Surprising Effects of Al2O3 Coating on Tribocatalytic Degradation of Organic Dyes by CdS Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Coating Al2O3 Ceramic Disks on the Bottoms of Glass Beakers
2.3. Tribocatalytic Degradation of RhB and MO Solutions
2.4. Detection of Radical Species
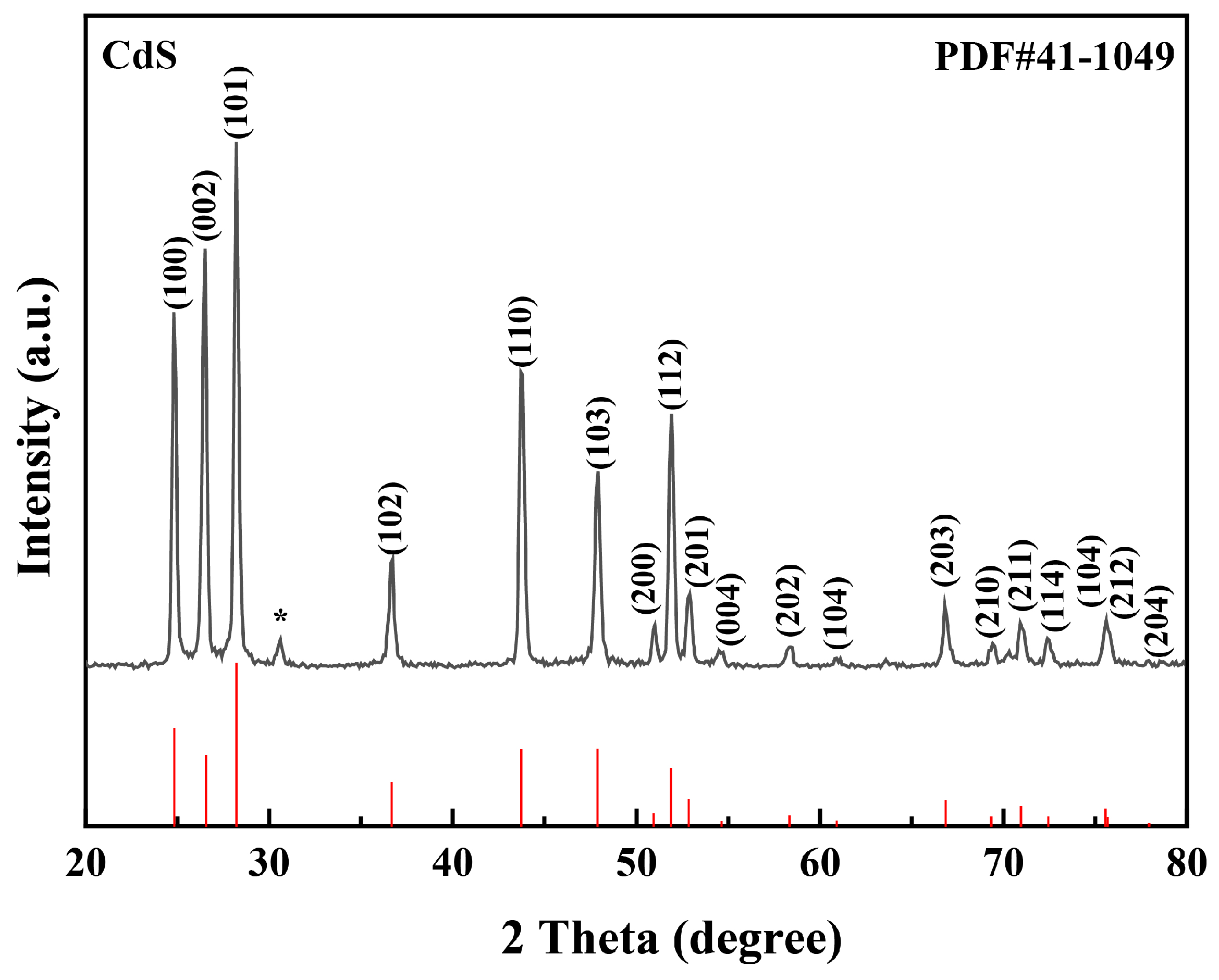
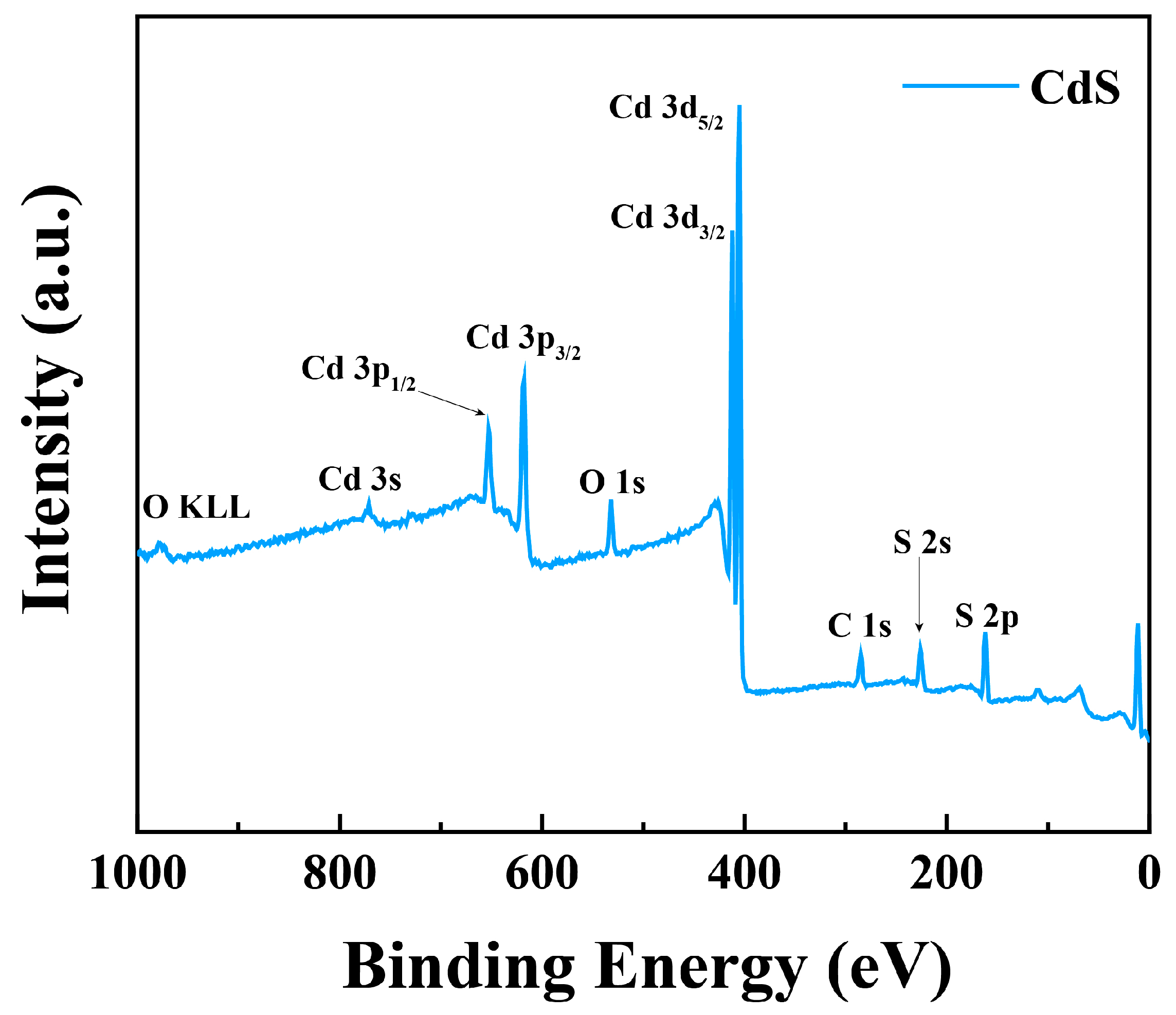
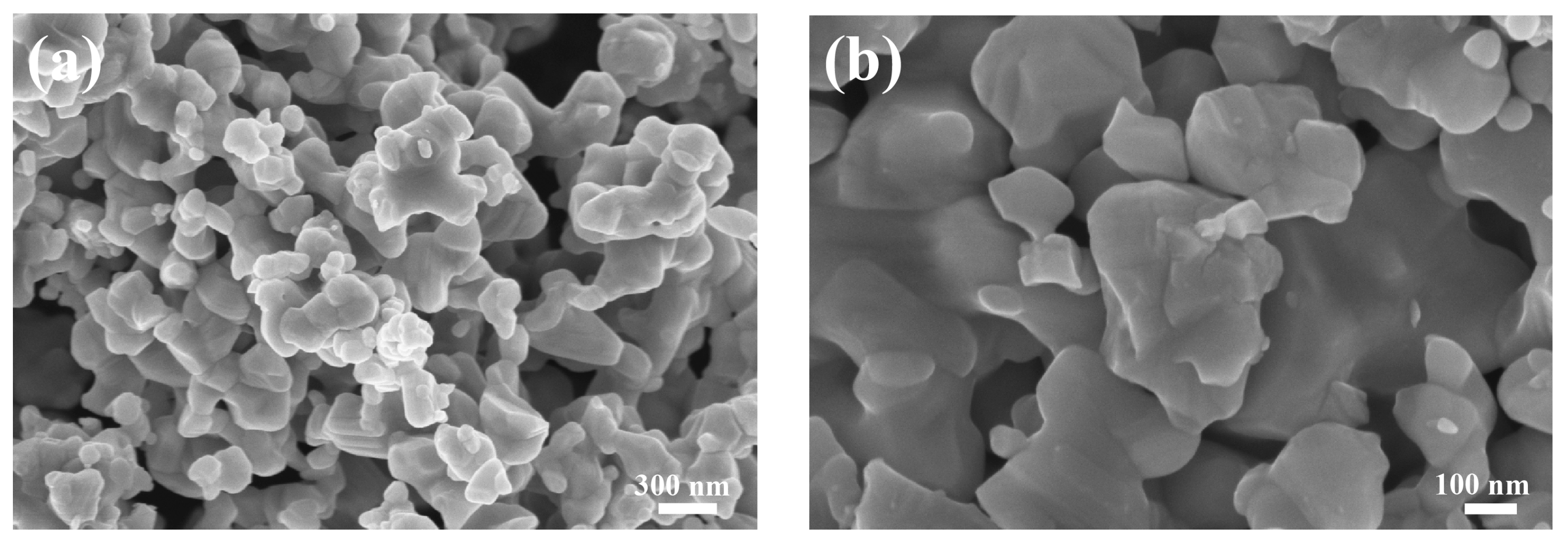
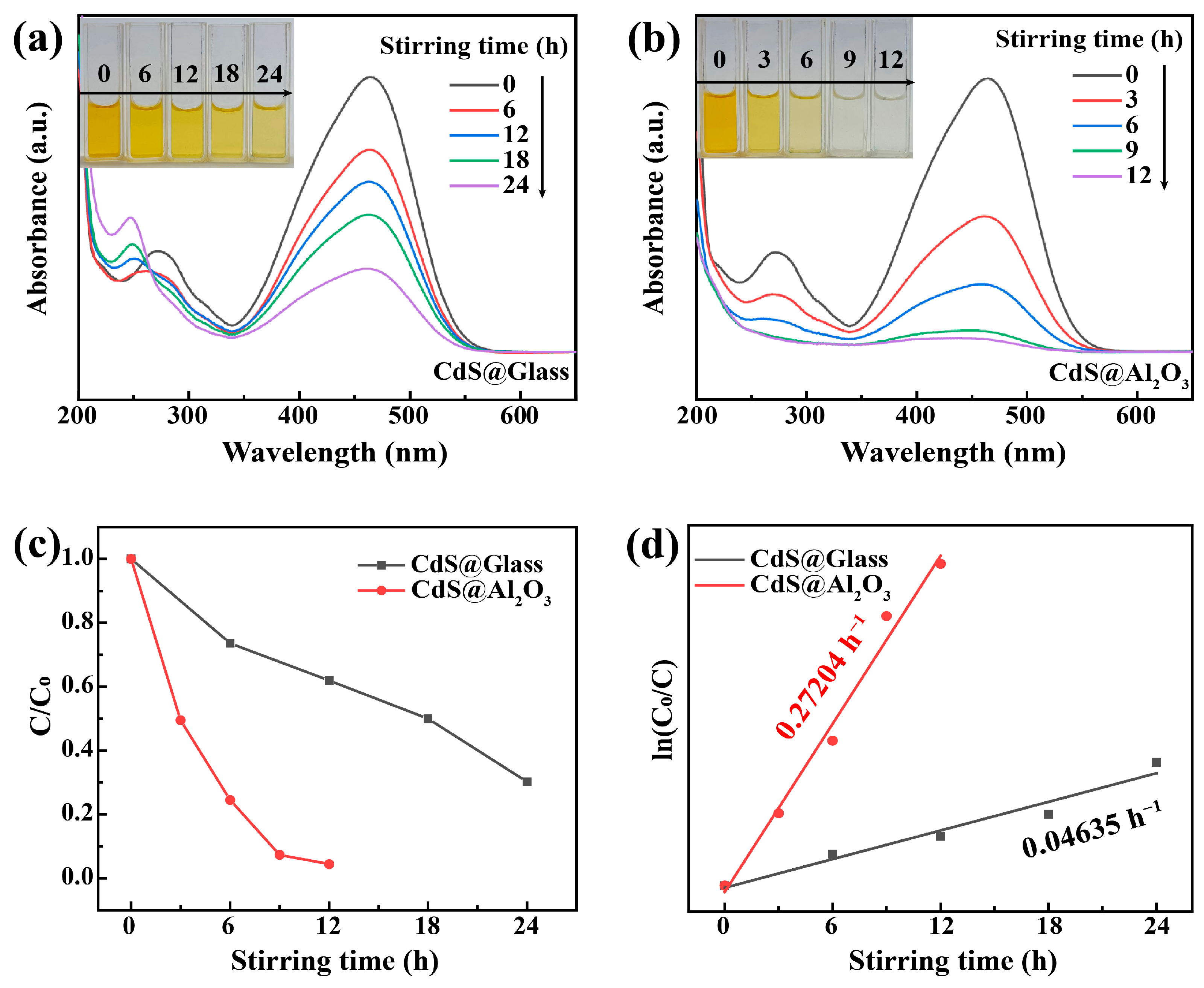
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.J.; Liao, Y.L.; Zhang, H.W. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Li, M.; Virguez, E.; Shan, R.; Tian, J.; Gao, S.; Patiño-Echeverri, D. High-resolution data shows China’s wind and solar energy resources are enough to support a 2050 decarbonized electricity system. Appl. Energy 2022, 306, 117996. [Google Scholar] [CrossRef]
- Daaboul, J.; Moriarty, P.; Honnery, D. Net green energy potential of solar photovoltaic and wind energy generation systems. J. Clean. Prod. 2023, 415, 137806. [Google Scholar] [CrossRef]
- Aljundi, K.; Figueiredo, A.; Vieira, A.; Lapa, J.; Cardoso, R. Geothermal energy system application: From basic standard performance to sustainability reflection. Renew. Energy 2024, 220, 119612. [Google Scholar] [CrossRef]
- Zhou, S.; Cao, S. Co-ordinations of ocean energy supported energy sharing between zero-emission cross-harbour buildings in the Greater Bay Area. Appl. Energy 2024, 359, 122718. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic degradation of organic pollutants through bismuth oxyiodate triboelectrically harvesting mechanical energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Li, Z.; Zhang, S.; Ren, P.; Xu, J.; Hui, Y.; Dai, Z. Contrasting piezocatalytic and tribocatalytic behavior of BaTiO3. Mater. Sci. Semicond. Process. 2024, 172, 108080. [Google Scholar] [CrossRef]
