Bimetallic Ni–Mn Electrocatalysts for Stable Oxygen Evolution Reaction in Simulated/Alkaline Seawater and Overall Performance in the Splitting of Alkaline Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Catalysts
2.3. Characterization of Catalysts
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Morphology and Microstructure Studies
3.2. Electrocatalytic Activity for OER in SSW
3.3. Electrocatalytic Performance for OER in ASW
3.4. Electrocatalytic Stability Investigations for OER
3.5. Performance of Overall Alkaline Seawater Splitting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and affordable hydrogen fuel from alkaline water splitting: Past, recent progress, and future prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Fu, X.; Wang, S.; Zhao, Y. Perfecting electrocatalysts via imperfections: Towards the large-scale deployment of water electrolysis technology. Energy Environ. Sci. 2021, 14, 1722–1770. [Google Scholar] [CrossRef]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Wilbraham, L.; Moniz, S.J.; Godin, R.; Zwijnenburg, M.A.; Durrant, J.R.; Cooper, A.I.; et al. Current understanding and challenges of solar-driven hydrogen generation using polymeric photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Ma, T.; Lutkenhaus, J.L. Hydrogen power gets a boost. Science 2022, 378, 138–139. [Google Scholar] [CrossRef]
- Tanç, B.; Arat, H.T.; Baltacıoğlu, E.; Aydın, K. Overview of the next quarter century vision of hydrogen fuel cell electric vehicles. Int. J. Hydrogen Energy 2019, 44, 10120–10128. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications. Energy Environ. Mater. 2023, 6, e12441. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Asghari, E.; Abdullah, M.I.; Foroughi, F.; Lamb, J.J.; Pollet, B.G. Advances, opportunities, and challenges of hydrogen and oxygen production from seawater electrolysis: An electrocatalysis perspective. Curr. Opin. Electrochem. 2022, 31, 100879. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, Y.; Kou, Z.; Wang, P.; Liu, X.; Zhang, J.; He, J.; Mu, S.; Wang, J. Synergizing aliovalent doping and interface in heterostructured NiV nitride@oxyhydroxide core-shell nanosheet arrays enables efficient oxygen evolution. Nano Energy 2021, 85, 105961. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.-J.; Zhu, X.-J.; Lu, S.; Long, L.-L.; Chen, J.-J. Nanostructured metallic FeNi2S4 with reconstruction to generate FeNi-based oxide as a highly-efficient oxygen evolution electrocatalyst. Nano Energy 2021, 81, 105619. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.; Li, J.; Yuan, Y.; Li, C.; Zhang, S.; Yang, L.; Bai, Z.; Lu, J. Lithiation-Induced Defect Engineering to Promote Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2209753. [Google Scholar] [CrossRef]
- Avcı, Ö.N.; Sementa, L.; Fortunelli, A. Mechanisms of the oxygen evolution reaction on NiFe2O4 and CoFe2O4 Inverse-Spinel Oxides. ACS Catal. 2022, 12, 9058–9073. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef]
- Dresp, S.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2023, 5, 101–116. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, X.; Yu, Q.; Liu, X.; Meng, X.; Wang, X.; Wang, L. Strategies of designing electrocatalysts for seawater splitting. J. Solid State Chem. 2022, 306, 122799. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Cui, B.; Shi, Y.; Li, G.; Chen, Y.; Chen, W.; Deng, Y.; Hu, W. Challenges and opportunities for seawater electrolysis: A mini-review on advanced materials in chlorine-involved electrochemistry. Acta Phys.-Chim. Syn. 2022, 38, 85–95. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, Y.; Qiu, H.; Su, C.; Shao, Z. High selectivity electrocatalysts for oxygen evolution reaction and anti-chlorine corrosion strategies in seawater splitting. Catalysts 2022, 12, 261. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.; Lee, K.; Kang, D.W.; Kwon, T. Emerging porous solids for electrocatalytic and photocatalytic seawater splitting. Coord. Chem. Rev. 2024, 514, 215935. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-free catalysts for proton exchange membrane fuel cells: Stability challenges and material solutions. Adv. Mater. 2021, 33, 1908232. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, T.; Shi, K.; Yu, H.; Deng, K.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Iridium-incorporated Co3O4 with lattice expansion for energy-efficient green hydrogen production coupled with glycerol valorization. Chem. Commun. 2023, 59, 1817–1820. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Xu, X.; Zhang, C. Advances in noble metal (Ru, Rh, and Ir) doping for boosting water splitting electrocatalysis. J. Mater. Chem. A 2021, 9, 13459–13470. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Qi, L.; Xing, C.; Zheng, X.; He, F.; Li, Y. Rhodium nanocrystals on porous graphdiyne for electrocatalytic hydrogen evolution from saline water. Nat. Commun. 2022, 13, 5227. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, X.; Li, J.; Xiao, Y.; Shi, X.; Rao, P.; Deng, P.; Wen, H.; Tian, X. Ni-based heterostructure with protective phosphide layer to enhance the oxygen evolution reaction for the seawater electrolysis. Int. J. Hydrogen Energy 2024, 51, 1373–1380. [Google Scholar] [CrossRef]
- Xiao, X.; Wei, Y.; Song, S.; McElhenny, B.; Zhang, F.; Jiang, X.; Zhang, Y.; Chen, S.; Wang, M.; Shen, Y.; et al. Boosting oxygen evolution in seawater media at large current density via boron-doped (Ni, Fe) OOH grown on Ni3N nanosheets. Appl. Catal. B Environ. Energy 2024, 349, 123871. [Google Scholar] [CrossRef]
- Yang, X.; Bu, H.; Qi, R.; Ye, L.; Wang, C.; Gao, H.; Zhan, T.; Chen, Z. A highly efficient and long-term durable electrocatalyst for oxygen evolution in alkaline seawater by growing Ni1.5Fe1.5B on the NiMoO4 nanorods. Mater. Today Chem. 2024, 35, 101849. [Google Scholar] [CrossRef]
- Wang, J.; Tran, D.T.; Chang, K.; Prabhakaran, S.; Zhao, J.; Kim, D.H.; Kim, N.H.; Lee, J.H. Hierarchical Ni@CNTs-bridged MoxC/Ni2P heterostructure micro-pillars for enhanced seawater splitting and Mg/seawater battery. Nano Energy 2023, 111, 108440. [Google Scholar] [CrossRef]
- Fan, J.; Ma, X.; Xia, J.; Zhang, L.; Bi, Q.; Hao, W. Corrosion resistance and earth-abundance FeS-based heterojunction catalyst for seawater splitting at industrial grade density. J. Colloid Interface Sci. 2024, 657, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Allison, C.A.; Srivastava, R.; Kumar, A.; Sim, M.; Horinek, J.; Lin, W.; de Souza, F.M.; Mishra, S.R.; Perez, F.; et al. Nanoneedles like FeP engineered on Ni-foam as an effective catalyst towards overall alkaline freshwater, urea, and seawater splitting. Fuel 2024, 369, 131725. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, S. Constructing the Fe/Cr double (oxy) hydroxides on Fe3O4 for boosting the electrochemical oxygen evolution in alkaline seawater and domestic sewage. Appl. Catal. B Environ. 2022, 302, 120847. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Zuo, Z.; Jin, M.; Peng, O.; Lian, Q.; Huang, Y.; Cheng, P.; Ai, Z.; Xiang, S.; et al. Robust and efficient iron-based electrodes for hydrogen production from seawater at high current density above 1000 mA cm−2. Chem. Eng. J. 2024, 490, 151705. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Qian, G.; Yu, T.; Wang, Z.; Luo, L.; Shen, F.; Yin, S. In situ growth of volcano-like FeIr alloy on nickel foam as efficient bifunctional catalyst for overall water splitting at high current density. Chem. Eng. J. 2021, 421, 129892. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Z.; Li, X.; Li, J.; Shangguan, E.; Qi, J. Engineering heterostructured and hierarchical CoP/CoFeP nanosheet for effective oxygen evolution reaction in alkaline freshwater and seawater. Int. J. Hydrogen Energy 2024, 71, 1342–1350. [Google Scholar] [CrossRef]
- Feng, S.; Rao, P.; Yu, Y.; Li, J.; Deng, P.; Kang, Z.; Wang, S.; Miao, Z.; Shen, Y.; Tian, X.; et al. Self-assembled heterojunction CoSe2@CoO catalysts for efficient seawater electrolysis. Electrochim. Acta 2023, 463, 142870. [Google Scholar] [CrossRef]
