Synthesis and Characterization of Titania-Coated Hollow Mesoporous Hydroxyapatite Composites for Photocatalytic Degradation of Methyl Red Dye in Water
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of HM-HAP Particles
2.3. Synthesis of TiO2/HM-HAP Composite
2.4. Characterization of TiO2/HM-HAP Composite
2.5. Point of Zero Charge (PZC)
2.6. Photocatalytic Studies of MR
2.7. Kinetics of MR Degradation
3. Results and Discussion
3.1. Synthesis of TiO2/HM-HAP Composite
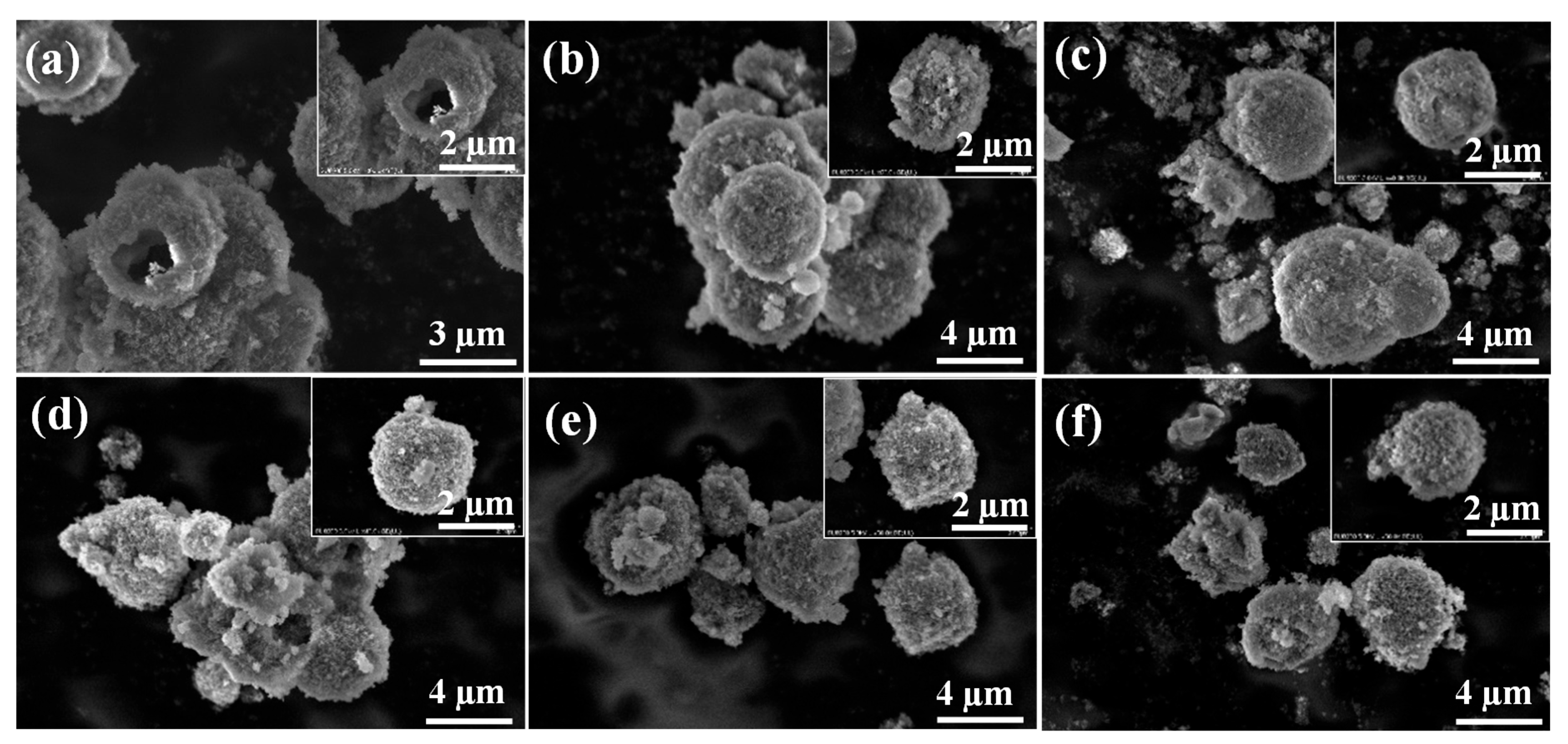
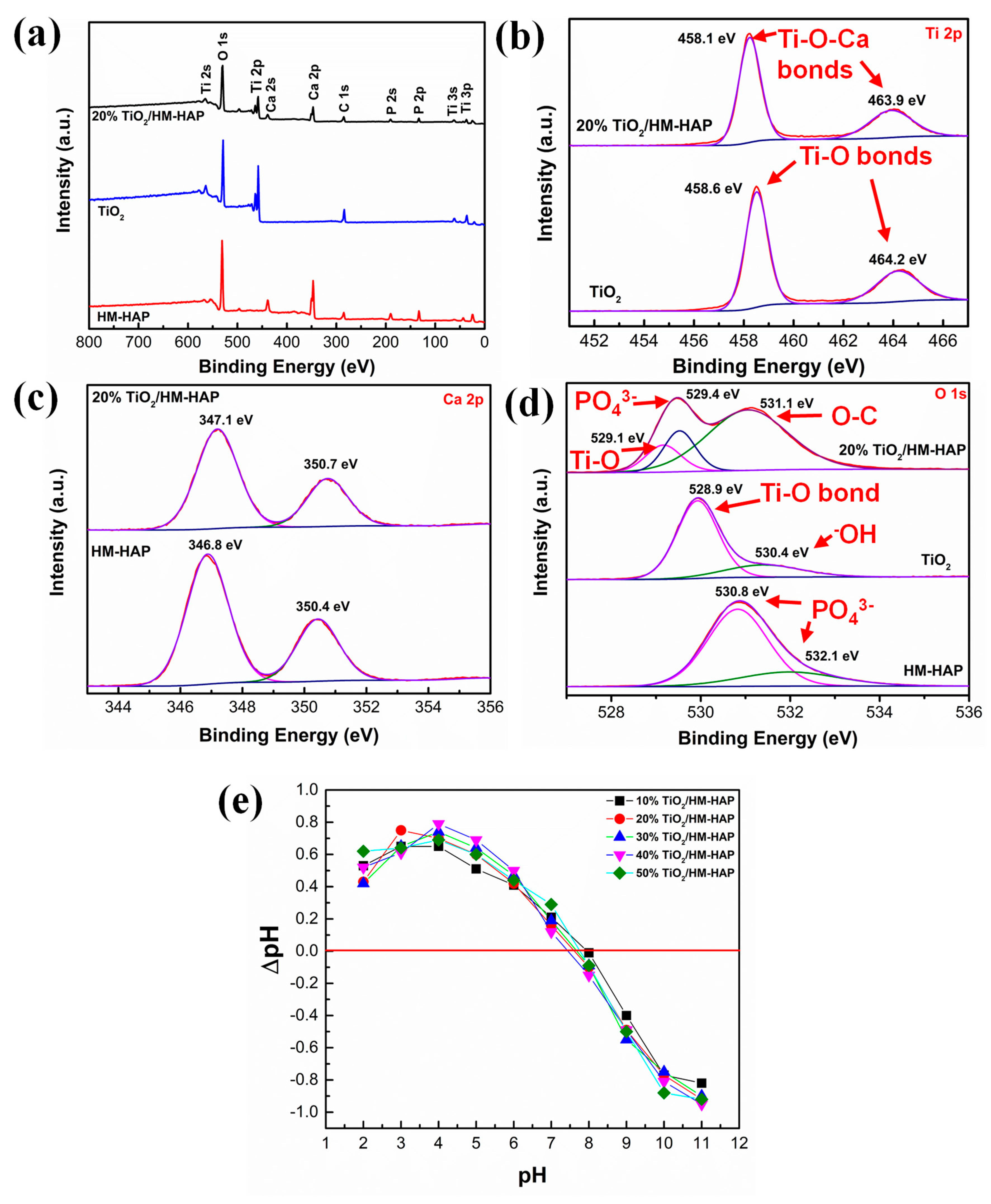
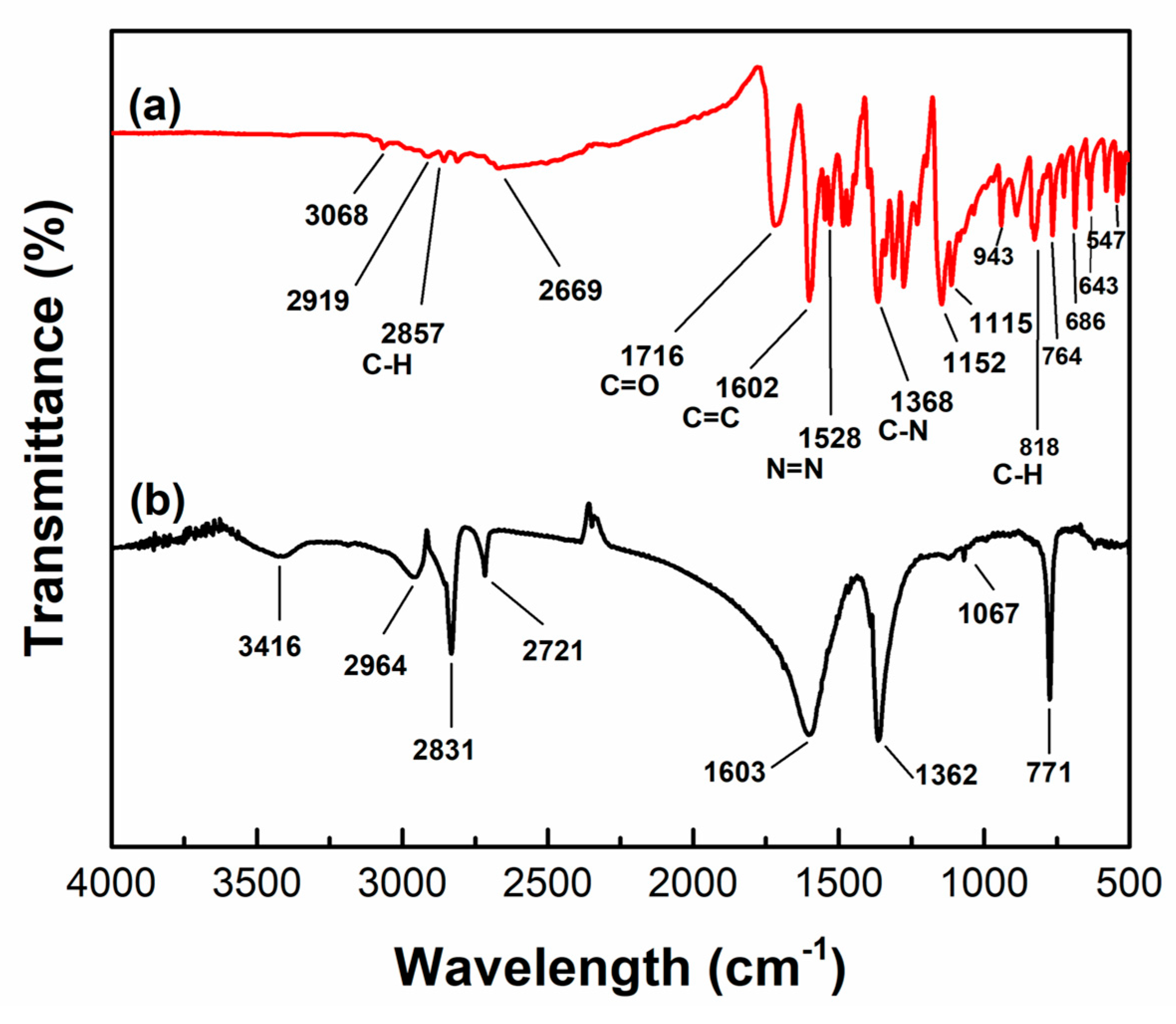
3.2. Characterization of the TiO2/HM-HAP Composite
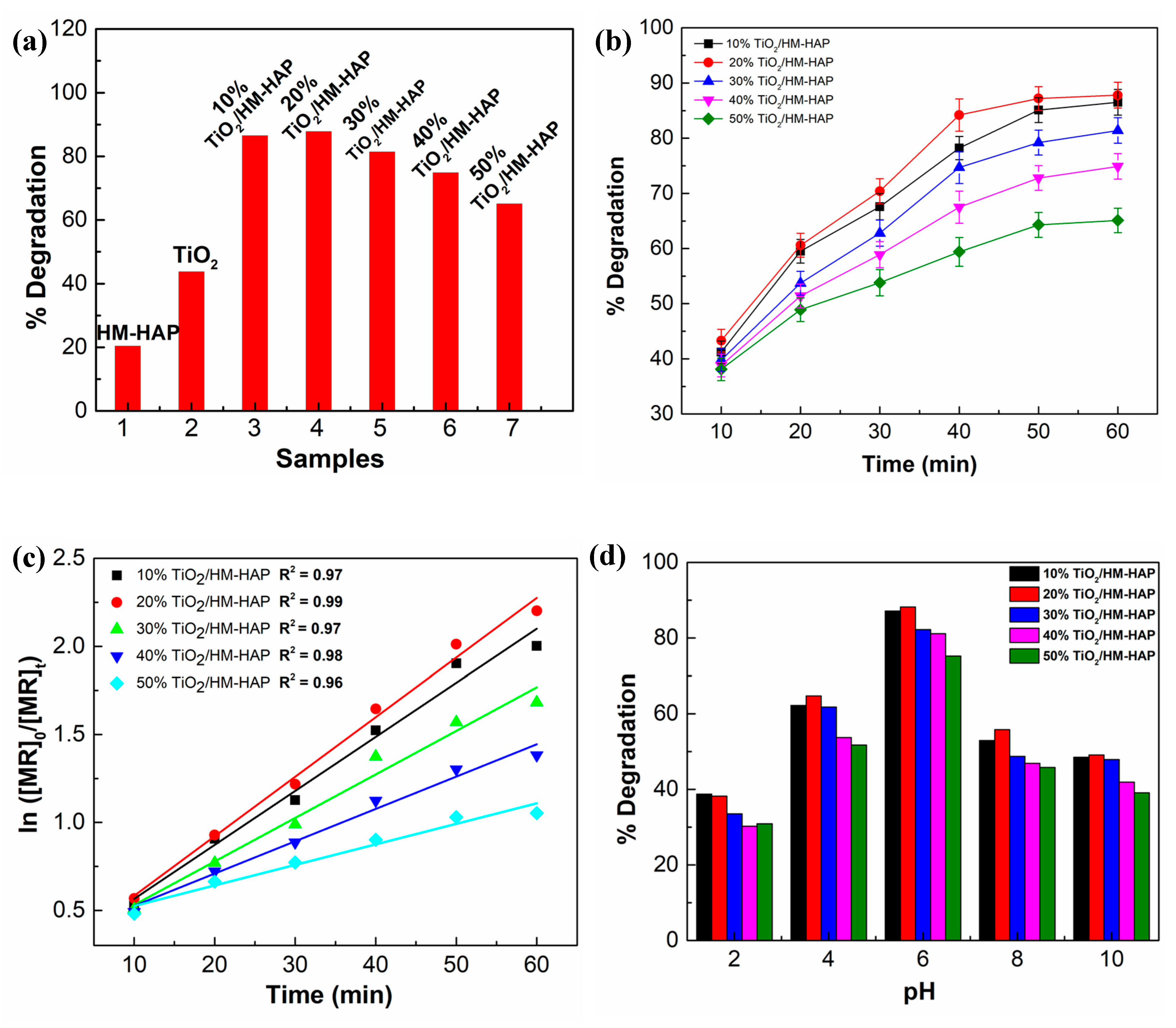
3.3. Evaluation of the Photocatalytic Performance of the Synthesized Composites for the Degradation of MR
3.3.1. Photocatalytic Degradation Kinetics
3.3.2. Effect of pH on the Degradation of MR
3.3.3. Post-Photodegradation FTIR Analysis and Mineralization Study of MR Dye
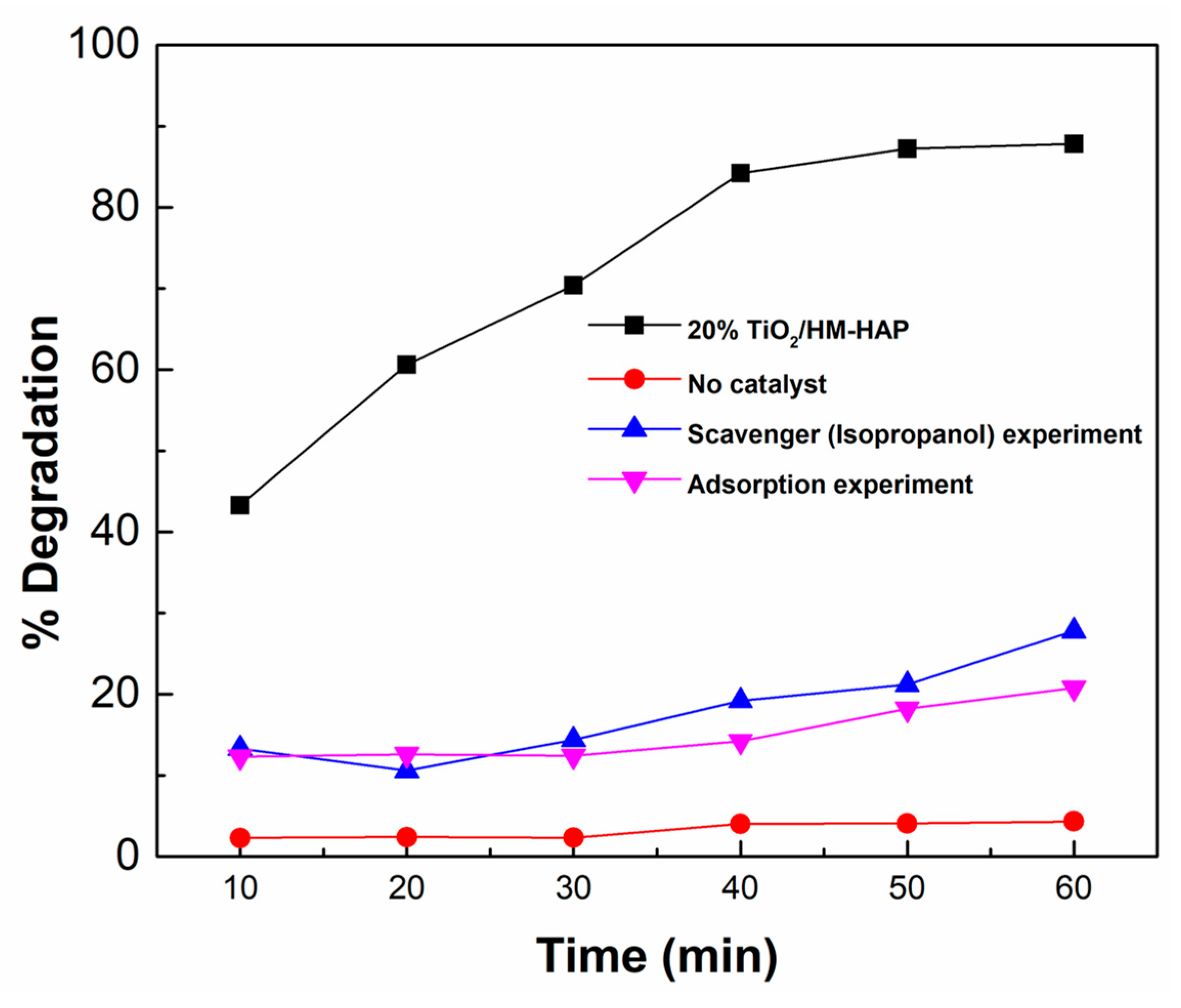
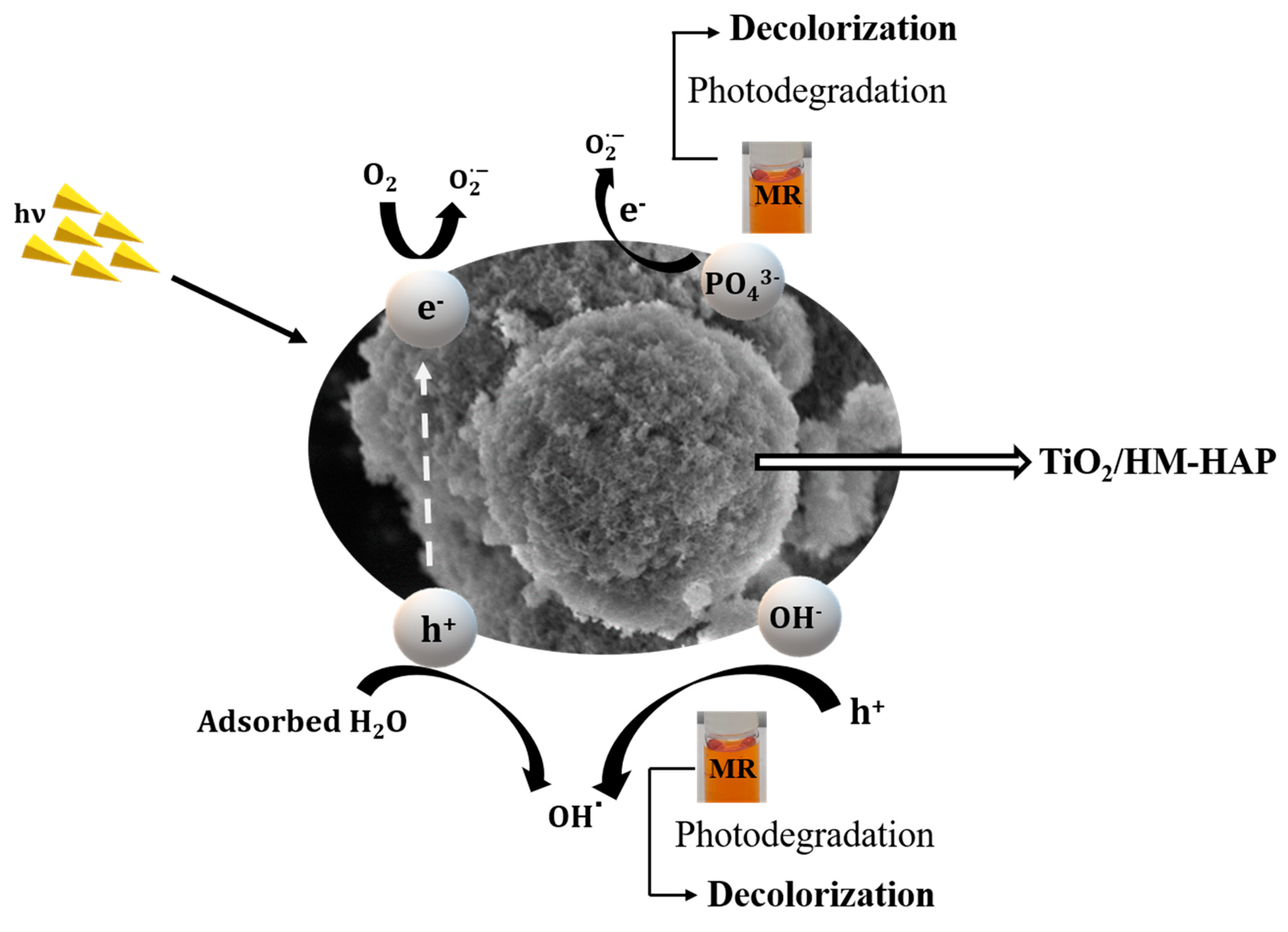
3.4. Photocatalytic Degradation Mechanism of TiO2/HM-HAP Composites on MR Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badr, Y.; Abd El-Wahed, M.G.; Mahmoud, M.A. Photocatalytic degradation of methyl red dye by silica nanoparticles. J. Hazard. Mater. 2008, 154, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, Z.; AL-Asfar, A.; Aazam, E.S. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J. Mol. Liq. 2019, 283, 287–298. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Majcen Le Marechal, A. Methods of decoloration of textile wastewaters. Dye. Pigment. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Gökçen, F.; Özbelge, T.A. Pre-ozonation of aqueous azo dye (Acid Red-151) followed by activated sludge process. Chem. Eng. J. 2006, 123, 109–115. [Google Scholar] [CrossRef]
- Khan, A.M.; Shafiq, F.; Khan, S.A.; Ali, S.; Ismail, B.; Hakeem, A.S.; Rahdar, A.; Nazar, M.F.; Sayed, M.; Khan, A.R. Surface modification of colloidal silica particles using cationic surfactant and the resulting adsorption of dyes. J. Mol. Liq. 2019, 274, 673–680. [Google Scholar] [CrossRef]