- Ma, J.; Ren, J.; Jia, Y.; Wu, Z.; Chen, L.; Haugen, N.O.; Huang, H.; Liu, Y. High efficiency bi-harvesting light/vibration energy using piezoelectric zinc oxide nanorods for dye decomposition. Nano Energy 2019, 62, 376–383. [Google Scholar] [CrossRef]
- Feng, R.; Xie, S.; Guan, W.; Zhong, Q. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar]
- Bie, C.; Wang, L.; Yu, J. Challenges for photocatalytic overall water splitting. Chem 2022, 8, 1567–1574. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.G. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5, 28. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, L.; Wu, Z.; Jia, Y.; Ye, X.; Wang, F.; Yuan, B.; Yu, Y.; Huang, H.; Zou, G. Harvesting vibration energy to piezo-catalytically generate hydrogen through Bi2WO6 layered-perovskite. Nano Energy 2020, 78, 105351. [Google Scholar] [CrossRef]
- Kajdas, C.; Hiratsuka, K. Tribochemistry, tribocatalysis, and the negative-ion-radical action mechanism. J. Eng. Tribol. 2009, 223, 827–848. [Google Scholar] [CrossRef]
- Song, W.; Li, J.F.; Zeng, C.Y.; Ouyang, C.K.; Sun, S.Y.; Wang, K.Q.; Li, J.J.; Luo, J.B. Tribo-catalysis triggered the in-situ formation of amphiphilic molecules to reduce friction and wear. Tribol. Int. 2023, 185, 10. [Google Scholar] [CrossRef]
- Gao, K.; Bin, W.; Berman, D.; Ren, Y.; Luo, J.; Xie, G. Self-Adaptive Macroscale Superlubricity Based on the Tribocatalytic Properties of Partially Oxidized Black Phosphorus. Nano Lett. 2023, 23, 6823–6830. [Google Scholar] [CrossRef]
