Fabrication of Vanadate-Exchanged Electrodeposited Zn-Al Layered Double Hydroxide (LDH) Coating on a ZX21 Mg Alloy to Improve the Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrications of the Zn-Al LDH Coatings
2.1.1. Electrodeposition of the Zn-Al LDH Coating
2.1.2. Vanadate-Exchange of the Electrodeposited Zn-Al LDH Coating
2.2. Microstructure Characterizations of the Zn-Al LDH Coatings
2.3. Corrosion and Electrochemical Measurements
2.3.1. Electrochemical Measurements
2.3.2. Artificial Scratch Tests
3. Results
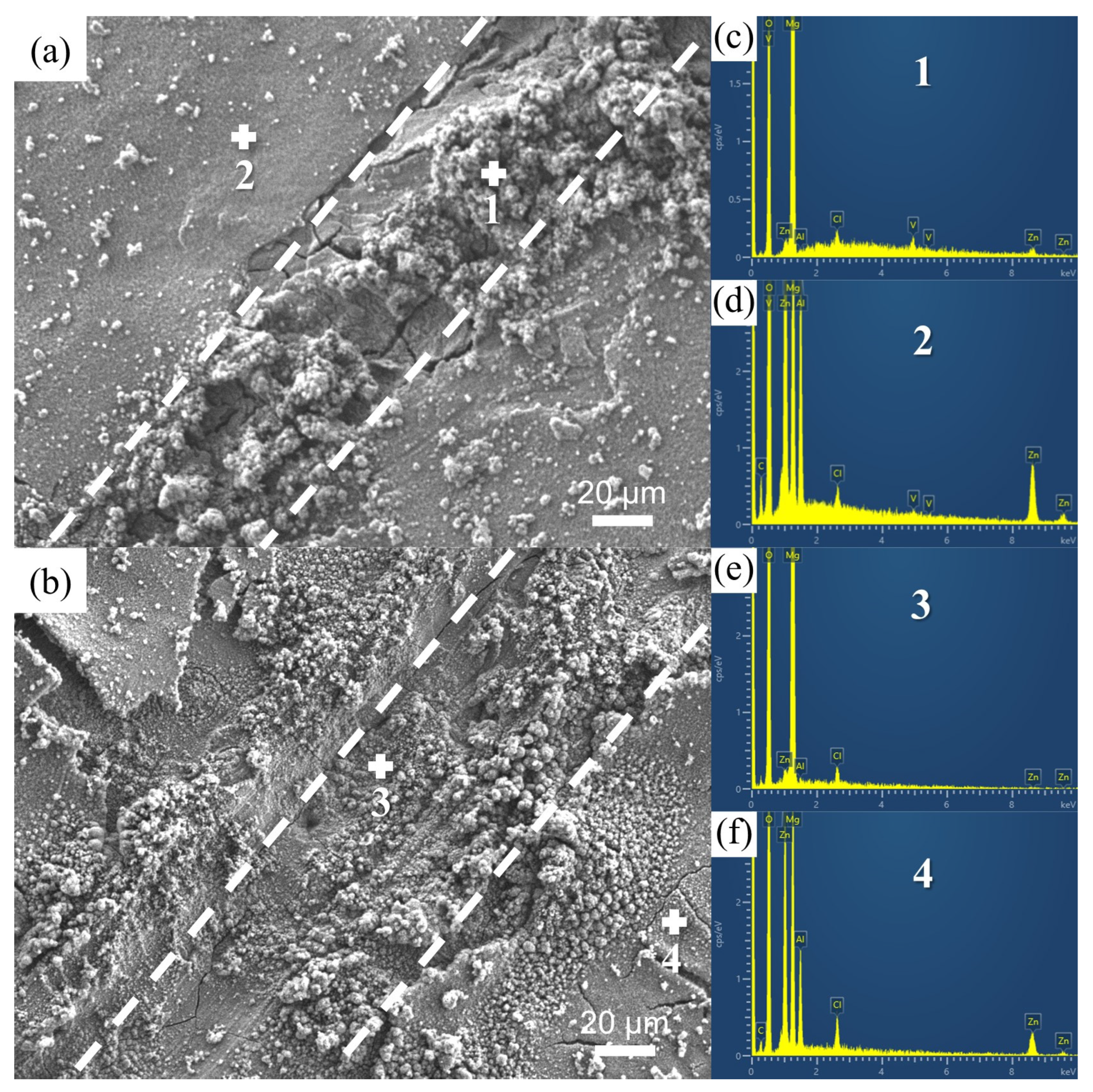
3.1. Microstructure Characterizations of the Zn-Al LDH Coatings
3.2. Electrochemical Measurements
