High-Temperature Hot Corrosion Resistance of CrAl/NiCoCrAlY/AlSiY Gradient Composite Coating on TiAl Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of CrAl/NiCoCrAlY/AlSiY Coating
2.2. Characterization
2.3. Hot Corrosion Tests
3. Results
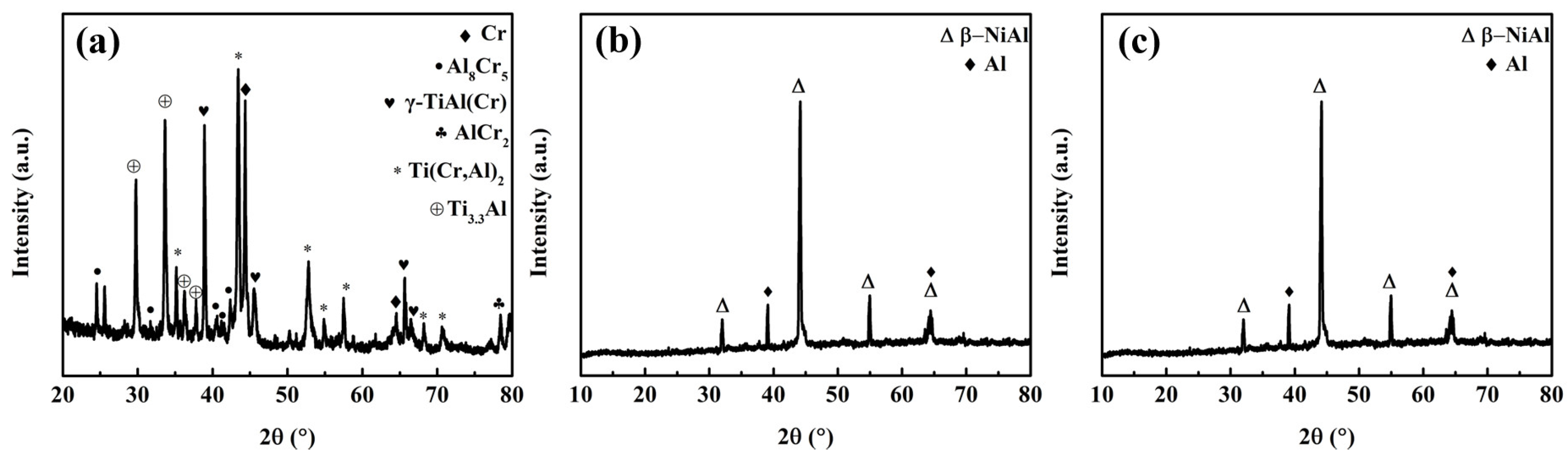
3.1. Phase Structure of CrAl/NiCoCrAlY/AlSiY Coating
3.2. Morphology CrAl/NiCoCrAlY/AlSiY Coating