- Li, P.C.; Wu, J.; Wu, Z.; Jia, Y.M.; Ma, J.P.; Chen, W.P.; Zhang, L.H.; Yang, J.; Liu, Y.S. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Lei, H.; Jia, X.; Wang, H.; Cui, X.; Jia, Y.; Fei, L.; Chen, W. Tribo-Catalytic Conversions of H2O and CO2 by NiO Particles in Reactors with Plastic and Metallic Coatings. Coatings 2023, 13, 396. [Google Scholar] [CrossRef]
- Cui, X.D.; Wang, H.B.; Lei, H.; Jia, X.C.; Jiang, Y.; Fei, L.F.; Jia, Y.M.; Chen, W.P. Surprising Tribo-catalytic Conversion of H2O and CO2 into Flammable Gases utilizing Frictions of Copper in Water. ChemistrySelect 2023, 8, e202204146. [Google Scholar] [CrossRef]
- Gaur, A.; Kumar Moharana, A.; Porwal, C.; Singh Chauhan, V.; Vaish, R. Degradation of organic dyes by utilizing CaCu3Ti4O12 (CCTO) nanoparticles via tribocatalysis process. J. Ind. Eng. Chem. 2024, 129, 341–351. [Google Scholar] [CrossRef]
- Gaur, A.; Porwal, C.; Chauhan, V.S.; Vaish, R. Tribocatalytic investigation of BaTiO3 for dye removal from water. J. Mater. Electron. 2023, 34, 14. [Google Scholar] [CrossRef]
- Mao, C.Y.; Lei, H.; Guo, Z.Y.; Jia, X.C.; Cui, X.D.; Huang, J.W.; Fei, L.F.; Jia, Y.M.; Chen, W.P. Exceptional tribo-catalytic degradation of concentrated methyl orange and methylene blue solutions by DXN-RT30 TiO2 nanoparticles. Ceram. Int. 2024, 50, 4737–4745. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Z.; Lei, H.; Jia, X.; Mao, C.; Ruan, L.; Zhou, X.; Wang, Z.; Chen, F.; Chen, W. Tribo-Catalytic Degradation of Methyl Orange Solutions Enhanced by Silicon Single Crystals. Coatings 2023, 13, 1804. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Yi, Y.; Zhou, B.; Sun, P.; Dong, X. Regulation of friction pair to promote conversion of mechanical energy to chemical energy on Bi2WO6 and realization of enhanced tribocatalytic activity to degrade different pollutants. J. Hazard. Mater. 2023, 459, 132147. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials 2023, 13, 46. [Google Scholar] [CrossRef]