3.2.1. Potentiodynamic Polarization Curves
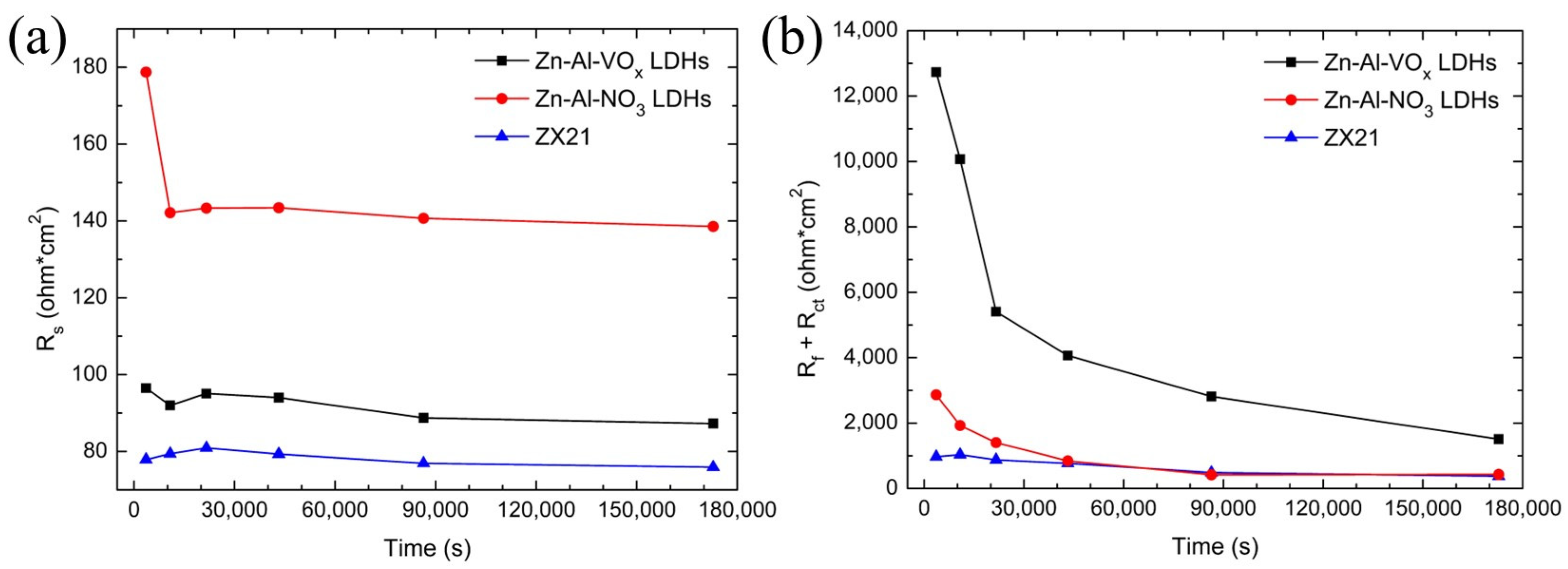
3.2.2. Electrochemical Impedance Spectroscopy (EIS) Measurements
3.3. Artificial Scratch Tests
4. Discussion
4.1. Microstructure of the Fabricated Zn-Al LDH Coatings
4.2. Corrosion Inhibition of the Vanadate-Exchanged Electrodeposited Zn-Al LDH Coating
5. Conclusions
- The fabricated vanadate-exchanged electrodeposited Zn-Al-VOx LDH coating contains complex ions in the interlayers, mainly NO3−, CO32−, and different vanadates (dominated by VO3(OH)2− and V2O74−). The vanadates in the interlayers play important roles in the corrosion inhibition of the ZX21 Mg alloy.