- Ullah, F.; Ji, G.; Irfan, M.; Gao, Y.; Shafiq, F.; Sun, Y.; Ain, Q.U.; Li, A. Adsorption performance and mechanism of cationic and anionic dyes by KOH activated biochar derived from medical waste pyrolysis. Environ. Pollut. 2022, 314, 120271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, H.; Xue, P.; Tian, S.; Sun, S.; Lv, X. Highly-efficient PVDF adsorptive membrane filtration based on chitosan@CNTs-COOH simultaneous removal of anionic and cationic dyes. Carbohydr. Polym. 2021, 274, 118664. [Google Scholar] [CrossRef] [PubMed]
- Saulat, H.; Yang, J.; Yan, T.; Raza, W.; Song, W.; He, G. Tungsten incorporated mobil-type eleven zeolite membranes: Facile synthesis and tuneable wettability for highly efficient separation of oil/water mixtures. Chin. J. Chem. Eng. 2023, 60, 242–252. [Google Scholar] [CrossRef]
- Badr, Y.; Mahmoud, M.A. Photocatalytic degradation of methyl orange by gold silver nano-core/silica nano-shell. J. Phys. Chem. Solids 2007, 68, 413–419. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Gil Lee, D.; Weon Roh, S.; Lee, C. Nitrogen-centered radical-mediated C–H imidation of arenes and heteroarenes via visible light induced photocatalysis. Chem. Commun. 2014, 50, 9273–9276. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Dias, A.A.; Sampaio, A.; Amaral, C.; Peres, J.A. Degradation of a textile reactive Azo dye by a combined chemical–biological process: Fenton’s reagent-yeast. Water Res. 2007, 41, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.Y.; Wei, J.H.; Xiong, R.; Pan, C.X.; Shi, J. Enhanced adsorption and visible-light-induced photocatalytic activity of hydroxyapatite modified Ag–TiO2 powders. Appl. Surf. Sci. 2010, 256, 6390–6394. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.K.; Tayade, R.J. Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal. Today 2017, 282, 13–23. [Google Scholar] [CrossRef]
- Wu, H.; Yang, X.; Zhao, S.; Zhai, L.; Wang, G.; Zhang, B.; Qin, Y. Encapsulation of atomically dispersed Pt clusters in porous TiO2 for semi-hydrogenation of phenylacetylene. Chem. Commun. 2022, 58, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, S.; Sun, Z.; Li, Z.; Wang, C.; Hu, Y.H. Thin-water-film-enhanced TiO2-based catalyst for CO2 hydrogenation to formic acid. Chem. Commun. 2022, 58, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lv, T.; Zhu, C.; Zhu, Z. Direct bandgap narrowing of TiO2/MoO3 heterostructure composites for enhanced solar-driven photocatalytic activity. Sol. Energy Mater. Sol. Cells 2016, 153, 1–8. [Google Scholar] [CrossRef]