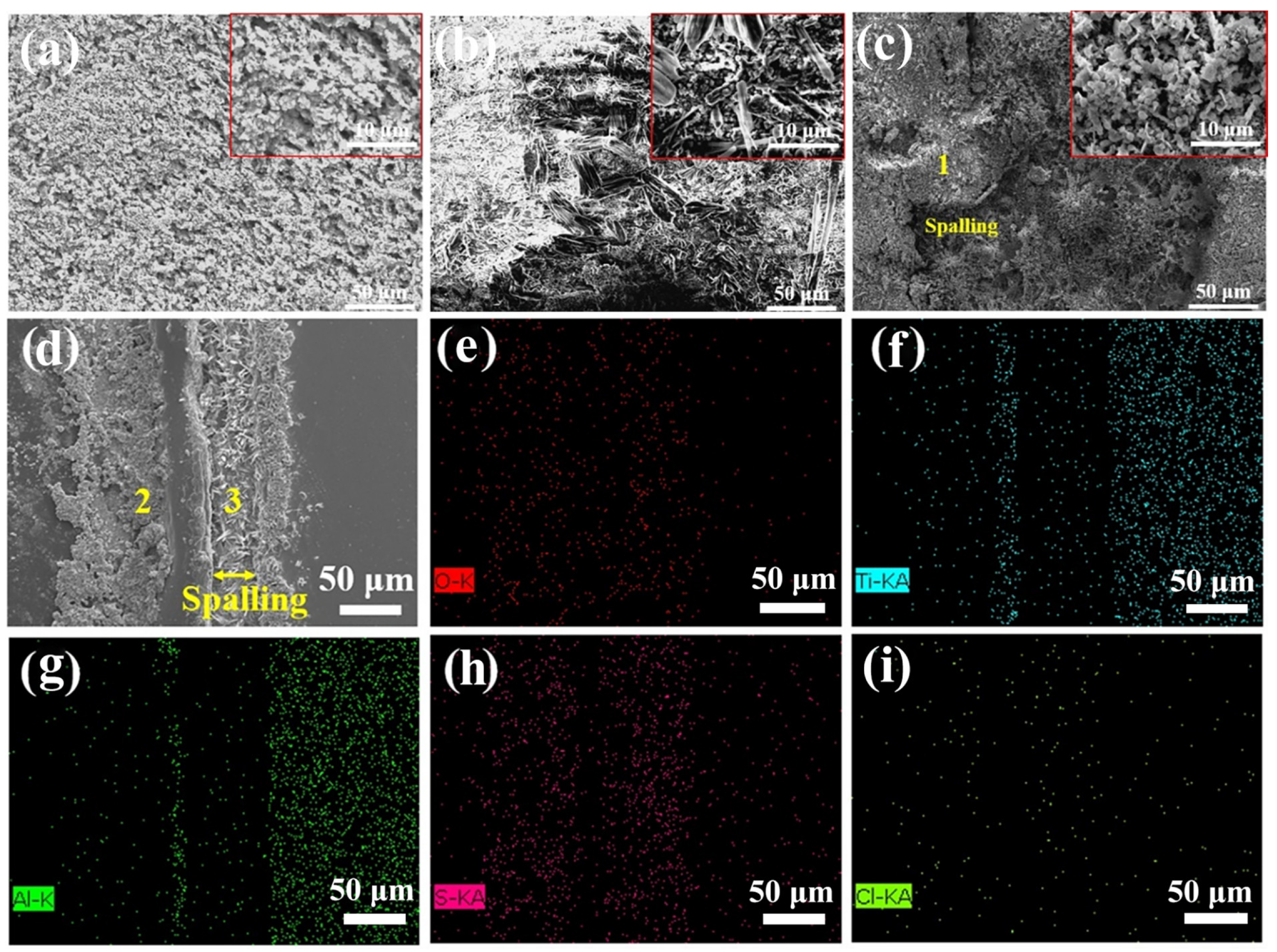
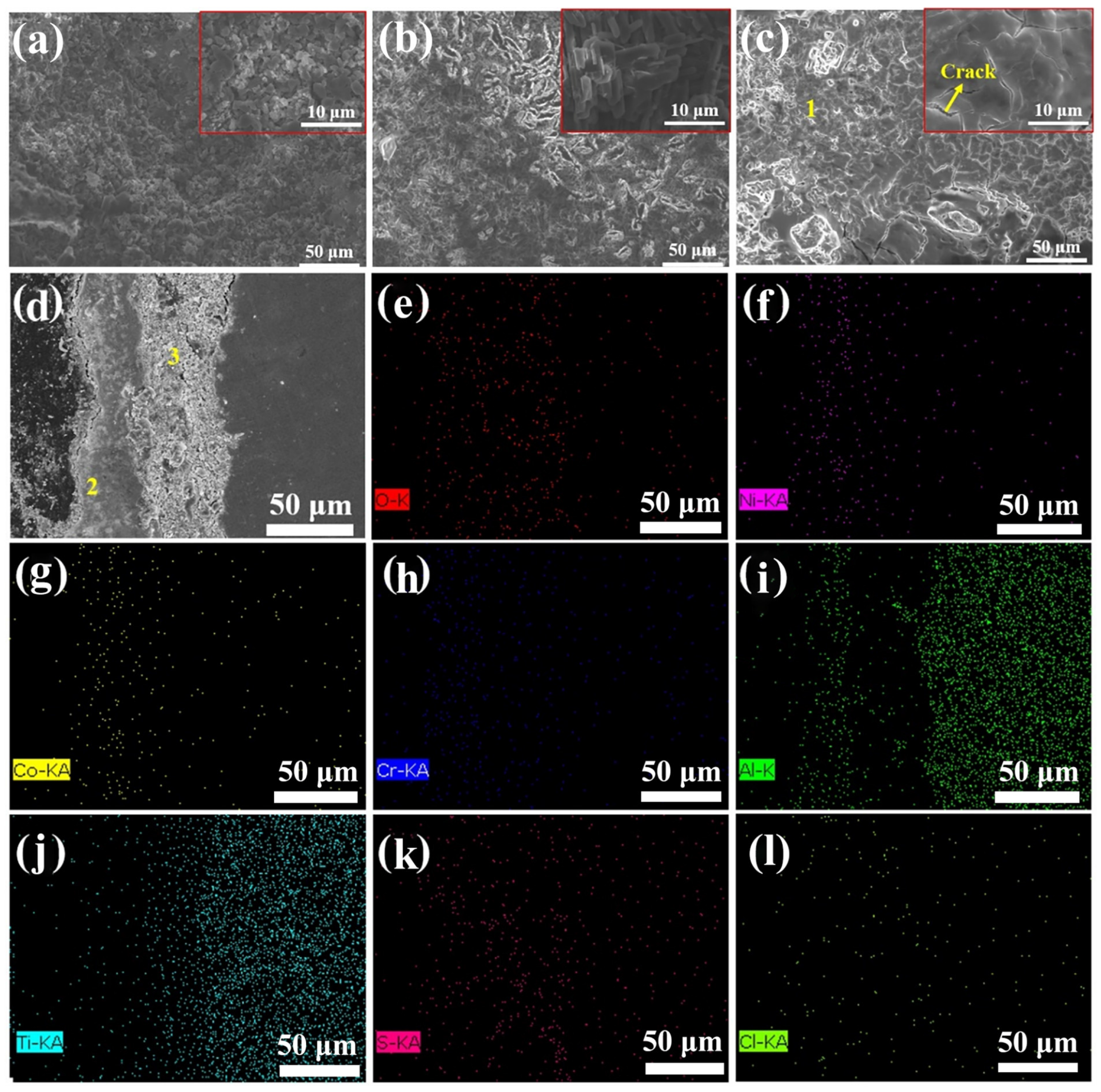
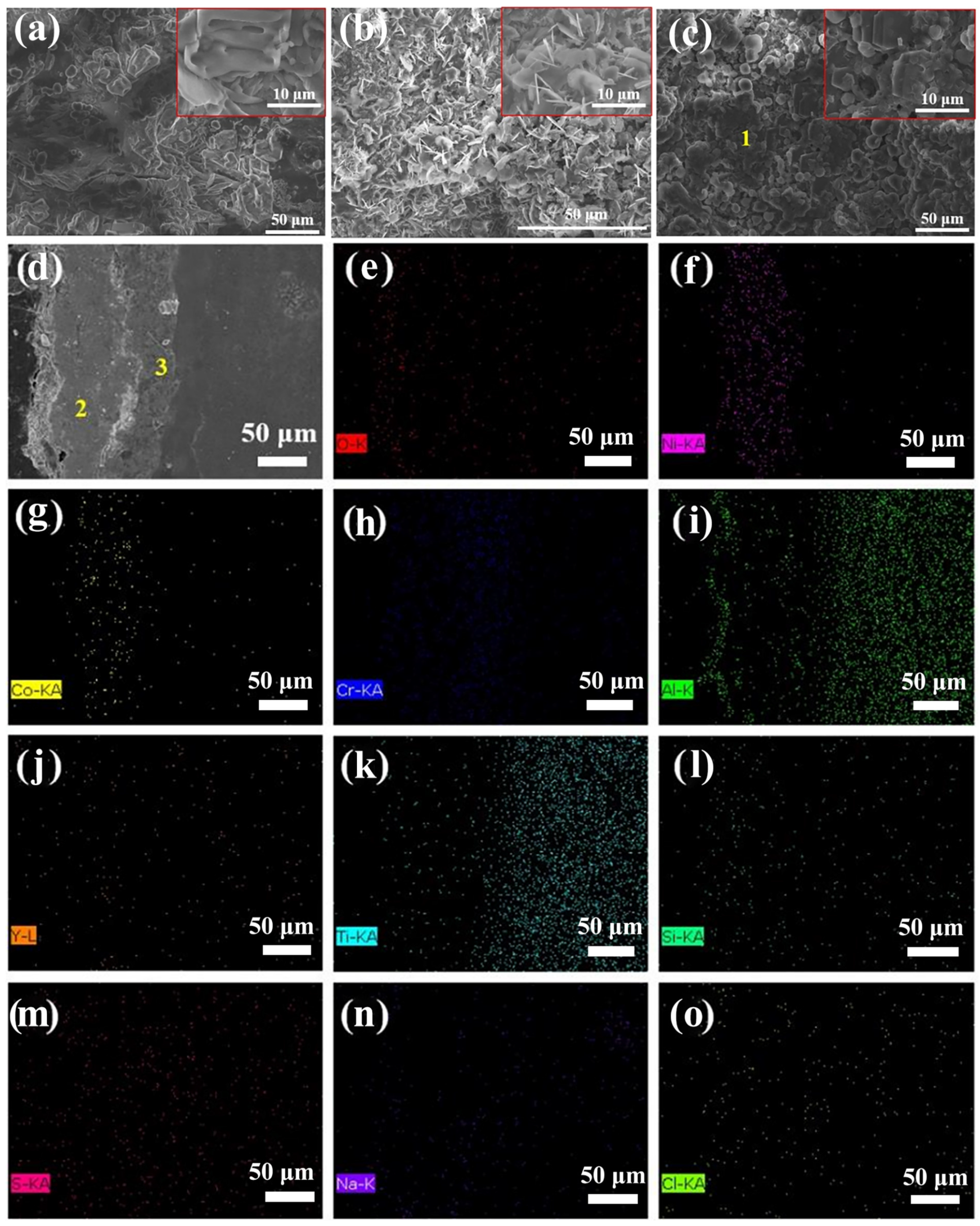
3.3. Hot Corrosion Tests of the Substrate and Coatings
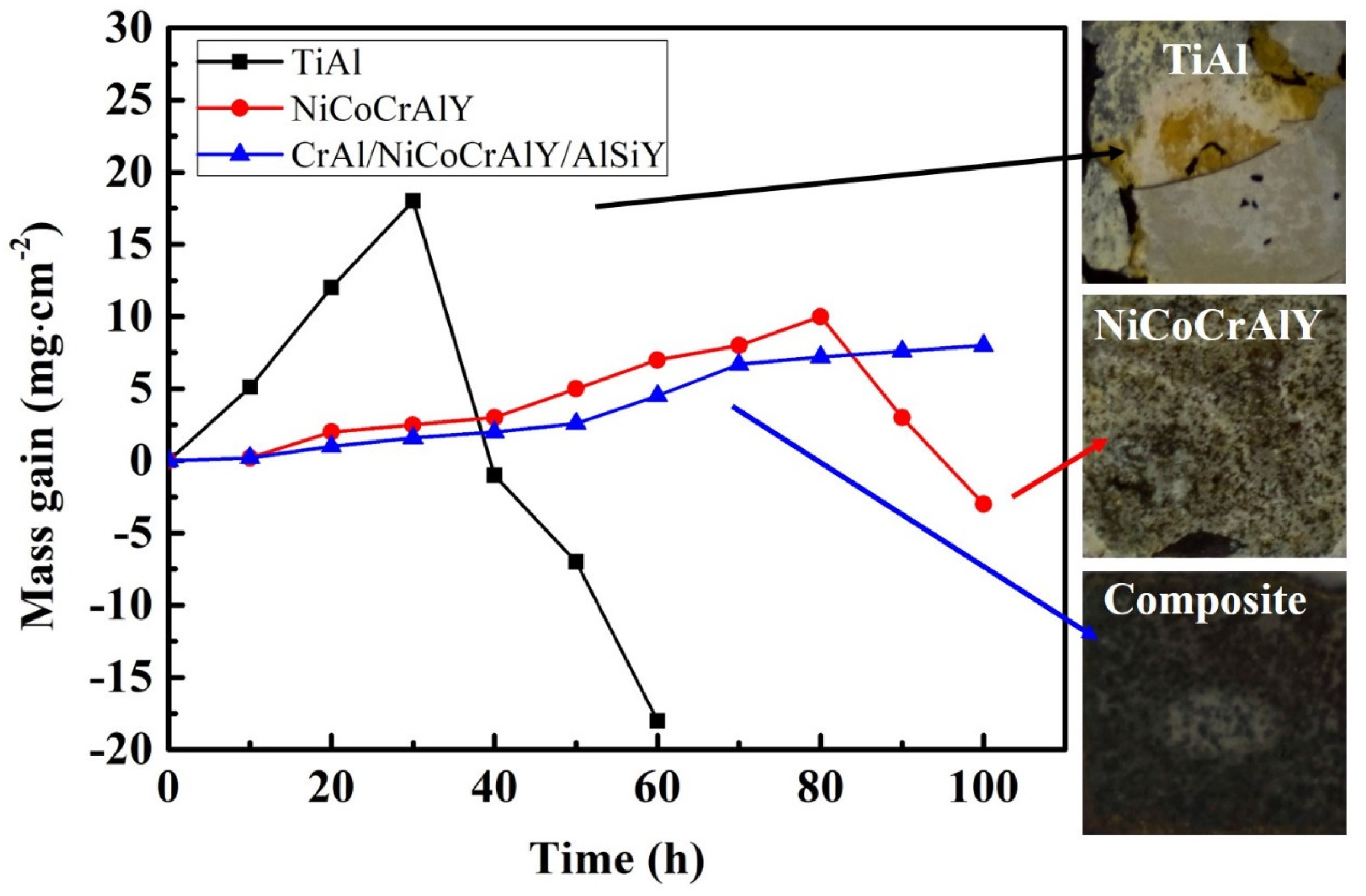
3.3.1. Corrosion Kinetic Curves
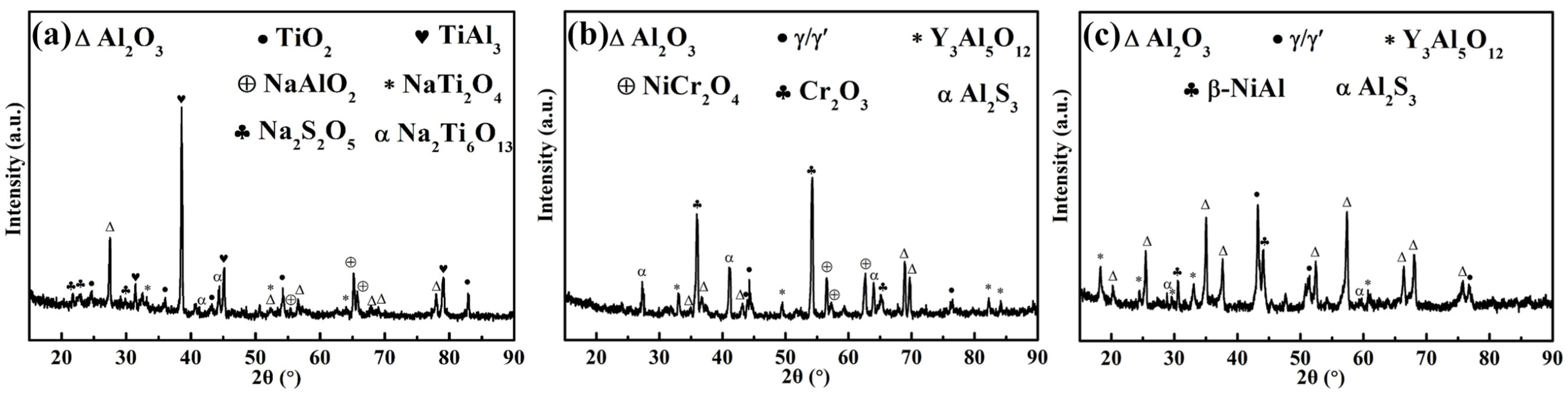
3.3.2. Structural Analysis
3.3.3. Morphology Analysis
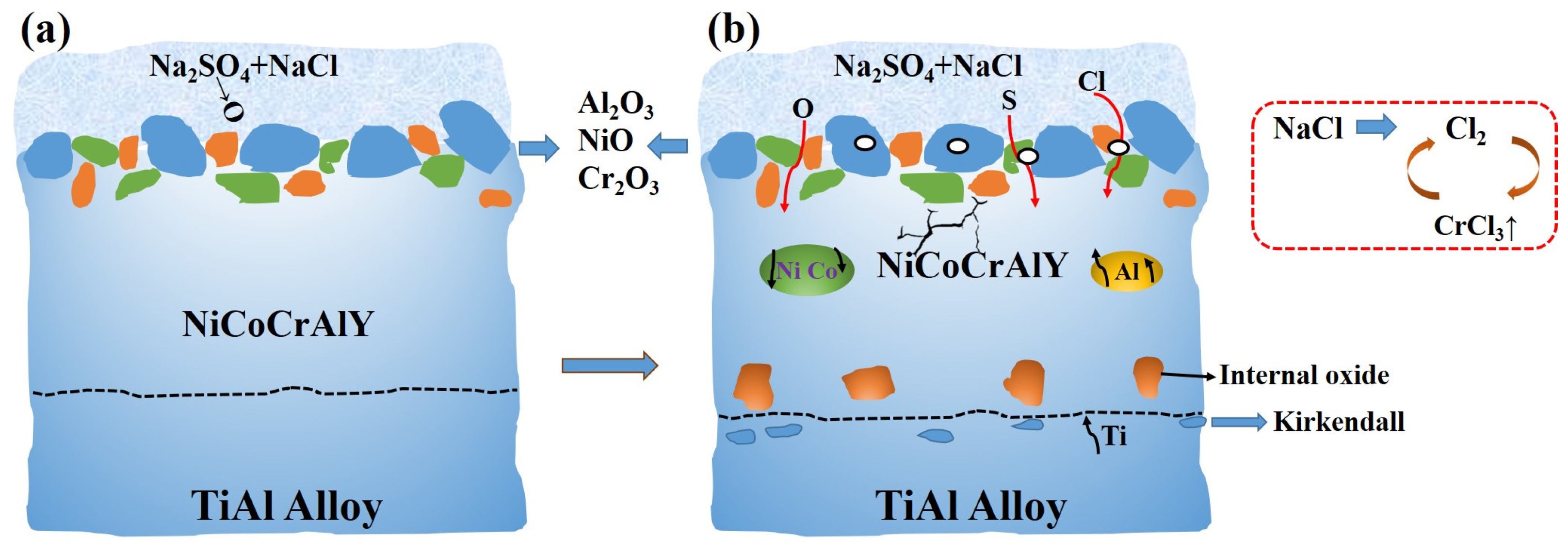