- The corrosion current density of the ZX21 Mg alloy decreases from 62.4 μA/cm2 to 3.32 μA/cm2 with the vanadate-exchanged electrodeposited Zn-Al-VOx LDH coating. The Zn-Al-VOx LDH coating not only serves as a physical barrier but also absorbs Cl− ions in the environment and inhibits corrosion with the reduction of the interlayer vanadates. Furthermore, the vanadates in the LDH coating can also be released to the damaged area of the coating.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avedesian, M.M.; Baker, H. Magnesium and Magnesium Alloys; ASM International: Park, OH, USA, 1999. [Google Scholar]
- Kainer, K.U. Magnesium Alloys and Their Applications; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Friedrich, H.E.; Mordike, B.L. Magnesium Technology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 212. [Google Scholar]
- Xu, T.C.; Yang, Y.; Peng, X.D.; Song, J.F.; Pan, F.S. Overview of Advancement and Development Trend on Magnesium Alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Corrosion Protection of Magnesium ZM21 Alloy with Polyaniline–TiO2 Composite Containing Coatings. Prog. Org. Coat. 2007, 59, 291–296. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Zhong, X.; Zhang, L.; Chen, B. Composite Anticorrosion Coatings for AZ91D Magnesium Alloy with Molybdate Conversion Coating and Silicon Sol–Gel Coatings. Prog. Org. Coat. 2009, 66, 199–205. [Google Scholar] [CrossRef]
- Yu, L.; Cao, J.; Cheng, Y. An Improvement of the Wear and Corrosion Resistances of AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation in a Silicate–Hexametaphosphate Electrolyte with the Suspension of SiC Nanoparticles. Surf. Coat. Technol. 2015, 276, 266–278. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, R.; Wang, J.; Li, Q.; Xing, X.; Liu, X.; Hu, W. Fabrication and Corrosion Resistance of Phosphate/ZnO Multilayer Protective Coating on Magnesium Alloy. Surf. Coat. Technol. 