- Sukhadeve, G.K.; Bandewar, H.; Janbandhu, S.Y.; Jayaramaiah, J.R.; Gedam, R.S. Photocatalytic hydrogen production, dye degradation, and antimicrobial activity by Ag-Fe co-doped TiO2 nanoparticles. J. Mol. Liq. 2023, 369, 120948. [Google Scholar] [CrossRef]
- Ali, F.; Moin-ud-Din, G.; Iqbal, M.; Nazir, A.; Altaf, I.; Alwadai, N.; Siddiqua, U.H.; Younas, U.; Ali, A.; Kausar, A.; et al. Ag and Zn doped TiO2 nano-catalyst synthesis via a facile green route and their catalytic activity for the remediation of dyes. J. Mater. Res. Technol. 2023, 23, 3626–3637. [Google Scholar] [CrossRef]
- Liza, T.Z.; Tusher, M.M.H.; Anwar, F.; Monika, M.F.; Amin, K.F.; Asrafuzzaman, F.N.U. Effect of Ag-doping on morphology, structure, band gap and photocatalytic activity of bio-mediated TiO2 nanoparticles. Results Mater. 2024, 22, 100559. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A. Silver nanoparticles decorated TiO2 nanoflakes for antibacterial properties. Inorg. Chem. Commun. 2023, 152, 110675. [Google Scholar] [CrossRef]
- Cabrera-Rodríguez, O.; Trejo-Valdez, M.D.; Torres-SanMiguel, C.R.; Pérez-Hernández, N.; Bañuelos-Hernández, Á.; Manríquez-Ramírez, M.E.; Hernández-Benítez, J.A.; Rodríguez-Tovar, A.V. Evaluation of the performance of TiO2 thin films doped with silver nanoparticles as a protective coating for metal prostheses. Surf. Coatings Technol. 2023, 458, 129349. [Google Scholar] [CrossRef]
- Meroni, D.; Galloni, M.G.; Cionti, C.; Cerrato, G.; Falletta, E.; Bianchi, C.L. Efficient Day-and-Night NO2 Abatement by Polyaniline/TiO2 Nanocomposites. Materials 2023, 16, 1304. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Jana, T.K.; Chatterjee, K. Silica supported TiO2 nanostructures for highly efficient photocatalytic application under visible light irradiation. Mater. Res. Bull. 2016, 76, 353–357. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Fazli, M.; Rasoulifard, M.H.; Choi, H. Decomposition of organic chemicals by zeolite-TiO2 nanocomposite supported onto low density polyethylene film under UV-LED powered by solar radiation. Appl. Catal. B Environ. 2016, 183, 407–416. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Ul Haq, E.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Zeng, G.; You, H.; Du, M.; Zhang, Y.; Ding, Y.; Xu, C.; Liu, B.; Chen, B.; Pan, X. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of aquatic naphthalene under sunlight irradiation. Chem. Eng. J. 2021, 412, 128498. [Google Scholar] [CrossRef]