- Kim, S.; Ji, S.; Yang, H.; Son, H.; Choi, H.; Kang, J.; Li, O.L. Near surface electric field enhancement: Pyridinic-N rich few-layer graphene encapsulating cobalt catalysts as highly active and stable bifunctional ORR/OER catalyst for seawater batteries. Appl. Catal. B Environ. 2022, 310, 121361. [Google Scholar] [CrossRef]
- Boakye, F.O.; Harrath, K.; Tabish, M.; Yasin, G.; Owusu, K.A.; Ajmal, S.; Zhang, W.; Zhang, H.; Wang, Y.-G.; Zhao, W. Phosphorus coordinated Co/Se2 heterointerface nanowires: In-situ catalyst reconstruction towards efficient overall water splitting in alkaline and seawater media. J. Alloys Compd. 2023, 969, 172240. [Google Scholar] [CrossRef]
- Khatun, S.; Roy, P. Cobalt chromium vanadium layered triple hydroxides as an efficient oxygen electrocatalyst for alkaline seawater splitting. Chem. Commun. 2022, 58, 1104–1107. [Google Scholar] [CrossRef]
- Yang, J.; An, Y.; Guo, K.; Ren, X.; Jiang, B. Nitrogen doped FeCoNiS nanoparticles on N, S-co-doped vertical graphene as bifunctional electrocatalyst for water splitting. Int. J. Hydrogen Energy 2023, 48, 4143–4157. [Google Scholar] [CrossRef]
- Ganesan, V.; Kim, J. Multi-shelled CoS2–MoS2 hollow spheres as efficient bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2020, 45, 13290–13299. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Y.; Jin, J.; Wang, Y.; Peng, Y.; Yin, J.; Shen, W.; Hou, Y.; Zhu, L.; An, L.; et al. Understanding the sulphur-oxygen exchange process of metal sulphides prior to oxygen evolution reaction. Nat. Commun. 2023, 14, 1949. [Google Scholar] [CrossRef]
- Pei, Z.; Qin, T.; Tian, R.; Ou, Y.; Guo, X. Construction of an amethyst-like MoS2@Ni9S8/Co3S4 rod electrocatalyst for overall water splitting. Nanomaterials 2023, 13, 2302. [Google Scholar] [CrossRef]
- Ding, X.; Uddin, W.; Sheng, H.; Li, P.; Du, Y.; Zhu, M. Porous transition metal phosphides derived from Fe-based Prussian blue analogue for oxygen evolution reaction. J. Alloys Compd. 2020, 814, 152332. [Google Scholar] [CrossRef]
- Li, Q.; Dong, S.; Xie, H.; Ren, J.; Hu, X.; Li, Y.; Zhao, H.; Liu, Z.; Sun, F. Controllable synthesis of crystal-amorphous heterostructures in transition metal phosphide and enhancement mechanism for overall water splitting. Appl. Surf. Sci. 2024, 647, 158961. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Du, X.; Zhang, X. Controlled synthesis of M (M = Cr, Cu, Zn and Fe)-NiCoP hybrid materials as environmentally friendly catalyst for seawater splitting. J. Alloys Compd. 2023, 966, 171516. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Xu, S.; Huang, M.; Wen, Y.; Shi, X.-R. DFT-assisted rational design of CoMxP/CC (M = Fe, Mn, and Ni) as efficient electrocatalyst for wide pH range hydrogen evolution and oxygen evolution. Nano Res. 2022, 15, 8897–8907. [Google Scholar] [CrossRef]
- Xiong, T.; Li, J.; Roy, J.C.; Koroma, M.; Zhu, Z.; Yang, H.; Zhang, L.; Ouyang, T.; Balogun, M.-S.; Al-Mamun, M. Hetero-interfacial nickel nitride/vanadium oxynitride porous nanosheets as trifunctional electrodes for HER, OER and sodium ion batteries. J. Energy Chem. 2023, 81, 71–81. [Google Scholar] [CrossRef]
- Liu, T.; Cai, S.; Zhao, G.; Gao, Z.; Liu, S.; Li, H.; Chen, L.; Li, M.; Yang, X.; Guo, H. Recycling valuable cobalt from spent lithium ion batteries for controllably designing a novel sea-urchin-like cobalt nitride-graphene hybrid catalyst: Towards efficient overall water splitting. J. Energy Chem. 2021, 62, 440–450. [Google Scholar] [CrossRef]
- Zhu, J.; Du, Q.; Khan, M.A.; Zhao, H.; Fang, J.; Ye, D.; Zhang, J. 2D porous Co-Mo nitride heterostructures nanosheets for highly effective electrochemical water splitting. Appl. Surf. Sci. 2023, 623, 156989. [Google Scholar] [CrossRef]