2018, 352, 74–83. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Kazempour, A. Salt-Nanoparticle Systems Incorporated into Sol-Gel Coatings for Corrosion Protection of AZ91 Magnesium Alloy. Prog. Org. Coat. 2019, 135, 475–482. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Belov, E.A.; Imshinetskiy, I.M.; Sinebrukhov, S.L.; Gnedenkov, S.V. Features of Composite Layers Created Using An Aqueous Suspension of A Fluoropolymer. Polymers 2022, 14, 4667. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Slade, R.C.T. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Duan, X., Evans, D.G., Eds.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2006; Volume 119, pp. 1–87. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T.; Dong, Z. Application of Layered Double Hydroxides for Removal of Oxyanions: A Review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, L.C.; Ling, H.; Diao, Y.P.; Pang, X.L.; Wang, Y.L.; Gao, K.W. Design and Fabrication of Enhanced Corrosion Resistance Zn-Al Layered Double Hydroxides Films Based Anion-Exchange Mechanism on Magnesium Alloys. Appl. Surf. Sci. 2017, 404, 246–253. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.F.; Zhang, F.; Zeng, R.C.; Zeng, J.M. Layered Double Hydroxide Coatings on Magnesium Alloys: A Review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Kasneryk, V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L. LDH Has Been Grown: What Is Next? Overview on Methods of Post-Treatment of LDH Conversion Coatings. Appl. Clay Sci. 2023, 232, 106774. [Google Scholar] [CrossRef]