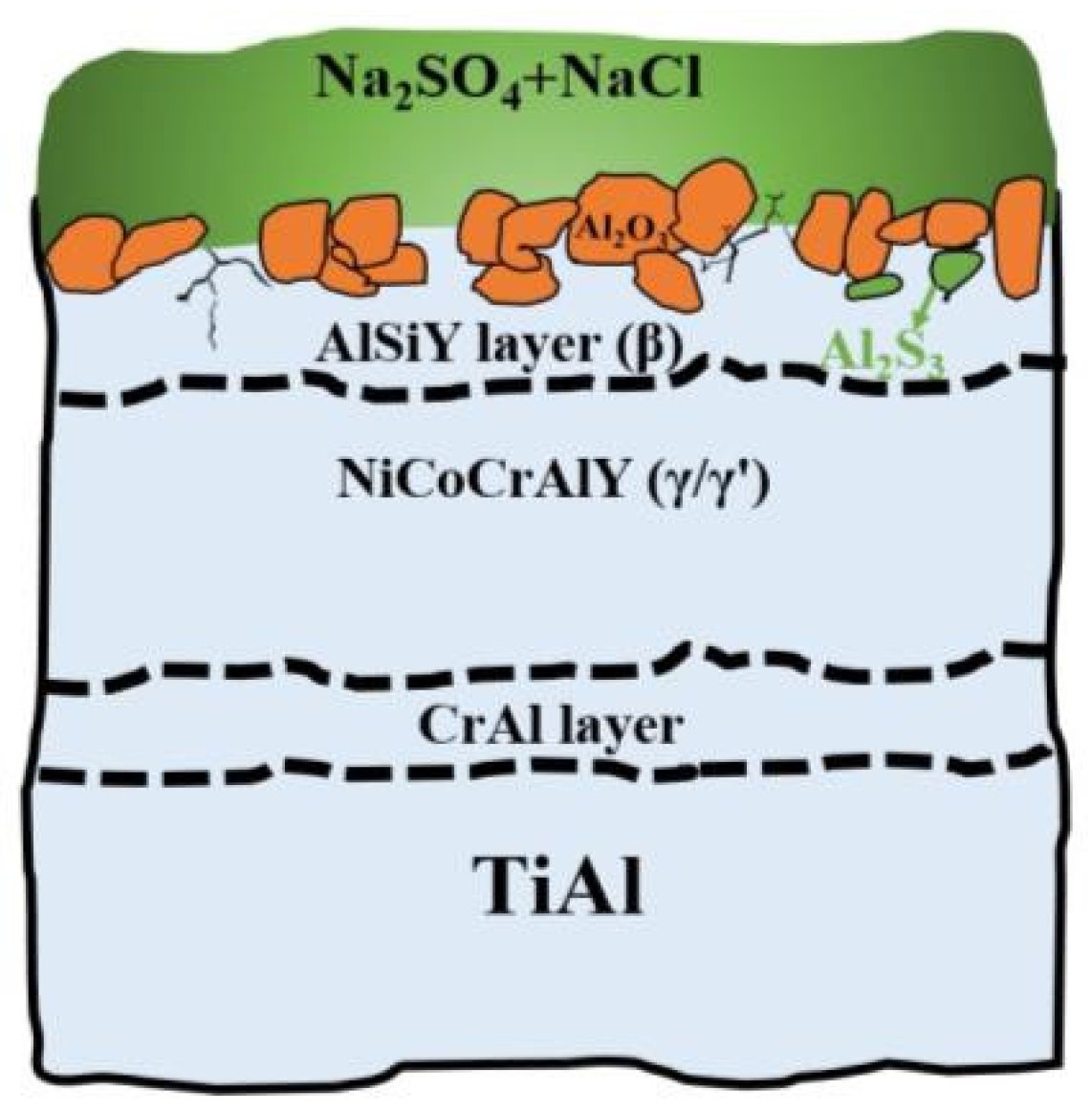
4. Discussion
5. Conclusions
- (1)
- The prepared CrAl/NiCoCrAlY/AlSiY gradient composite coating is dense with 10 [9.06, 10.94] μm, 45 [42.49, 47.51] μm, and 5 [4.52, 5.48] μm for the CrAl layer, NiCoCrAlY layer, and AlSiY layer, respectively. The elements in the composite coating show a gradient distribution.
- (2)
- After corrosion at 950 °C, the TiAl substrate and NiCoCrAlY coating exhibit obvious corrosion loss, while the composite coating shows no weight loss and remains stable.
- (3)
- Corrosion tests reveal that the TiAl substrate spalled largely and corroded completely. The NiCoCrAlY-coated sample also showed peeling phenomena. No obvious cracks occurred in the composite coating, demonstrating better hot corrosion resistance.
- (4)
- The main hot corrosion products of the TiAl substrate are TiO2 and Al2O3, with the loose porous TiO2 on the surface rendering the substrate completely ineffective. The NiCoCrAlY coating mainly produces uneven Cr2O3 and a variety of loose mixtures. The corrosion product of the composite coating is continuous Al2O3, which provides good hot corrosion resistance and effective protection for the substrate.