- Sans, J.; Arnau, M.; Sanz, V.; Turon, P.; Alemán, C. Hydroxyapatite-based biphasic catalysts with plasticity properties and its potential in carbon dioxide fixation. Chem. Eng. J. 2022, 433, 133512. [Google Scholar] [CrossRef]
- Sayed, I.R.; Farhan, A.M.; AlHammadi, A.A.; El-Sayed, M.I.; Abd El-Gaied, I.M.; El-Sherbeeny, A.M.; Al Zoubi, W.; Ko, Y.G.; Abukhadra, M.R. Synthesis of novel nanoporous zinc phosphate/hydroxyapatite nano-rods (ZPh/HPANRs) core/shell for enhanced adsorption of Ni2+ and Co2+ ions: Characterization and application. J. Mol. Liq. 2022, 360, 119527. [Google Scholar] [CrossRef]
- Ding, Z.; Han, H.; Fan, Z.; Lu, H.; Sang, Y.; Yao, Y.; Cheng, Q.; Lu, Q.; Kaplan, D.L. Nanoscale Silk-Hydroxyapatite Hydrogels for Injectable Bone Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 16913–16921. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Liu, C.; Liu, C.; Wang, L.; Shafiq, F.; Liu, X.; Sun, G.; Song, Q.; Qiao, W. Biomimetic hydroxyapate/polydopamine composites with good biocompatibility and efficiency for uncontrolled bleeding. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef] [PubMed]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Darmokoesoemo, H.; Kusuma, H.S. Hydroxyapatite-based adsorbents: Applications in sequestering heavy metals and dyes. J. Environ. Manag. 2022, 302, 113989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, B.; Chen, T.; Tu, S.; Li, H.; Yang, Z.; Hao, T.; Chen, C. Piezoelectric hydroxyapatite synthesized from municipal solid waste incineration fly ash and its underlying mechanism for high efficiency in degradation of xanthate. Chem. Eng. J. 2024, 493, 152601. [Google Scholar] [CrossRef]
- Kumar Yadav, M.; Hiren Shukla, R.; Prashanth, K.G. A comprehensive review on development of waste derived hydroxyapatite (HAp) for tissue engineering application. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tong, H.; Shi, D.; Cai, H.; Liu, J.; Lv, M.; Gu, L.; Luo, L.; Wang, B. Novel hydroxyapatite (HAP)-assisted hydrothermal solidification of heavy metals in fly ash from MSW incineration: Effect of HAP liquid-precursor and HAP seed crystal derived from eggshell waste. Fuel Process. Technol. 2022, 236, 107400. [Google Scholar] [CrossRef]