- Lin, J.K.; Uan, J.Y. Formation of Mg,Al-Hydrotalcite Conversion Coating on Mg Alloy in Aqueous HCO3−/CO32− and Corresponding Protection against Corrosion by the Coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Wang, L.D.; Zhang, K.Y.; Sun, W.; Wu, T.T.; He, H.R.; Liu, G.C. Hydrothermal Synthesis of Corrosion Resistant Hydrotalcite Conversion Coating on AZ91D Alloy. Mater. Lett. 2013, 106, 111–114. [Google Scholar] [CrossRef]
- Yarger, M.S.; Steinmiller, E.M.P.; Choi, K.S. Electrochemical Synthesis of Zn-Al Layered Double Hydroxide (LDH) Films. Inorg. Chem. 2008, 47, 5859–5865. [Google Scholar] [CrossRef]
- Syu, J.H.; Uan, J.Y.; Lin, M.C.; Lin, Z.Y. Optically Transparent Li-Al-CO3 Layered Double Hydroxide Thin Films on An AZ31 Mg Alloy Formed by Electrochemical Deposition and Their Corrosion Resistance in a Dilute Chloride Environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Wu, F.X.; Liang, J.; Peng, Z.J.; Liu, B.X. Electrochemical Deposition and Characterization of Zn-Al Layered Double Hydroxides (LDHs) Films on Magnesium Alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- He, Q.Q.; Zhou, M.J.; Hu, J.M. Electrodeposited Zn-Al Layered Double Hydroxide Films for Corrosion Protection of Aluminum Alloys. Electrochim. Acta 2020, 355, 136796. [Google Scholar] [CrossRef]
- Chu, P.W.; Fan, C.W.; Yang, C.H. Corrosion Behavior and Microstructure of the Surface Corrosion Film of Biodegradable WE43 and ZX21 Mg Alloys in Hanks’ Balanced Salt Solution. Mater. Chem. Phys. 2024, 312, 128609. [Google Scholar] [CrossRef]
- Feliu, S.F. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Benbouzid, A.Z.; Gomes, M.P.; Costa, I.; Gharbi, O.; Pébère, N.; Rossi, J.L.; Tran, M.T.T.; Tribollet, B.; Turmine, M.; Vivier, V. A New Look on the Corrosion Mechanism of Magnesium: An EIS Investigation at Different pH. Corros. Sci. 2022, 205, 110463–110474. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S.F. A Comparative Study and Optimization of Corrosion Resistance of ZnAl Layered Double Hydroxides Films Intercalated with Different Anions on AZ31 Mg Alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Zhao, X.J.; Zhu, Y.Q.; Xu, S.M.; Liu, H.M.; Yin, P.; Feng, Y.L.; Yan, H. Anion Exchange Behavior of (MAl)-Al-II Layered Double Hydroxides: A Molecular Dynamics and DFT Study. Phys. Chem. Chem. Phys. 2020, 22, 19758–19768. [Google Scholar] [CrossRef] [PubMed]
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Jensen, O.E.; Cliffe, K.A.; Maroto-Valer, M.M. A Model of Carbon Dioxide Dissolution and Mineral Carbonation Kinetics. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2010, 466, 1265–1290. [Google Scholar] [CrossRef]
- Wijitwongwan, R.; Intasa-Ard, S.; Ogawa, M. Preparation of Layered Double Hydroxides toward Precisely Designed Hierarchical Organization. ChemEngineering 2019, 3, 68. [Google Scholar] [CrossRef]
- Jang, W.; Yoon, S.; Song, J.; Kim, J.; An, K.; Cho, S. Selective Phase Transformation of Layered Double Hydroxides into Mixed Metal Oxides for Catalytic CO Oxidation. Cell Rep. Phys. Sci. 2021, 2, 100628. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Sajieddine, M.; Ounacer, M.; Fnidiki, A.; Richomme, F.; Juraszek, J.; Mansori, M.; Dib, M.; Essoumhi, A. Structural, Morphological, and Magnetic Studies of Spinel Ferrites Derived from Layered Double Hydroxides. Appl. Phys. A-Mater. Sci. Process. 2022, 128, 406. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Farashi, S. Fabrication and Characterization of PO43− Intercalated Zn-Al-Layered Double Hydroxide Nanocontainer. J. Electrochem. Soc. 2016, 163, C495–C505. [Google Scholar] [CrossRef]
