetracycline (TC) by biochar-supported zerovalent iron composite prepared by co-pyrolysis of hematite and pinewood. Environ. Pollut. Bioavailab. 2021, 33, 247–254. [Google Scholar] [CrossRef]
- Xu, S.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.; Hu, Y.; Wang, Q.; Nwakaire, J.N. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process. Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Wang, B.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J. Mater. Res. Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Zhang, Y.; Li, H. Pulse-electrodeposited Ni/W-Al2O3 nanocomposites at different current densities. J. Nanoparticle Res. 2023, 25, 208. [Google Scholar] [CrossRef]
- Almezgagi, M.; Kaya, G.G.; Kar, Y.; Deveci, H. Biochar produced from co-pyrolysis of olive pomace & crude oil as an adsorbent for Cr (vi) removal from aqueous solutions. Iran. J. Chem. Chem. Eng.-Int. Engl. Ed. 2022, 41, 1199–1210. [Google Scholar]
- Ma, C.; He, H.; Xia, F.; Xiao, Z.; Liu, Y. Performance of Ni-SiC composites deposited using magnetic-field-assisted electrodeposition under different magnetic-field directions. Ceram. Int. 2023, 49, 35907–35916. [Google Scholar] [CrossRef]








| CP-CAL/BC | Icorr (A/cm2) | Ecorr (V) |
|---|---|---|
| 650 °C | 9.732 × 10−5 | −0.71 |
| 700 °C | 4.581 × 10−5 | −0.44 |
| 750 °C | 7.298 × 10−6 | −0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, H.; Pan, T.; Zhong, T.; Jiang, J.; Wei, L.; Jin, P. Study on Co-Pyrolysis and Characteristics of Calcite/Biochar Composites. Coatings 2024, 14, 1044. https://doi.org/10.3390/coatings14081044
Li Y, Wang H, Pan T, Zhong T, Jiang J, Wei L, Jin P. Study on Co-Pyrolysis and Characteristics of Calcite/Biochar Composites. Coatings. 2024; 14(8):1044. https://doi.org/10.3390/coatings14081044
Chicago/Turabian StyleLi, Yaxuan, Haoyang Wang, Tuo Pan, Tianran Zhong, Jing Jiang, Lihui Wei, and Pen Jin. 2024. "Study on Co-Pyrolysis and Characteristics of Calcite/Biochar Composites" Coatings 14, no. 8: 1044. https://doi.org/10.3390/coatings14081044
APA StyleLi, Y., Wang, H., Pan, T., Zhong, T., Jiang, J., Wei, L., & Jin, P. (2024). Study on Co-Pyrolysis and Characteristics of Calcite/Biochar Composites. Coatings, 14(8), 1044. https://doi.org/10.3390/coatings14081044