- Sinha, N.; Das, C.; Roy, P. Iron-doped cobalt nitride as an efficient electrocatalyst towards energy saving hydrazine assisted seawater splitting in near neutral to highly alkaline pH achieving industry level current density. Int. J. Hydrogen Energy 2024, 51, 1011–1021. [Google Scholar] [CrossRef]
- Sharma, L.; Katiyar, N.K.; Parui, A.; Das, R.; Kumar, R.; Tiwary, C.S.; Singh, A.K.; Halder, A.; Biswas, K. Low-cost high entropy alloy (HEA) for high-efficiency oxygen evolution reaction (OER). Nano Res. 2022, 15, 4799–4806. [Google Scholar] [CrossRef]
- Zhang, Q.; Lian, K.; Liu, Q.; Qi, G.; Zhang, S.; Luo, J.; Liu, X. High entropy alloy nanoparticles as efficient catalysts for alkaline overall seawater splitting and Zn-air batteries. J. Colloid Interface Sci. 2023, 646, 844–854. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Li, C.; Li, W.; Wang, T.; Liang, C. Hierarchical CoNi2S4@NiMn-layered double hydroxide heterostructure nanoarrays on superhydrophilic carbon cloth for enhanced overall water splitting. Electrochim. Acta 2020, 345, 136247. [Google Scholar] [CrossRef]
- He, S.; Yue, R.; Liu, W.; Ding, J.; Zhu, X.; Liu, N.; Guo, R.; Mo, Z. Nano-NiFe LDH assembled on CNTs by electrostatic action as an efficient and durable electrocatalyst for oxygen evolution. J. Electroanal. Chem. 2023, 946, 117718. [Google Scholar] [CrossRef]
- Gupta, A.; Sadhanala, H.K.; Gedanken, A. Iron doped cobalt nickel layered double hydroxide supported on nickel foam as a robust electrocatalyst for highly efficient water oxidation in alkaline sea water. Electrochim. Acta 2023, 470, 143269. [Google Scholar] [CrossRef]
- Luo, X.; Ji, P.; Wang, P.; Tan, X.; Chen, L.; Mu, S. Spherical Ni3S2/Fe-NiPx Magic Cube with Ultrahigh Water/Seawater Oxidation Efficiency. Adv. Sci. 2022, 9, 2104846. [Google Scholar] [CrossRef]
- Luo, X.; Ji, P.; Wang, P.; Cheng, R.; Chen, D.; Lin, C.; Zhang, J.; He, J.; Shi, Z.; Li, N.; et al. Interface engineering of hierarchical branched Mo-doped Ni3S2/NixPy hollow heterostructure nanorods for efficient overall water splitting. Adv. Energy Mater. 2020, 10, 1903891. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, Y.; Zhou, X.; Ye, Y.; Nie, K.; Wang, J.; Xie, M.; Zhang, Z.; Liu, Z.; Cheng, T.; et al. Promoting nickel oxidation state transitions in single-layer NiFeB hydroxide nanosheets for efficient oxygen evolution. Nat. Commun. 2022, 13, 6094. [Google Scholar] [CrossRef]
- Li, M.-X.; Xiao, B.; Zhao, Z.-Y.; Ma, Y.; Zhou, Y.-N.; Zhang, X.-Y.; Wang, F.-G.; Chai, Y.-M.; Dong, B. Morphology evolution regulation of dual-doped S, Fe-NiMoO4 microrods based on precipitation-dissolution equilibrium for oxygen evolution. Fuel 2023, 336, 126769. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, Z.; Gao, J.; Wang, E. Surface-oxidized iron–cobalt–nickel alloy with continuous variable composition for hydrogen and oxygen evolution reaction. ACS Sustain. Chem. Eng. 2022, 10, 14926–14934. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, L.; Yang, Z.; Wang, Y. Self-supported hierarchical porous FeNiCo-based amorphous alloys as high-efficiency bifunctional electrocatalysts towards overall water splitting. Int. J. Hydrogen Energy 2021, 46, 36731–36741. [Google Scholar] [CrossRef]
- Chen, J.; Ling, Y.; Qu, D.; Huang, L.; Li, J.; Tang, P.; He, A.; Jin, X.; Zhou, Y.; Xu, M.; et al. Enhanced electrocatalysis of NiMnIn Heusler alloy films for hydrogen evolution reaction by magnetic field. J. Alloys Compd. 2021, 877, 160271. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Li, C.; Pham, B.T.; Zhang, D. Electrodeposition of Ni-Fe-Mn ternary nanosheets as affordable and efficient electrocatalyst for both hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2020, 45, 24670–24683. [Google Scholar] [CrossRef]
- Srivastava, R.; Bhardwaj, S.; Kumar, A.; Robinson, A.N.; Sultana, J.; Mishra, S.R.; Perez, F.; Gupta, R.K. Bimetallic MnNi-hydroxide electrodeposited on Ni-foam for superior water-splitting and energy storage. Int. J. Hydrogen Energy 2024, 49, 971–983. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, L. The rise of manganese as catalysts for acidic water oxidation: A mini review. Electrochem. Commun. 2023, 151, 107505. [Google Scholar] [CrossRef]