- Nishikawa, H. Surface changes and radical formation on hydroxyapatite by UV irradiation for inducing photocatalytic activation. J. Mol. Catal. A Chem. 2003, 206, 331–338. [Google Scholar] [CrossRef]
- El, A.; Boumanchar, I.; Zbair, M.; Chhiti, Y.; Sahibed-Dine, A.; Bentiss, F.; Bensitel, M. The photocatalytic degradation of methylene bleu over TiO2 catalysts supported on hydroxyapatite. JMES 2017, 8, 1301–1311. Available online: http://www.jmaterenvironsci.com/ (accessed on 7 October 2022).
- Sharifat, S.; Zolgharnein, H.; Hamidifalahi, A.; Enayati-Jazi, M.; Hamid, E. Preparation and Characterization of HAp/TiO2 Nanocomposite for Photocatalytic Degradation of Methyl Orange under UV-Irradiation. Adv. Mater. Res. 2014, 829, 594–599. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Wang, Y.; Chen, M.; Huang, Y.; Cao, J.; Ho, W.; Lee, S.C. Enhanced photocatalytic removal of NO over titania/hydroxyapatite (TiO2/HAp) composites with improved adsorption and charge mobility ability. RSC Adv. 2017, 7, 24683–24689. [Google Scholar] [CrossRef]
- Narayan, R.B.; Goutham, R.; Srikanth, B.; Gopinath, K.P. A novel nano-sized calcium hydroxide catalyst prepared from clam shells for the photodegradation of methyl red dye. J. Environ. Chem. Eng. 2018, 6, 3640–3647. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Xu, Q.; Feng, J.; Li, L.; Xiao, Q.; Wang, J. Hollow ZnFe2O4/TiO2 composites: High-performance and recyclable visible-light photocatalyst. J. Alloys Compd. 2015, 641, 110–118. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Jose, M. Dielectric functionalities of anatase phase titanium dioxide nanocrystals synthesized using water-soluble complexes. Appl. Phys. A 2017, 123, 512. [Google Scholar] [CrossRef]
- Wei, J.; Shi, J.; Wu, Q.; Yang, L.; Cao, S. Hollow hydroxyapatite/polyelectrolyte hybrid microparticles with controllable size, wall thickness and drug delivery properties. J. Mater. Chem. B 2015, 3, 8162–8169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, J.; Ma, H.; Chen, R.; Li, J.; Cao, S. Hierarchical hydroxyapatite/polyelectrolyte microcapsules capped with AuNRs for remotely triggered drug delivery. Mater. Sci. Eng. C 2019, 99, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, F.; Liu, C.; Zhou, H.; Chen, H.; Yu, S.; Qiao, W. Adsorption mechanism and synthesis of adjustable hollow hydroxyapatite spheres for efficient wastewater cationic dyes adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 672, 131713. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, S. Photodegradation of methyl orange by photocatalyst of CNTs/P-TiO2 under UV and visible-light irradiation. J. Hazard. Mater. 2011, 185, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, B.; Wang, Y.; Xiao, G.; Chen, X.; Xu, W.; Lu, Y.P. A facile strategy for one-step hydrothermal preparation of porous hydroxyapatite microspheres with core–shell structure. J. Mater. Res. Technol. 2022, 17, 320–328. [Google Scholar] [CrossRef]
- Shafiq, F.; Liu, C.; Zhou, H.; Chen, H.; Yu, S.; Qiao, W. Stearic acid-modified hollow hydroxyapatite particles with enhanced hydrophobicity for oil adsorption from oil spills. Chemosphere 2024, 348, 140651. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Yao, Y.B.; Guo, Y.J.; Ning, C.Q. Hydrothermal fabrication of mesoporous carbonated hydroxyapatite microspheres for a drug delivery system. Microporous Mesoporous Mater. 2012, 155, 245–251. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Li, H.; Zhao, C.; Zhang, B.; Zhang, H.; Geng, W.; Zhang, Q. Novel BiOCl/TiO2 hierarchical composites: Synthesis, characterization and application on photocatalysis. Appl. Catal. A Gen. 2016, 516, 81–89. [Google Scholar] [CrossRef]
- Abbasi, S.; Bayati, M.R.; Golestani-Fard, F.; Rezaei, H.R.; Zargar, H.R.; Samanipour, F.; Shoaei-Rad, V. Micro arc oxidized HAp–TiO2 nanostructured hybrid layers-part I: Effect of voltage and growth time. Appl. Surf. Sci. 2011, 257, 5944–5949. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-situ-reduced synthesis of Ti3+ self-doped TiO2/g-C3N4 heterojunctions with high photocatalytic performance under LED light irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.K.; Charpentier, P.A. Hydroxyapatite-TiO2-based Nanocomposites Synthesized in Supercritical CO2 for Bone Tissue Engineering: Physical and Mechanical Properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, U.; Priyadarshini, B.; Arul Xavier Stango, S.; Chellappa, M.; Geetha, M.; Vijayalakshmi, U. Preparation and characterisation of sol–gel-derived hydroxyapatite nanoparticles and its coatings on medical grade Ti-6Al-4V alloy for biomedical applications. Mater. Technol. 2017, 32, 800–814. [Google Scholar] [CrossRef]