- (5)
- In subsequent studies, researchers could focus on improving the mechanical properties of the coating while enhancing its hot corrosion resistance. The coefficients of thermal expansion between layers of the composite coating also need to be considered when applying these coatings to high-temperature components of engines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tetsui, T. Identifying low-cost, machinable, impact-resistant TiAl alloys suitable for last-stage turbine blades of jet engines. Intermetallics 2024, 168, 108263. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, R.; Zou, H.; Zhou, M.; Luo, X. Insight into the Ta alloying effects on the oxidation behavior and mechanism of cast TiAl alloy. Mater. Design 2024, 241, 112941. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chen, Y.J.; Gesang, Y.; Pan, M.H.; Liang, Y. Tribological Properties of γ-TiAl Alloy Fretting against Nickel-Based Superalloy at Elevated Temperature. Tribol. Trans. 2024, 67, 259–269. [Google Scholar] [CrossRef]
- Swadźba, R.; Swadźba, L.; Mendala, B.; Bauer, P.P.; Laska, N.; Schulz, U. Microstructure and cyclic oxidation resistance of Si-aluminide coatings on γ-TiAl at 850 °C. Surf. Coat. Technol. 2020, 403, 126361. [Google Scholar] [CrossRef]
- Garip, Y. Investigation of isothermal oxidation performance of TiAl alloys sintered by different processing methods. Intermetallics 2020, 127, 106985. [Google Scholar] [CrossRef]
- Dai, J.; Sun, C.; Wang, A.; Zhang, H.; Li, S.; Zhang, H. High temperature oxidation and hot corrosion behaviors of Ti2AlNb alloy at 923 K and 1023 K. Corros. Sci. 2021, 184, 109336. [Google Scholar] [CrossRef]
- Kosieniak, E.; Biesiada, K.; Kaczorowski, J.; Innocenti, M. Corrosion failures in gas turbine hot components. J. Fail. Anal. Prev. 2013, 12, 330–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Zhang, S.; Yang, Z.; Zhang, T.; Tian, S. Hot salt corrosion behavior of the Ti-44Al-4Nb-1.5Mo-(B,Y) alloy in the temperature range of 750-950 °C. J. Mater. Res. Technol. 2023, 26, 4887–4901. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.B.; Zhang, X.H.; Song, L. Corrosion resistance and interfacial morphologies of a high Nb-containing TiAl alloy with and without thermal barrier coatings in molten salts. Corrosion Sci. 2019, 156, 139–146. [Google Scholar] [CrossRef]
- Yao, Z.; Marek, M. NaCl-induced hot corrosion of a titanium aluminide alloy. Mat. Sci. Eng. A-Struct. 1995, 192, 994–1000. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Xu, B.W.; Ma, Y.; Gong, S.K. Inter-phase selective corrosion of γ-TiAl alloy in molten salt environment at high temperature. Prog. Nat. Sci. 2011, 21, 322–329. [Google Scholar] [CrossRef]
- Gong, X.; Chen, R.R.; Wang, Y.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and Oxidation Behavior of NiCoCrAlY Coating with Different Sm2O3 Concentration on TiAl Alloy. Front. Mater. 2021, 8, 710431. [Google Scholar] [CrossRef]
- Tian, S.W.; Zhang, Y.F.; Liu, J.H.; Zeng, S.W.; Jiang, H.T. Interdiffusion mechanism at the interface between TiAl alloy and NiCoCrAlY bond coating. Surf. Coat. Technol. 2022, 444, 128687. [Google Scholar] [CrossRef]
- Han, D.J.; Liu, D.D.; Niu, Y.R.; Qi, Z.X.; Pan, Y.Y.; Xu, H.; Zheng, X.B.; Chen, G. Interface stability of NiCrAlY coating without and with a Cr or Mo diffusion barrier on Ti-42Al-5Mn alloy. Corros. Sci. 2021, 188, 109538. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Z.; Xiang, L.; Xiao, H.; Zhao, Y.; Wan, Y.; Ning, H.; Xie, Z. Enhancing hot corrosion performance of NiCoCrAlY/AlSiY coating by arc enhanced glow discharge. Mater. Lett. X 2022, 13, 100130. [Google Scholar] [CrossRef]