- Cao, J.; Jia, Y.; Wan, X.; Li, B.; Zhang, Y.; Huang, S.; Yang, H.; Yuan, G.; Li, G.; Cui, X.; et al. Strong tribocatalysis of strontium titanate nanofibers through harvesting friction energy for dye decomposition. Ceram. Int. 2022, 48, 9651–9657. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Wang, X.; Zhang, L.; Yuan, G.; Wu, Z. Efficient tribocatalysis of magnetically recyclable cobalt ferrite nanoparticles through harvesting friction energy. Sep. Purif. Technol. 2023, 307, 122846. [Google Scholar] [CrossRef]
- Jie, L.F.; Gao, X.; Cao, X.Q.; Wu, S.; Long, X.X.; Ma, Q.Y.; Su, J.X. A review of CdS photocatalytic nanomaterials: Morphology, synthesis methods, and applications. Mater. Sci. Semicond. Process. 2024, 176, 18. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, H. Comparative study on the mechanism in photocatalytic degradation of different-type organic dyes on SnS2 and CdS. Appl. Catal. B Environ. 2012, 123–124, 174–181. [Google Scholar] [CrossRef]
- Fard, N.E.; Fazaeli, R.; Ghiasi, R. Band Gap Energies and Photocatalytic Properties of CdS and Ag/CdS Nanoparticles for Azo Dye Degradation. Chem. Eng. Technol. 2016, 39, 149–157. [Google Scholar] [CrossRef]
- Yang, B.A.; Chen, H.B.; Guo, X.D.; Wang, L.; Xu, T.; Bian, J.H.; Yang, Y.D.; Liu, Q.D.; Du, Y.P.; Lou, X.J. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Stoev, M.; Katerski, A. XPS and XRD study of photoconductive CdS films obtained by a chemical bath deposition process. J. Mater. Chem. 1996, 6, 377–380. [Google Scholar] [CrossRef]