- Cao, Y.H.; Zheng, D.J.; Dong, S.G.; Zhang, F.; Lin, J.Y.; Wang, C.; Lin, C.J. A Composite Corrosion Inhibitor of MgAl Layered Double Hydroxides Co-Intercalated with Hydroxide and Organic Anions for Carbon Steel in Simulated Carbonated Concrete Pore Solutions. J. Electrochem. Soc. 2019, 166, C3106–C3113. [Google Scholar] [CrossRef]
- Iuzviuk, M.H.; Bouali, A.C.; Serdechnova, M.; Yasakau, K.A.; Wieland, D.C.F.; Dovzhenko, G.; Mikhailau, A.; Blawert, C.; Zobkalo, I.A.; Ferreira, M.G.S.; et al. In Situ Kinetics Studies of Zn-Al LDH Intercalation with Corrosion Related Species. Phys. Chem. Chem. Phys. 2020, 22, 17574–17586. [Google Scholar] [CrossRef]
- Roy, A.S.; Pillai, S.K.; Ray, S.S. A Comparison of Nitrate Release from Zn/Al-, Mg/Al-, and Mg-Zn/Al Layered Double Hydroxides and Composite Beads: Utilization as Slow-Release Fertilizers. ACS Omega 2023, 8, 8427–8440. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Thakur, K.P. Reappraisal of Thermochemical Radii for Complex-Ions. J. Chem. Educ. 1979, 56, 576–577. [Google Scholar] [CrossRef]
- Ralston, K.D.; Chrisanti, S.; Young, T.L.; Buchheit, R.G. Corrosion Inhibition of Aluminum Alloy 2024-T3 by Aqueous Vanadium Species. J. Electrochem. Soc. 2008, 155, C350–C359. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Poznyak, S.K.; Rodrigues, L.M.; Raps, D.; Hack, T.; Dick, L.F.; Nunes, T.; Ferreira, M.G.S. Active Protection Coatings with Layered Double Hydroxide Nanocontainers of Corrosion Inhibitor. Corros. Sci. 2010, 52, 602–611. [Google Scholar] [CrossRef]
- Salak, A.N.; Tedim, J.; Kuznetsova, A.I.; Ribeiro, J.L.; Vieira, L.G.; Zheludkevich, M.L.; Ferreira, M.G.S. Comparative X-ray Diffraction and Infrared Spectroscopy Study of Zn-Al Layered Double Hydroxides: Vanadate vs. Nitrate. Chem. Phys. 2012, 397, 102–108. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. Mechanisms of Corrosion Inhibition of AA2024-T3 by Vanadates. Corros. Sci. 2007, 49, 2371–2391. [Google Scholar] [CrossRef]