- Chang, S.-Q.; Cheng, C.-C.; Cheng, P.-Y.; Huang, C.-L.; Lu, S.-Y. Pulse electrodeposited FeCoNiMnW high entropy alloys as efficient and stable bifunctional electrocatalysts for acidic water splitting. Chem. Eng. J. 2022, 446, 137452. [Google Scholar] [CrossRef]
- Huang, H.; Hu, X.; Hou, Z.; Yang, D.; Xiang, D.; Hu, L. Interfacial construction and lattice distortion-triggered bifunctionality of Mn-NiS/Mn-Ni3S4 for H2 production. Fuel 2022, 328, 125337. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, B.; Zhang, Q. Deep eutectic solvent mediated synthesis of Mn-based hybrid electrocatalyst for oxygen evolution reaction: Insights into the effect of anion on the evolution of structure-activity. Appl. Surf. Sci. 2024, 645, 158843. [Google Scholar] [CrossRef]
- Zhou, B.; Shao, Y.; Li, Z.; Yang, W.; Ren, X.; Hao, Y. Efficient energy-saving hydrogen production on binder-free electrodeposited hierarchical Ni-Mn-P@Ni-Co nanostructure. Int. J. Hydrogen Energy 2024, 51, 1022–1032. [Google Scholar] [CrossRef]
- Priamushko, T.; Guggenberger, P.; Mautner, A.; Lee, J.; Ryoo, R.; Kleitz, F. Enhancing OER activity of Ni/Co oxides via Fe/Mn substitution within tailored mesoporous frameworks. ACS Appl. Energy Mater. 2022, 5, 13385–13397. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Facile electrodeposition of Mn-CoP nanosheets on Ni foam as high-rate and ultrastable electrodes for supercapacitors. ACS Appl. Energy Mater. 2021, 5, 186–195. [Google Scholar] [CrossRef]
- Guo, D.; Duan, D.; Gao, J.; Zhou, X.; Liu, S.; Wang, Y. Synthesis of nest-like porous MnCo-P electrocatalyst by electrodeposition on nickel foam for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 6620–6630. [Google Scholar] [CrossRef]
- Aaboubi, O.; Ali-Omar, A.-Y.; Dzoyem, E.; Marthe, J.; Boudifa, M. Ni-Mn based alloys as versatile catalysts for different electrochemical reactions. J. Power Sources 2014, 269, 597–607. [Google Scholar] [CrossRef]
- Xiao, T.; Sun, C.; Wang, R. Electrodeposited CrMnFeCoNi Oxy-carbide film and effect of selective dissolution of Cr on oxygen evolution reaction. J. Mater. Sci. Technol. 2024, 200, 176–184. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Rajendiran, R.; Li, O.L.; Prabakar, K. Mn-Co bimetallic phosphate on electrodeposited PANI nanowires with composition modulated structural morphology for efficient electrocatalytic water splitting. Appl. Catal. B Environ. 2021, 292, 120202. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.; Du, X.; Zhang, X. Mn-doped nickel-copper phosphides as oxygen evolution reaction electrocatalyst in alkaline seawater solution. Int. J. Hydrogen Energy 2024, 69, 895–904. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, H.; Du, X.; Zhang, X. Fe, Mn-Ni3S2 directly grown on nickel foam as an environmentally friendly electrocatalyst for seawater splitting. Surf. Interfaces 2024, 46, 104079. [Google Scholar] [CrossRef]
- Barua, S.; Balčiūnaitė, A.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Bimetallic 3D nickel-manganese/titanium bifunctional electrocatalysts for efficient hydrogen and oxygen evolution reaction in alkaline and acidic media. Coatings 2023, 13, 1102. [Google Scholar] [CrossRef]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Li, W.; Wang, T.; Liang, C. Three-dimensional heterostructured NiCoP@NiMn-layered double hydroxide arrays supported on Ni foam as a bifunctional electrocatalyst for overall water splitting. ACS Appl. Mater. Interfaces 2020, 12, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, J.; Song, Q.; Liu, H. Double functionalization of Mo2C and NiMn-LDH assembling g-C3N4 as efficient bifunctional electrocatalysts for selective electrocatalytic reactions and overall water splitting. Int. J. Energy Res. 2022, 46, 12406–12416. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Harish, S.; Kumar, E.S.; Navaneethan, M. Interface engineering of heterogeneous NiMn layered double hydroxide/vertically aligned NiCo2S4 nanosheet as highly efficient hybrid electrocatalyst for overall seawater splitting. Chemosphere 2024, 350, 141016. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Subrata, K. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.E.; Zhang, Z.; Baranova, E.A. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Zabielaite, A.; Balciunaite, A.; Upskuviene, D.; Simkunaite, D.; Levinas, R.; Niaura, G.; Vaiciuniene, J.; Jasulaitiene, V.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Investigation of hydrogen and oxygen evolution on cobalt-nanoparticles-supported graphitic carbon nitride. Materials 2023, 16, 5923. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, H.; Yang, Y.; Gong, S.; Li, Y.; Wang, Y.; Luo, W.; Li, Z. Nickel and manganese oxide heterostructure nanoparticles supported by carbon nanotube for highly efficient oxygen evolution reaction catalysis. Appl. Surf. Sci. 2022, 575, 151699. [Google Scholar] [CrossRef]
- Sana Fathima, T.K.; Ghosh, A.; Ramaprabhu, S. ZIF67-derived Co-CoO@C nanocomposites as highly efficient and selective oxygen evolution reaction (OER) catalysts for seawater electrolysis. Int. J. Hydrogen Energy 2024, 49, 780–793. [Google Scholar] [CrossRef]
- Wang, H.; Ying, J.; Xiao, Y.X.; Chen, J.B.; Li, J.H.; He, Z.Z.; Yang, H.J.; Yang, X.Y. Ultrafast synthesis of Cu2O octahedrons inlaid in Ni foam for efficient alkaline water/seawater electrolysis. Electrochem. Commun. 2022, 134, 107177. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Li, Z.; Li, C.; Ren, G.; Zhang, Z.; Meng, X. Modulating the electronic structure on cobalt sites by compatible heterojunction fabrication for greatly improved overall water/seawater electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 9980–9990. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Petrulevičienė, M.; Butkutė, S.; Jasulaitienė, V.; Selskis, A.; Ramanauskas, R. Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution. Int. J. Hydrogen Energy 2019, 44, 5929–5939. [Google Scholar] [CrossRef]
- Cheng, F.; Feng, X.; Chen, X.; Lin, W.; Rong, J.; Yang, W. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 2017, 251, 336–343. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Ramani, V. Selective seawater splitting using pyrochlore electrocatalyst. ACS Appl. Energy Mater. 2020, 3, 3978–3983. [Google Scholar] [CrossRef]
- Wang, N.; Ou, P.; Hung, S.-F.; Huang, J.E.; Ozden, A.; Abed, J.; Grigioni, I.; Chen, C.; Miao, R.K.; Yan, Y.; et al. Strong-proton-adsorption Co-based electrocatalysts achieve active and stable neutral seawater splitting. Adv. Mater. 2023, 35, 2210057. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lin, C.-Y. Iron phosphate modified calcium iron oxide as an efficient and robust catalyst in electrocatalyzing oxygen evolution from seawater. Farad. Disc. 2019, 215, 205–215. [Google Scholar] [CrossRef]
- Zhuang, L.; Li, J.; Wang, K.; Li, Z.; Zhu, M.; Xu, Z. Structural buffer engineering on metal oxide for long-term stable seawater splitting. Adv. Funct. Mater. 2022, 32, 2201127. [Google Scholar] [CrossRef]
- Liu, F.; Hu, R.; Qiu, H.; Miao, H.; Wang, Q.; Yuan, J. Constructing high-activity cobalt-based perovskite hybrid by a top-down phase evolution method for the direct seawater electrolysis anode. J. Alloys Compd. 2022, 913, 165342. [Google Scholar] [CrossRef]
- Barua, S.; Balčiūnaitė, A.; Upskuvienė, D.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. 3D Nickel-manganese bimetallic electrocatalysts for an enhanced hydrogen evolution reaction performance in simulated seawater/alkaline natural seawater. Int. J. Hydrogen Energy 2024, 79, 1490–1500. [Google Scholar] [CrossRef]
- Saquib, M.; Arora, P.; Bhosale, A.C. Nickel molybdenum selenide on carbon cloth as an efficient bifunctional electrocatalyst for alkaline seawater splitting. Fuel 2024, 365, 131251. [Google Scholar] [CrossRef]