- Li, S.M.; Fu, L.B.; Zhang, W.L.; Li, W.; Sun, J.; Wang, T.G.; Jiang, S.M.; Gong, J.; Sun, C. Formation process and oxidation behavior of MCrAlY + AlSiY composite coatings on a Ni-based superalloy. J. Mate. Sci. Technol. 2022, 120, 65–77. [Google Scholar] [CrossRef]
- Xue, G.M.; Wang, Z.G.; Wang, E.L.; Tang, Y.; Zhao, Y.H.; Wang, Y.H.; Hu, S.Y.; Xiang, L.; Xie, Z.W. Enhanced Hot Salt-Water Corrosion Resistance of NiCoCrAlY-AlSiY Coating by Ion-Beam-Assisted Deposition. Coatings 2021, 11, 1062. [Google Scholar] [CrossRef]
- Zeng, S.; Li, J.; Chen, C.; Meng, Y.; Zhu, C.W.; Bao, Y.W.; Han, X.C.; Zhang, H.B. Oxidation behavior of CrAl coating with and without Nb addition in high temperature steam environment. J. Nucl. Mater. 2023, 581, 154437. [Google Scholar] [CrossRef]
- Xu, M.; Li, S.; Dong, Z.; Liu, H.; Wang, J.; Bao, Z.; Zhu, S.; Wange, F.H. High temperature performance and interface evolution of a NiCoCrAlY coating with Pt modification and Re-based diffusion barrier. Surf. Coat. Technol. 2023, 463, 129510. [Google Scholar] [CrossRef]
- Du, Y.; Clavaguera, N. Thermodynamic assessment of the Al-Ni system. J. Alloys Compd. 1996, 237, 20–32. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Q.; Liang, G.; Liu, G.; Liu, S. Microstructure and hot corrosion properties of an Al-Y coating on TiAl alloy. J. Cent. South Univ. 2024, 31, 330–345. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, R.; Qu, G.; Zhou, X. Influences of Laser Remelting on Hot Corrosion Resistance of Plasma-sprayed Nanostructured Al2O3-13wt.%TiO2 Coating. J. Phys. Conf. Ser. 2021, 1965, 012084. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Lv, W.; Ming, B.; Li, Y.; Liu, X.; Lai, S.; Liu, F. Structure and Hot Corrosion Behavior of an Al-Co-Y Co-deposited Coating on TiAl Alloy. J. Mat. Eng. Perf. 2023, 32, 4796–4806. [Google Scholar] [CrossRef]
- Li, Y.; Xie, F.; Yang, S. Microstructure and hot corrosion resistance of Si-Al-Y coated TiAl alloy. J. Cent. South Univ. 2020, 27, 2530–2537. [Google Scholar] [CrossRef]
- Liao, Y.; Feng, M.; Chen, M.; Geng, Z.; Liu, Y.; Wang, F.; Zhu, S. Comparative study of hot corrosion behavior of the enamel based composite coatings and the arc ion plating NiCrAlY on TiAl alloy. Acta Metall. Sin. 2019, 55, 229–237. [Google Scholar]
- Wang, D.; Tian, Z.; Shen, L.; Huang, Y. Hot corrosion resistance of plasma-sprayed MCrAlY coatings by laser remelting on TiAl alloy surface TiAl. Trans. China Weld. Inst. 2014, 35, 17–20. [Google Scholar]
- Tian, Z.J.; Gao, X.S.; Huang, Y.H.; Liu, Z.D.; Shen, L.D.; Wang, D.S. Study on Hot Corrosion Behavior of Plasma-Sprayed NiCoCrAl-Y2O3 Coating on TiAl Alloy Surface. Rare Met. Mater. Eng. 2010, 39, 1439–1442. [Google Scholar]
- Rapp, R.A. Hot corrosion of materials: A fluxing mechanism? Corros. Sci. 2002, 44, 209–221. [Google Scholar] [CrossRef]
- Guo, M.H.; Wang, Q.M.; Ke, P.L.; Gong, J.; Sun, C.; Huang, R.F.; Wen, L.S. The preparation and hot corrosion resistance of gradient NiCoCrAlYSiB coatings. Surf. Coat. Technol. 2006, 200, 3942–3949. [Google Scholar] [CrossRef]
- Xu, Y.; Miao, Q.; Wang, L.; Yu, X.; Jiang, Q.; Ren, B. Oxidation behavior of γ-TiAl alloy with NiCrAlY/Al duplex coating at 950 °C. Rare Met. Mat. Eng. 2014, 43, 1407–1411. [Google Scholar]
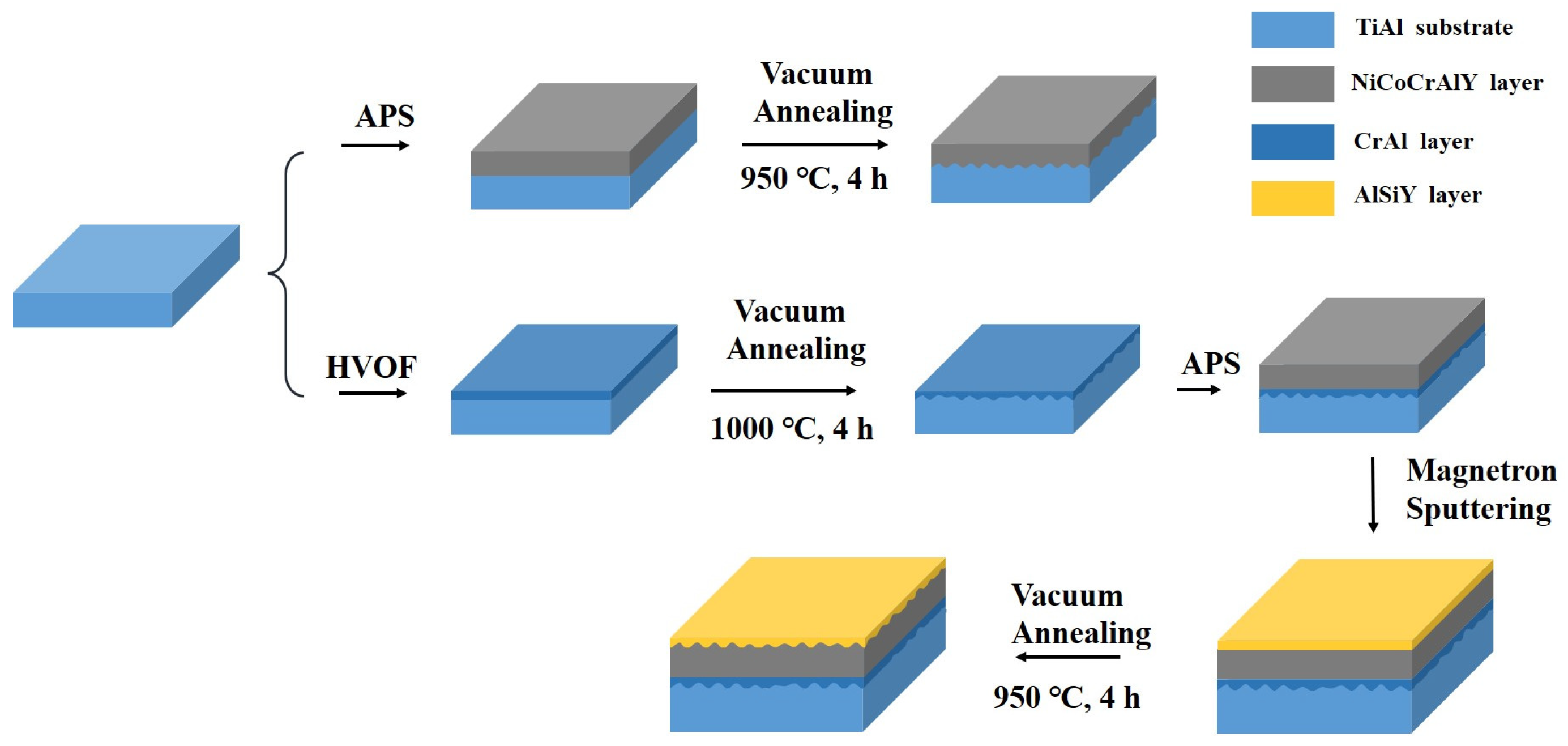


| Parameters | CrAl Layer | NiCoCrAlY Layer | AlSiY Layer |
|---|---|---|---|
| Oxygen pressure/MPa | 1.42 | - | - |
| Argon flow rate/sccm | 5.37 × 107 | - | 30 |