- Rath, P.C.; Singh, B.P.; Besra, L.; Bhattacharjee, S. Multiwalled Carbon Nanotubes Reinforced Hydroxyapatite-Chitosan Composite Coating on Ti Metal: Corrosion and Mechanical Properties. J. Am. Ceram. Soc. 2012, 95, 2725–2731. [Google Scholar] [CrossRef]
- Goto, T.; Cho, S.H.; Ohtsuki, C.; Sekino, T. Selective adsorption of dyes on TiO2-modified hydroxyapatite photocatalysts morphologically controlled by solvothermal synthesis. J. Environ. Chem. Eng. 2021, 9, 105738. [Google Scholar] [CrossRef]
- Lin, Y.C.; Fang, Y.P.; Hung, C.F.; Yu, H.P.; Alalaiwe, A.; Wu, Z.Y.; Fang, J.Y. Multifunctional TiO2/SBA-15 mesoporous silica hybrids loaded with organic sunscreens for skin application: The role in photoprotection and pollutant adsorption with reduced sunscreen permeation. Colloids Surf. B Biointerfaces 2021, 202, 111658. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Saha, S.; Pal, A. Methyl red degradation under UV illumination and catalytic action of commercial ZnO: A parametric study. New Pub Balaban 2014, 56, 1066–1076. [Google Scholar] [CrossRef]
- Bouzid, T.; Grich, A.; Naboulsi, A.; Regti, A.; Alaoui Tahiri, A.; El Himri, M.; El Haddad, M. Adsorption of Methyl Red on porous activated carbon from agriculture waste: Characterization and response surface methodology optimization. Inorg. Chem. Commun. 2023, 158, 111544. [Google Scholar] [CrossRef]
- Amari, A.; Yadav, V.K.; Pathan, S.K.; Singh, B.; Osman, H.; Choudhary, N.; Khedher, K.M.; Basnet, A. Remediation of Methyl Red Dye from Aqueous Solutions by Using Biosorbents Developed from Floral Waste. Adsorpt. Sci. Technol. 2023, 2023, 1532660. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.H.; Govindwar, S.P. Sequential photocatalysis and biological treatment for the enhanced degradation of the persistent azo dye methyl red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Balu, P.; Asharani, I.V.; Thirumalai, D. Catalytic degradation of hazardous textile dyes by iron oxide nanoparticles prepared from Raphanus sativus leaves’ extract: A greener approach. J. Mater. Sci. Mater. Electron. 2020, 31, 10669–10676. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Rahim, A.; Hanafiah, M.M.; Oyekanmi, A.A.; Shah, A.B.; Mahnashi, M.H.; Al Ali, A.; Jalal, N.A.; et al. Biodegradation of Azo Dye Methyl Red by Pseudomonas aeruginosa: Optimization of Process Conditions. Int. J. Environ. Res. Public Health 2022, 19, 9962. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Wang, Y.; Yi, Y.; Shao, S.; Sun, P.; Xiao, Y.; Wang, W.; Dong, X. Enhanced photocatalytic activity of S-doped graphitic carbon nitride hollow microspheres: Synergistic effect, high-concentration antibiotic elimination and antibacterial behavior. J. Colloid Interface Sci. 2023, 643, 256–266. [Google Scholar] [CrossRef]