- Yang, T.; Lv, H.; Quan, Q.; Li, X.; Lu, H.; Cui, X.; Liu, G.; Jiang, L. Electronic structure modulation of MoO2 via Er-doping for efficient overall water/seawater splitting and Mg/seawater batteries. Appl. Surf. Sci. 2023, 615, 156360. [Google Scholar] [CrossRef]
- Austeria, M.; Dao, H.T.; Mai, M.; Kim, D.H. Dual-phase cobalt phosphide/phosphate hybrid interactions via iridium nanocluster interfacial engineering toward efficient overall seawater splitting. Appl. Catal. B Environ. 2023, 327, 122467. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, X.; He, L.; Zheng, Y.; Pang, J.; Wang, L.; Jiang, R.; Hou, J.; Guo, X.; Chen, L. Structural and electronic modulation of iron-based bimetallic metal-organic framework bifunctional electrocatalysts for efficient overall water splitting in alkaline and seawater environments. ACS Appl. Mater. Interfaces 2022, 14, 46374–46385. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Saranya, V.; Harish, S.; Kumar, E.S.; Navaneethan, M. Heterogeneous bimetallic oxysulfide nanostructure (Ni-Co) as hybrid bifunctional electrocatalyst for sustainable overall alkaline simulated seawater splitting. J. Alloys Compd. 2023, 965, 171124. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.; Kim, S.H.; Park, J.; Kim, S.; Kwon, S.-H.; Bae, J.-S.; Park, Y.S.; Kim, Y. Cobalt-iron-phosphate hydrogen evolution reaction electrocatalyst for solar-driven alkaline seawater electrolyzer. Nanomaterials 2021, 11, 2989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, L.; Yan, B.; Zhang, F.; Shi, Y.; Guo, X. Regeneration of textile sludge into Cu8S5 decorated N, S self-doped interconnected porous carbon as an advanced bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2023, 451, 138497. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen generation from seawater electrolysis over a sandwich-like NiCoN|NixP|NiCoN microsheet array catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Zhang, G.; Wu, S.; Chen, Z.; Ranganathan, H.; Sun, S.; Shi, Z. Mn-doped nickel-iron phosphide heterointerface nanoflowers for efficient alkaline freshwater/seawater splitting at high current densities. Chem. Eng. J. 2023, 454, 140061. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Hu, H.; Zheng, Y.; Yuan, Z.; Dai, Y.; Zhang, T.; Yang, D.; Xue, S.; Qiu, F. Unique sandwich-cookie-like nanosheet array heterojunction bifunctional electrocatalyst towards efficient overall water/seawater splitting. J. Colloid Interface Sci. 2024, 669, 935–943. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Luo, J.; Kang, Z.; Jia, C.; Liu, Z.; Huang, W.; Chen, Q.; Deng, P.; Shen, Y.; et al. Mo-decorated cobalt phosphide nanoarrays as bifunctional electrocatalysts for efficient overall water/seawater splitting. Mater. Today Nano 2022, 18, 100216. [Google Scholar] [CrossRef]
- Debnath, B.; Parvin, S.; Dixit, H.; Bhattacharyya, S. Oxygen-defect-rich cobalt ferrite nanoparticles for practical water electrolysis with high activity and durability. ChemSusChem 2020, 13, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.-Z. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef] [PubMed]
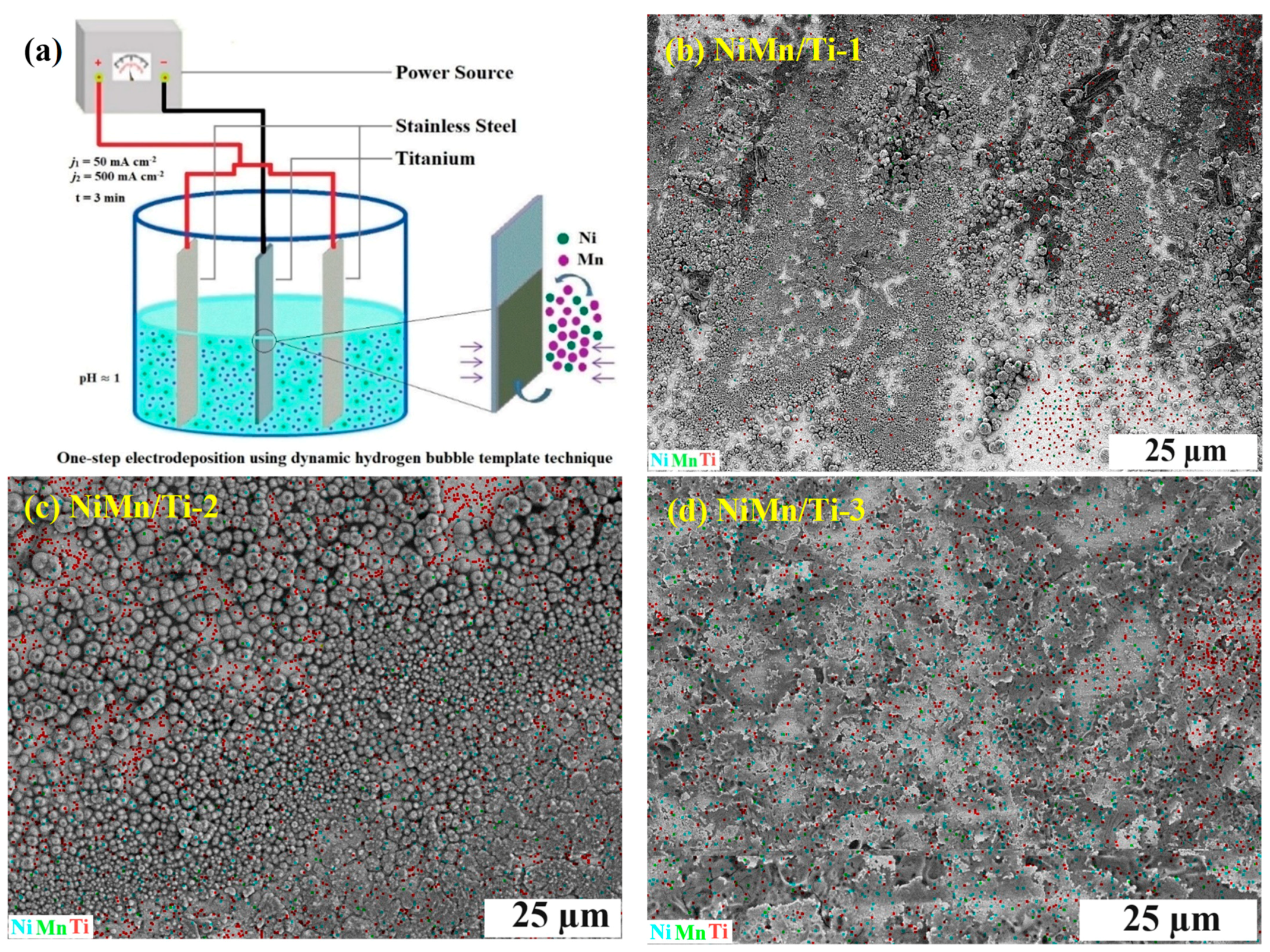
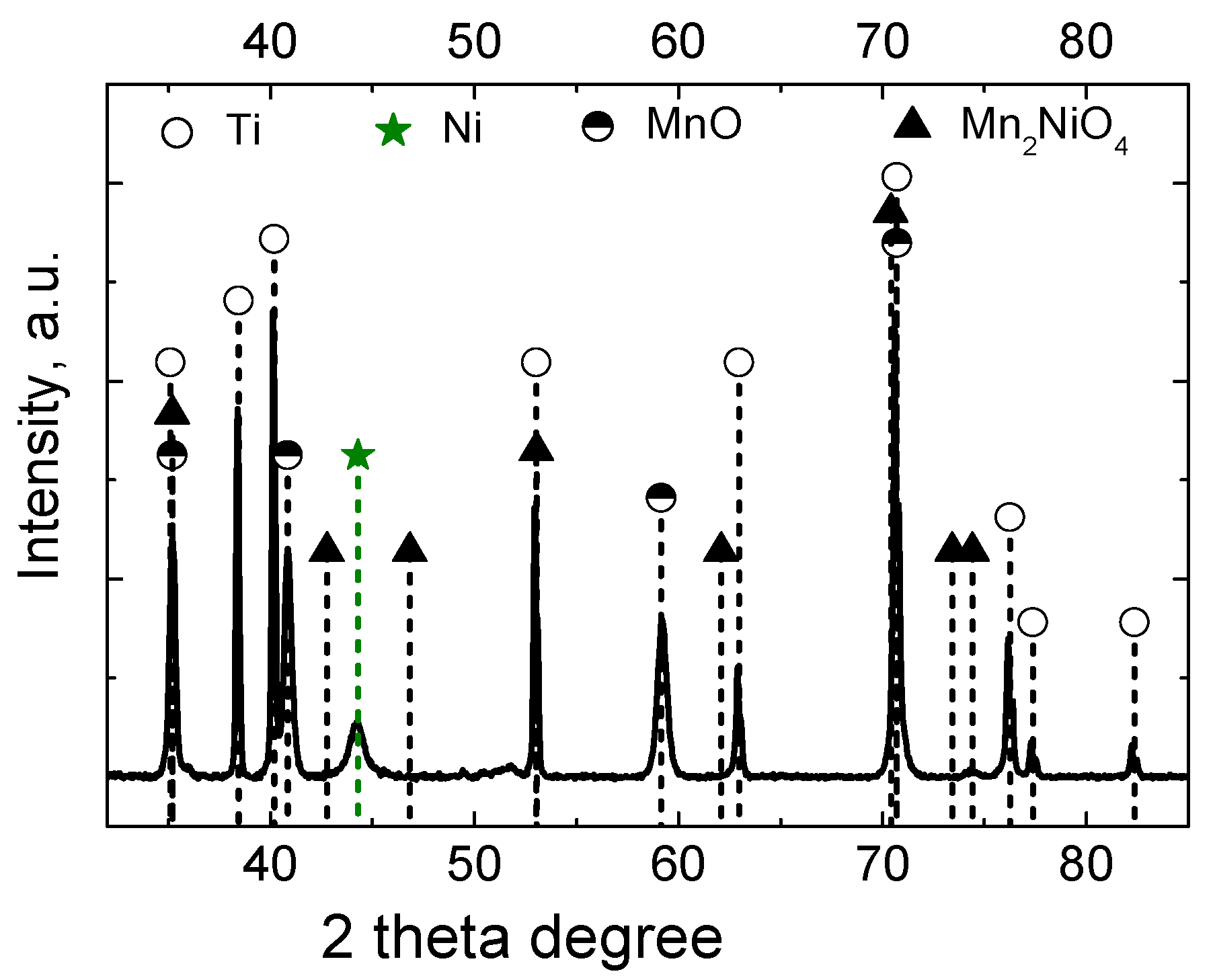

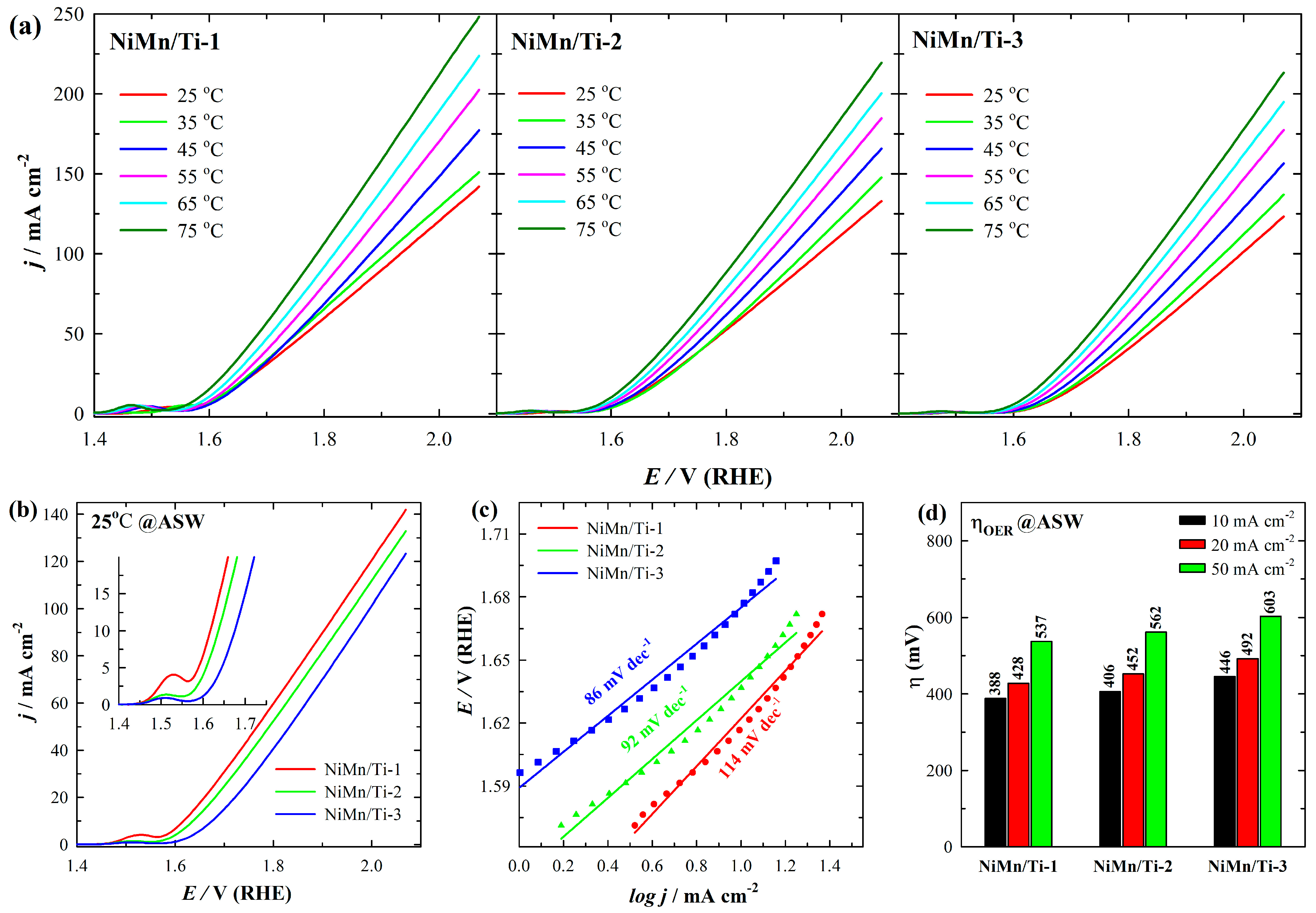



| Catalyst | Concentration (M) | Plating Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| NiSO4 | MnCl2 | (NH4)2SO4 | H3BO3 | pH * | T (°C) | j (mA cm−2) | t (min) | |
| NiMn/Ti-1 NiMn/Ti-2 NiMn/Ti-3 | 0.2 0.2 0.2 | 0.2 0.4 0.6 | 0.5 0.5 0.5 | 0.3 0.3 0.3 | ~1 | 25 | 50 500 | 3 3 |
| Catalyst | Ni Loadings (µgNicm−2) | Mn Loadings (µgMncm−2) | Total Metal Loading (µgmetalcm−2) | Wt% | |
|---|---|---|---|---|---|
| Ni | Mn | ||||
| NiMn/Ti-1 | 86.55 | 13.43 | 99.98 | 86.56 | 13.44 |
| NiMn/Ti-2 | 126.4 | 40.55 | 166.95 | 75.71 | 24.29 |
| NiMn/Ti-3 | 269.7 | 105.25 | 374.95 | 71.93 | 28.07 |
| Catalysts | j at 2.06 V (mA cm−2) | η10 at 25 °C (mV) | Tafel Slope (mV dec−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | 65 °C | 75 °C | |||