- Song, J.; Zhao, J.; Liu, Y.; Hu, Y.; Chen, W. Room-temperature hydrogen sensitive Pt—SnO2 composite nanoceramics: Dormancy and a practicable regeneration method. Ceram. Int. 2024, 50, 31357–31363. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Xu, L.; Wang, L.; Ju, Z.; Li, G.; Qian, Y. High yield synthesis of novel boron nitride submicro-boxes and their photocatalytic application under visible light irradiation. Catal. Sci. Technol. 2011, 1, 1159–1165. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scalese, S. Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489–499. [Google Scholar] [CrossRef]
- Jia, X.C.; Wang, H.B.; Lei, H.; Mao, C.Y.; Cui, X.D.; Liu, Y.; Jia, Y.M.; Yao, W.Q.; Chen, W.P. Boosting tribo-catalytic conversion of H2O and CO2 by Co3O4 nanoparticles through metallic coatings in reactors. J. Adv. Ceram. 2023, 12, 1833–1843. [Google Scholar] [CrossRef]
- Mao, C.Y.; Zhang, Y.C.; Lei, H.; Jia, X.C.; Chen, F.; Yao, W.Q.; Liu, P.T.; Chen, W.P. Boosting tribo-catalytic degradation of organic pollutants by BaTiO3 nanoparticles through metallic coatings. Appl. Surf. Sci. 2024, 663, 160172. [Google Scholar] [CrossRef]
- Xu, X.; Mao, C.; Song, J.; Ke, S.; Hu, Y.; Chen, W.; Pan, C. Surprising Effects of Ti and Al2O3 Coatings on Tribocatalytic Degradation of Organic Dyes by GaN Nanoparticles. Materials 2024, 17, 3487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Berbille, A.; Feng, Y.W.; Li, S.; Zhu, L.P.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 2022, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, Y.; Wang, L.; Zhang, Y.; Wang, D.; Yang, M.; Yang, J.; Zhang, B.; Jiang, Z.; Li, C. Highly efficient photocatalytic oxidation of sulfur-containing organic compounds and dyes on TiO2 with dual cocatalysts Pt and RuO2. Appl. Catal. B Environ. 