| Working pressure/Pa | 1.24 | - | 1 |
| Fuel flow/sccm | 2.12 × 105 | - | - |
| Carrier flow/sccm | 7.07 × 105 | - | - |
| Electricity/A | - | 600 | - |
| Voltage/V | - | 40 | - |
| Main gas (Ar)/MPa | - | 0.5 | - |
| Auxiliary gas (He)/MPa | - | 0.8 | - |
| Carrier gas (Ar)/MPa | - | 0.3 | - |
| Powder feed rate/r·min−1 | - | 3 | - |
| Spraying distance/mm | - | 100 | - |
| Spray gun moving speed/mm·s−1 | - | 80 | - |
| Distance (target − workpiece)/mm | - | - | 15 |
| Sputtering time/h | - | - | 3 |
| Areas | Ni | Co | Cr | Al | Y | O | Ti |
|---|---|---|---|---|---|---|---|
| 1 | 28.2 | 1.2 | 2.2 | 65.5 | 0.3 | 2.6 | -- |
| 2 | 36.4 | 12.4 | 7.6 | 40.4 | 1.1 | 1.1 | 1.0 |
| 3 | 47.5 | 11.9 | 9.2 | 25.5 | 2.0 | 3.2 | 0.7 |
| 4 | 21.1 | 6.3 | 37.8 | 23.8 | 2.6 | 2.4 | 6.0 |
| 5 | 0.5 | 0.3 | 1.4 | 41.5 | 1.5 | 1.1 | 53.8 |
| Coating | Substrate (at.%) | Preparation Technology | Molten Salt Composition (wt.%) | Exposure | Max. Mass Gain (mg/cm2) | |
|---|---|---|---|---|---|---|
| Al-based | Al-Y | Ti−48Al−2Nb−2Cr | Pack cementation process | 75% Na2SO4 + 25% NaCl | 850 °C, 35 h | −2 [21] |
| Al2O3-TiO2 | Ti−46.5Al−2.5V−1Cr | Plasma spraying | 75% Na2SO4 + 25% NaCl | 850 °C, 50 h | 5.5 [22] | |
| Al-Co-Y | Ti−45Al−8Nb−0.1Y | Pack cementation process | 75% K2SO4 + 25% NaCl | 850 °C, 25 h | 30.66 [23] | |
| Si-Al-Y | Ti−48Al−2Cr−2Nb | Pack cementation process | 75% Na2SO4 + 25% K2SO4 | 900 °C, 20 h | 6.01 [24] | |
| MCrAlY | NiCrAlY | Ti−45Al−2Mn−2Nb | Multi arc ion plating | 75% Na2SO4 + 25% NaCl | 850 °C, 160 h | 5.456 [25] |
| NiCoCrAlY | Ti−46.5Al−2.5V−1Cr | Plasma spraying | 75% Na2SO4 + 25% NaCl | 850 °C, 40 h | 5 [26] | |
| Composite | NiCoCrAl-Y2O3 | Ti−46.5Al−2.5V−1Cr | Plasma spraying | 75% Na2SO4 + 25% NaCl | 850 °C, 20 h | 4 [27] |
| CrAl/NiCoCrAlY/AlSiY | Ti−46.5Al−1Cr−0.6V | Various | 75% Na2SO4 + 25% NaCl | 950 °C, 100 h | 8 (This work) |
| Points | O | Ti | Al | S | Cl | Na |
|---|---|---|---|---|---|---|
| 1 | 62.6 | 21.4 | 14.3 | 0.9 | 0.2 | 0.6 |
| 2 | 62.0 | 21.2 | 15.3 | 0.7 | 0.3 | 0.5 |
| 3 | 52.3 | 15.7 | 10.5 | 6.7 | 7.2 | 7.6 |
| Areas | O | Ni | Al | Cr | Co | Y | Ti | S | Cl | Na |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52.1 | 21.5 | 10.7 | 4.8 | 3.7 | 1.3 | -- | 3.0 | 0.4 | 2.5 |
| 2 | 18.6 | 39.3 | 18.9 | 7.3 | 6.7 | 1.7 | -- | 5.3 | 0.3 | 1.9 |
| 3 | 41.3 | 12.7 | 15.9 | 5.7 | 2.3 | 0.3 | 11.2 | 9.5 | 0.1 | 1.0 |
| Areas | O | Ni | Al | Cr | Co | Y | Ti | Si | S | Cl | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50.7 | 7.8 | 31.7 | 2.8 | 1.2 | 0.6 | -- | 1.2 | 3.1 | 0.8 | 0.1 |
| 2 | 3.6 | 53.3 | 21.4 | 10.9 | 7.9 | 1.2 | -- | -- | 0.5 | -- | 1.2 |
| 3 | 4.6 | 2.8 | 23.7 | 31.8 | 3.4 | 0.1 | 32.7 | -- | 0.9 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Miao, Q.; Sun, S.; Liang, W.; Ding, Z.; Niu, J.; Jia, F.; Xu, J.; Gao, J. High-Temperature Hot Corrosion Resistance of CrAl/NiCoCrAlY/AlSiY Gradient Composite Coating on TiAl Alloy. Coatings 2024, 14, 1067. https://doi.org/10.3390/coatings14081067
Sun Y, Miao Q, Sun S, Liang W, Ding Z, Niu J, Jia F, Xu J, Gao J. High-Temperature Hot Corrosion Resistance of CrAl/NiCoCrAlY/AlSiY Gradient Composite Coating on TiAl Alloy. Coatings. 2024; 14(8):1067. https://doi.org/10.3390/coatings14081067
Chicago/Turabian StyleSun, Yuanyuan, Qiang Miao, Shijie Sun, Wenping Liang, Zheng Ding, Jiangqi Niu, Feilong Jia, Jianyan Xu, and Jiumei Gao. 2024. "High-Temperature Hot Corrosion Resistance of CrAl/NiCoCrAlY/AlSiY Gradient Composite Coating on TiAl Alloy" Coatings 14, no. 8: 1067. https://doi.org/10.3390/coatings14081067