- Feng, Z. Corrosion Inhibition Study of AZ31 Mg Alloy by Vanadate, Selenite and Phosphate; The Ohio State University: Columbus, OH, USA, 2019. [Google Scholar]
- Kuznetsov, B.; Serdechnova, M.; Tedim, J.; Starykevich, M.; Kallip, S.; Oliveira, M.P.; Hack, T.; Nixon, S.; Ferreira, M.G.S.; Zheludkevich, M.L. Sealing of Tartaric Sulfuric (TSA) Anodized AA2024 with Nanostructured LDH Layers. RSC Adv. 2016, 6, 13942–13952. [Google Scholar] [CrossRef]
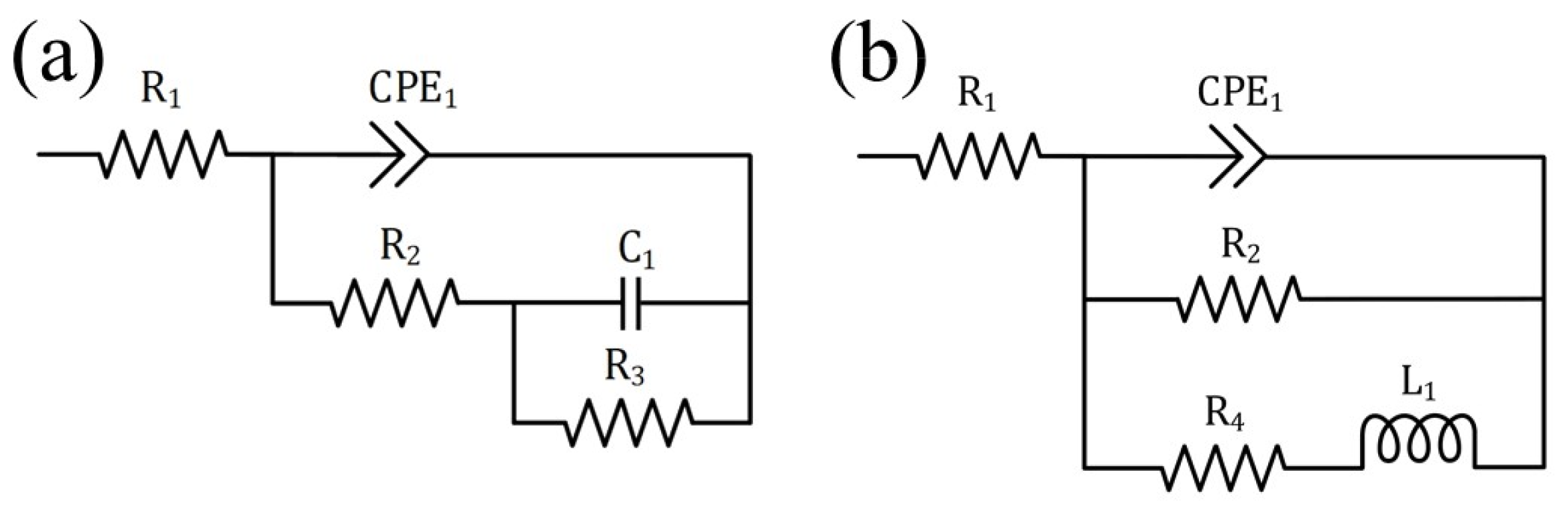
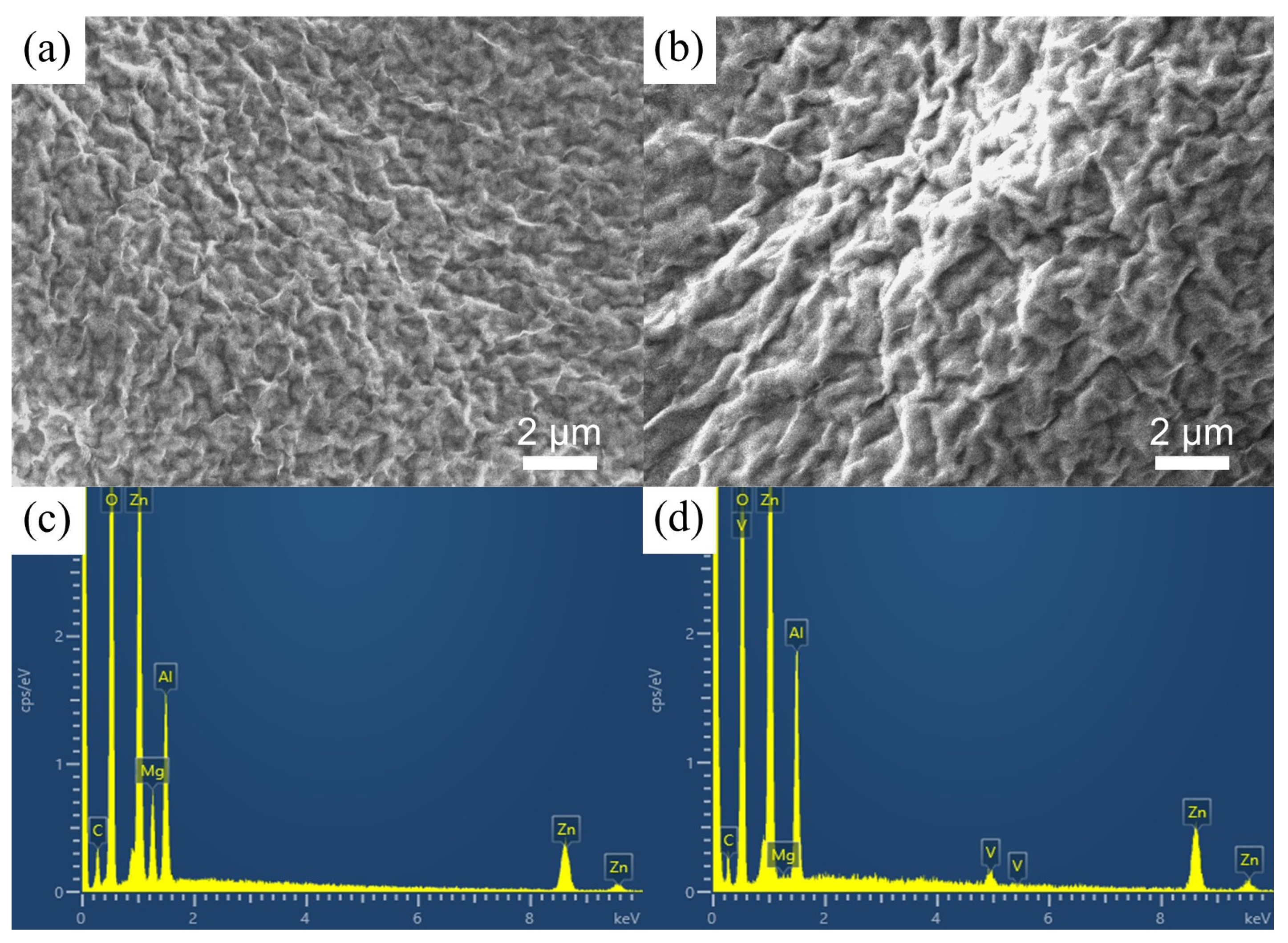

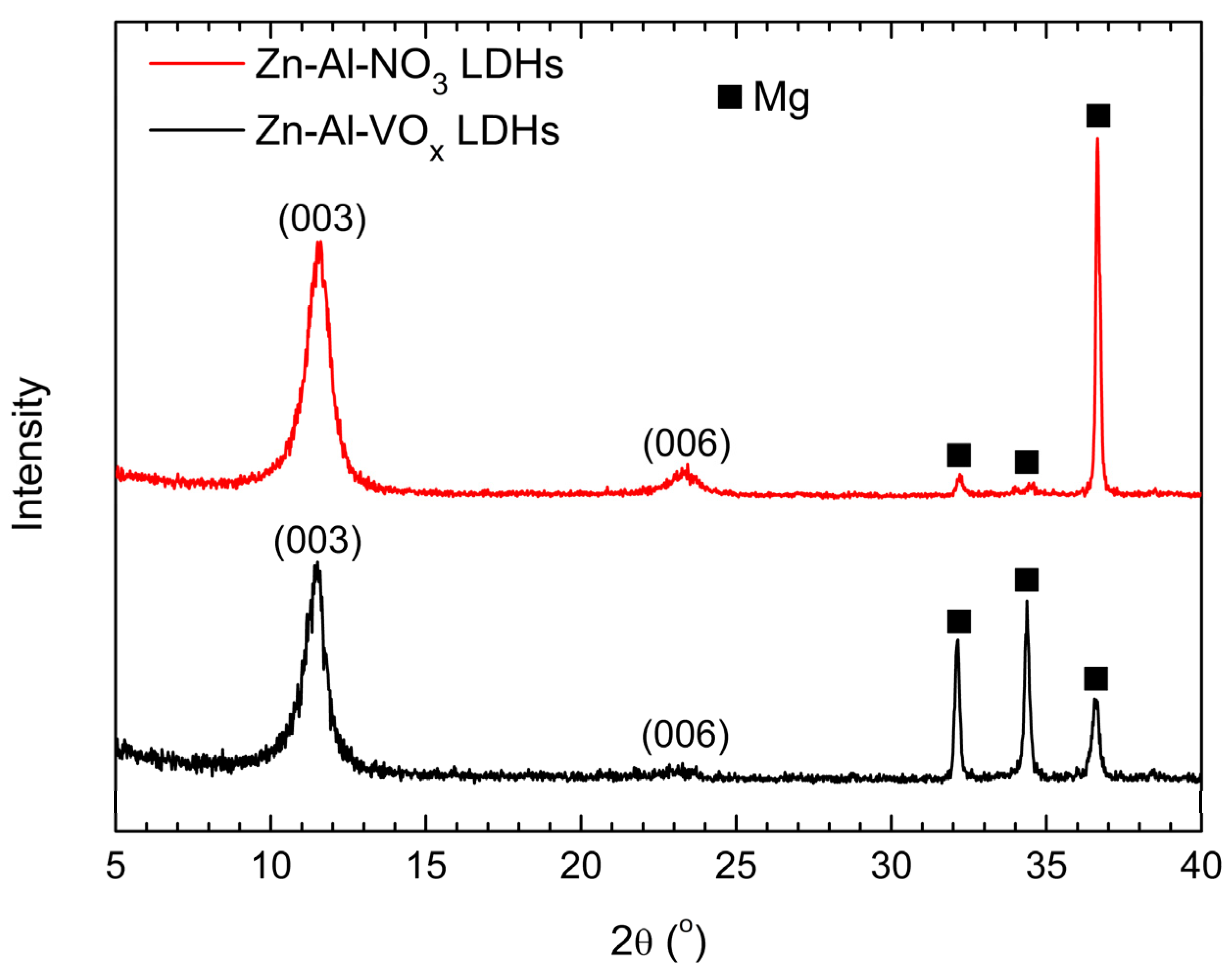
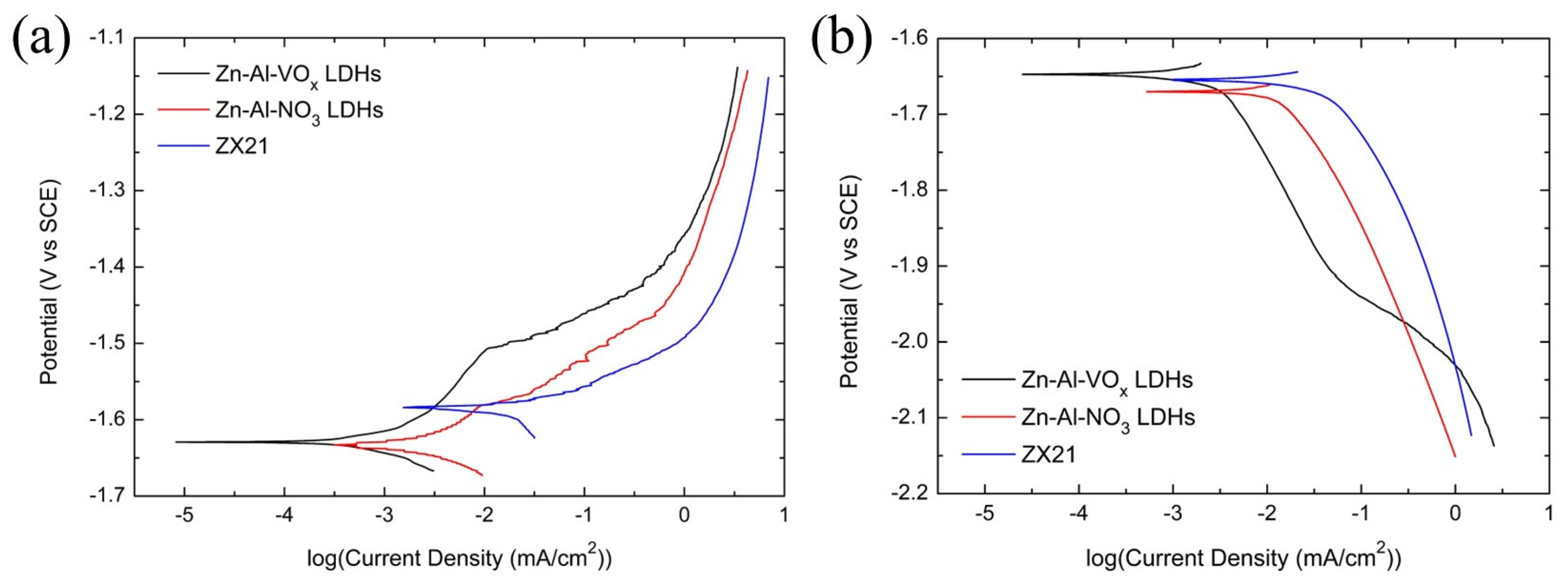

| Item | (003) Diffraction Peak Position (2θ) | FWHM |
|---|---|---|
| Zn-Al-NO3 LDHs | 11.52° | 0.876° |
| Zn-Al-VOx LDHs | 11.42° | 0.943° |
| Item | Ecorr (V vs. SCE) | βc (mV/dec) | icorr (μA/cm2) |
|---|---|---|---|
| Zn-Al-VOx LDHs | −1.647 | −230 | 3.32 |
| Zn-Al-NO3 LDHs | −1.670 | −239 | 18.4 |
| ZX21 Mg Alloy | −1.664 | −250 | 62.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, W.-L.; Chu, P.-W. Fabrication of Vanadate-Exchanged Electrodeposited Zn-Al Layered Double Hydroxide (LDH) Coating on a ZX21 Mg Alloy to Improve the Corrosion Resistance. Coatings 2024, 14, 1047. https://doi.org/10.3390/coatings14081047
Hsiao W-L, Chu P-W. Fabrication of Vanadate-Exchanged Electrodeposited Zn-Al Layered Double Hydroxide (LDH) Coating on a ZX21 Mg Alloy to Improve the Corrosion Resistance. Coatings. 2024; 14(8):1047. https://doi.org/10.3390/coatings14081047
Chicago/Turabian StyleHsiao, Wei-Lun, and Peng-Wei Chu. 2024. "Fabrication of Vanadate-Exchanged Electrodeposited Zn-Al Layered Double Hydroxide (LDH) Coating on a ZX21 Mg Alloy to Improve the Corrosion Resistance" Coatings 14, no. 8: 1047. https://doi.org/10.3390/coatings14081047


.jpg)