2012, 127, 363–370. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, J.; Zhou, S.; Mao, X.; Shah, M.W.; Wang, F.; Chen, Z.; Wang, C. TiO2-supported Ag nanoclusters with enhanced visible light activity for the photocatalytic removal of NO. Appl. Catal. B Environ. 2018, 234, 206–212. [Google Scholar] [CrossRef]
- Rej, S.; Hejazi, S.M.H.; Badura, Z.; Zoppellaro, G.; Kalytchuk, S.; Kment, Š.; Fornasiero, P.; Naldoni, A. Light-Induced Defect Formation and Pt Single Atoms Synergistically Boost Photocatalytic H2 Production in 2D TiO2-Bronze Nanosheets. ACS Sustain. Chem. Eng. 2022, 10, 17286–17296. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Wang, H.; Mao, C.; Guo, Z.; Fei, L.; Chen, W. Converting H2O and CO2 into chemical fuels by nickel via friction. Surf. Interfaces 2024, 46, 104203. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, S.; Mao, C.; Luo, R.; Zhou, Z.; Hu, Y.; Zhao, W.; Chen, W. Surprising Effects of Al2O3 Coating on Tribocatalytic Degradation of Organic Dyes by CdS Nanoparticles. Coatings 2024, 14, 1057. https://doi.org/10.3390/coatings14081057
Ke S, Mao C, Luo R, Zhou Z, Hu Y, Zhao W, Chen W. Surprising Effects of Al2O3 Coating on Tribocatalytic Degradation of Organic Dyes by CdS Nanoparticles. Coatings. 2024; 14(8):1057. https://doi.org/10.3390/coatings14081057
Chicago/Turabian StyleKe, Senhua, Chenyue Mao, Ruiqing Luo, Zeren Zhou, Yongming Hu, Wei Zhao, and Wanping Chen. 2024. "Surprising Effects of Al2O3 Coating on Tribocatalytic Degradation of Organic Dyes by CdS Nanoparticles" Coatings 14, no. 8: 1057. https://doi.org/10.3390/coatings14081057


.jpg)