| NiMn/Ti-1 | 182.57 | 203.48 | 231.38 | 250.69 | 269.53 | 286.1 | 386 | 130 |
| NiMn/Ti-2 | 144.36 | 158.77 | 177.15 | 195.43 | 210.07 | 232.39 | 413 | 120 |
| NiMn/Ti-3 | 127.68 | 141.57 | 164.31 | 182.45 | 197.2 | 219.21 | 454 | 86 |
| Catalysts | j at 2.06 V(mA cm−2) | η10 at 25 °C(mV) | Tafel Slope (mV dec−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | 65 °C | 75 °C | |||
| NiMn/Ti-1 | 141.97 | 151.05 | 177.2 | 202.47 | 223.75 | 248.18 | 388 | 114 |
| NiMn/Ti-2 | 132.92 | 147.65 | 165.8 | 184.67 | 200.39 | 219.45 | 406 | 92 |
| NiMn/Ti-3 | 123.3 | 136.9 | 156.51 | 177.4 | 195 | 213.23 | 446 | 86 |
| Catalysts | η10 (mV) | Tafel Slope (mV dec−1) | Electrolytes | Ref. |
|---|---|---|---|---|
| NiMn/Ti-1 | 386 388 | 130 114 | 1 M KOH + 0.5 M NaCl 1 M KOH + Seawater | This work |
| Co-CoO@C (denoted as ZIF67-600Ar/GF) | 374 | – | 1 M KOH + Seawater | [90] |
| oct_Cu2O-NF | 354 | 90 | 1 M KOH + 0.5 M NaCl | [91] |
| CoSe/MoSe2/NF | 350 | – | 1 M KOH + Seawater | [92] |
| FTO/NiO | 340 | - | 1 M KOH + 0.5 M NaCl | [93] |
| CoFe-LDH | 530 | – | Simulated seawater (pH 8.0) | [94] |
| Pb2Ru2O7−x | 500 | ~48 | Natural simulated seawater (only 0.6M NaCl) | [95] |
| Co3O4 Co3−xPdxO4 | 440 370 | – | 1 M PBS + 0.5 M NaCl | [96] |
| CaFeOx|FePO4 | ~710 | – | Phosphate-buffered (0.5 M, pH 7) seawater | [97] |
| Co(OH)3Cl | 379 | – | 1 M KOH + 0.6 M NaCl | [98] |
| ER-RP/P-SNCF-5 | 332 346 | – | 1 M KOH + 0.5 M NaCl 1 M KOH + Seawater | [99] |
| Electrodes (−||+) | Potential@Current Density (V@mA cm−2) | Electrolyte | Ref. |
|---|---|---|---|
| NiMn/Ti-5||NiMn/Ti-1 Pt||NiMnTi-1 | 1.619@10 1.694@10 | 1 M KOH + Seawater | This work |
| FeNiCoMnRu@CNT (−||+) | 1.6@10 | 1 M KOH + Seawater | [56] |
| Pt/C/GF||ZIF67-600Ar/GF | 1.63@20 | 1 M KOH + Seawater | [90] |
| oct_Cu2O-NF||oct_Cu2O-NF | 1.71@10 | 1 M KOH + 0.5 M NaCl | [91] |
| CoSe/MoSe2/NF||CoSe/MoSe2/NF | 1.69@10 1.77@10 | 1 M KOH + 0.5 M NaCl 1 M KOH + Seawater | [92] |
| NiMoSe@CC||NiMoSe@CC | 1.63@10 | 1 M KOH + 0.5 M NaCl | [101] |
| 3%Er-MoO2||3%Er-MoO2 | 1.67@10 | 1.0 M KOH + 3.5% NaCl | [102] |
| Ir0.05-Co2P/Co2P2O7 NW/NF (−||+) | 1.6@10 1.67@10 | 1 M KOH + Seawater Seawater | [103] |
| CdFe-BDC||CdFe-BDC | 1.68@10 | Seawater | [104] |
| Co-Ni-S/NF||Co-Ni-S/NF | 1.67@10 | 1 M KOH + 0.5 M NaCl | [105] |
| NiFeOOH||(Co,Fe)PO4 | 1.625@10 | 1 M KOH + Seawater | [106] |
| Cu8S5/NSC-900||Cu8S5/NSC-900 | 1.58@10 1.65@10 | 1 M KOH + 0.5 M NaCl 1 M KOH + Seawater | [107] |
| NiCoN|NixP|NiCoN||S-(Ni,Fe)OOH | 1.81@10 | Seawater | [108] |
| Mn-doped Ni2P/Fe2P (−||+) | 1.64@10 | 1 M KOH + 0.5 M NaCl | [109] |
| RNPOH/NF||RNPOH/NF | 1.75@50 | 1 M KOH + Seawater | [110] |
| Mo-CoPx/NF||Mo-CoPx/NF | 1.59@10 1.61@10 | 1 M KOH + 0.5 M NaCl 1 M KOH + Seawater | [111] |
| CoF-2||CoF-3 | 1.72@10 1.76@10 | 1 M KOH + 0.6 M NaCl 1 M KOH + Seawater | [112] |
| Ni-SN@C||Ni-SN@C | 1.72@10 | 1 M KOH + Seawater | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, S.; Balčiūnaitė, A.; Upskuvienė, D.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Bimetallic Ni–Mn Electrocatalysts for Stable Oxygen Evolution Reaction in Simulated/Alkaline Seawater and Overall Performance in the Splitting of Alkaline Seawater. Coatings 2024, 14, 1074. https://doi.org/10.3390/coatings14081074
Barua S, Balčiūnaitė A, Upskuvienė D, Vaičiūnienė J, Tamašauskaitė-Tamašiūnaitė L, Norkus E. Bimetallic Ni–Mn Electrocatalysts for Stable Oxygen Evolution Reaction in Simulated/Alkaline Seawater and Overall Performance in the Splitting of Alkaline Seawater. Coatings. 2024; 14(8):1074. https://doi.org/10.3390/coatings14081074
Chicago/Turabian StyleBarua, Sukomol, Aldona Balčiūnaitė, Daina Upskuvienė, Jūrate Vaičiūnienė, Loreta Tamašauskaitė-Tamašiūnaitė, and Eugenijus Norkus. 2024. "Bimetallic Ni–Mn Electrocatalysts for Stable Oxygen Evolution Reaction in Simulated/Alkaline Seawater and Overall Performance in the Splitting of Alkaline Seawater" Coatings 14, no. 8: 1074. https://doi.org/10.3390/coatings14081074