- Shahzad, W.; Badawi, A.K.; Rehan, Z.A.; Khan, A.M.; Khan, R.A.; Shah, F.; Ali, S.; Ismail, B. Enhanced visible light photocatalytic performance of Sr0.3(Ba,Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 2022, 48, 24979–24988. [Google Scholar] [CrossRef]
- Yeasmin, Z.; Alim, A.; Ahmed, S.; Rahman, M.M.; Masum, S.M.; Ghosh, A.K. Synthesis, Characterization and Efficiency of HAp-TiO2-ZnO Composite as a Promising Photocatalytic Material. Trans. Indian Ceram. Soc. 2018, 77, 161–168. [Google Scholar] [CrossRef]
- Hu, A.; Li, M.; Chang, C.; Mao, D. Preparation and characterization of a titanium-substituted hydroxyapatite photocatalyst. J. Mol. Catal. A Chem. 2007, 267, 79–85. [Google Scholar] [CrossRef]



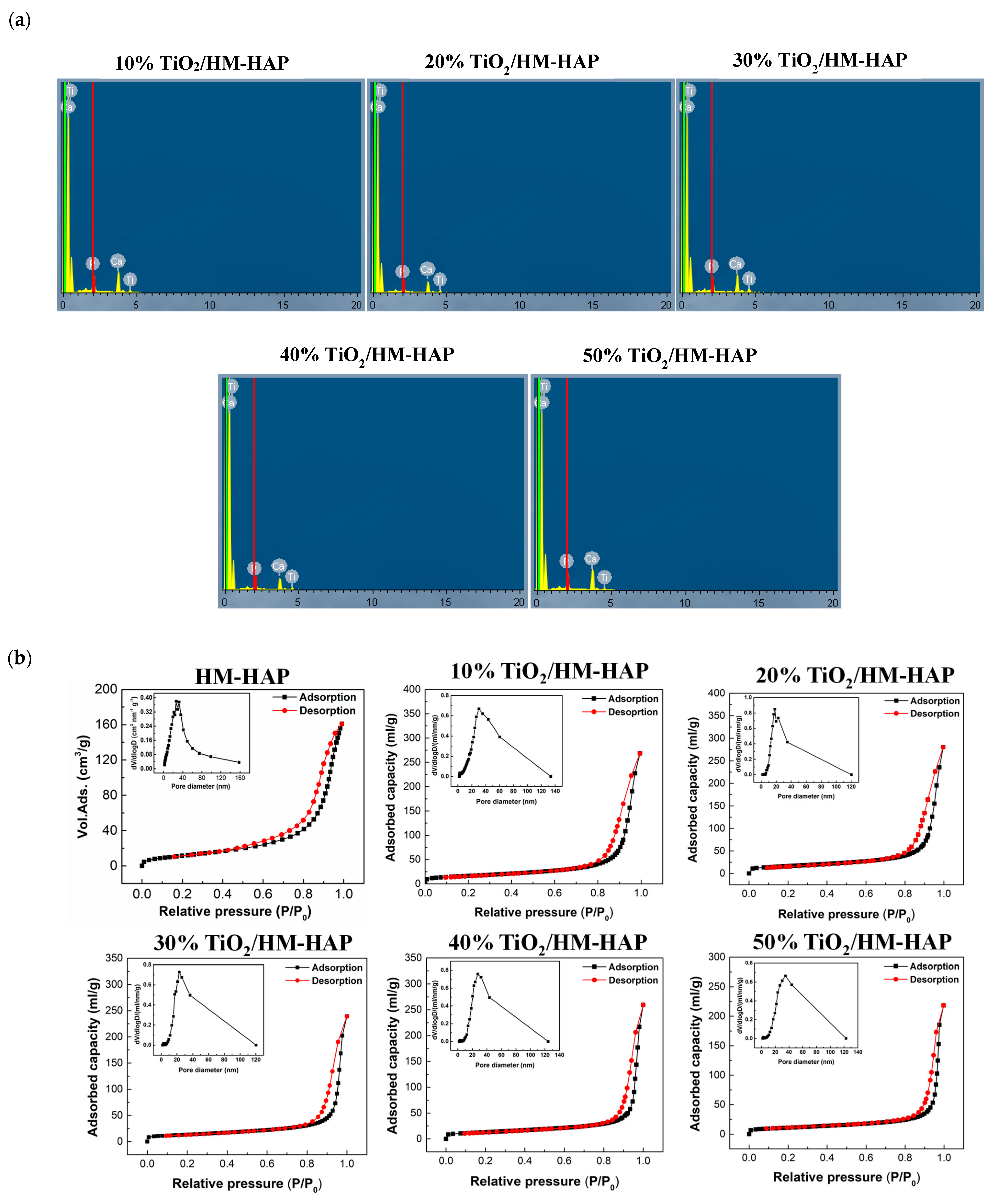
| Sample | BET Specific Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|
| HM-HAP | 44 | 12.47 |
| 10% TiO2/HM-HAP | 56 | 27.82 |
| 20% TiO2/HM-HAP | 57 | 28.19 |
| 30% TiO2/HM-HAP | 47 | 29.28 |
| 40% TiO2/HM-HAP | 46 | 31.94 |
| 50% TiO2/HM-HAP | 38 | 33.43 |
| Sample | Rate Constant k1 (min−1) | R2 |
|---|---|---|
| 10% TiO2/HM-HAP | 0.030 | 0.97 |
| 20% TiO2/HM-HAP | 0.033 | 0.99 |
| 30% TiO2/HM-HAP | 0.024 | 0.97 |
| 40% TiO2/HM-HAP | 0.018 | 0.98 |
| 50% TiO2/HM-HAP | 0.011 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiq, F.; Yu, S.; Pan, Y.; Qiao, W. Synthesis and Characterization of Titania-Coated Hollow Mesoporous Hydroxyapatite Composites for Photocatalytic Degradation of Methyl Red Dye in Water. Coatings 2024, 14, 921. https://doi.org/10.3390/coatings14080921
Shafiq F, Yu S, Pan Y, Qiao W. Synthesis and Characterization of Titania-Coated Hollow Mesoporous Hydroxyapatite Composites for Photocatalytic Degradation of Methyl Red Dye in Water. Coatings. 2024; 14(8):921. https://doi.org/10.3390/coatings14080921
Chicago/Turabian StyleShafiq, Farishta, Simiao Yu, Yongxin Pan, and Weihong Qiao. 2024. "Synthesis and Characterization of Titania-Coated Hollow Mesoporous Hydroxyapatite Composites for Photocatalytic Degradation of Methyl Red Dye in Water" Coatings 14, no. 8: 921. https://doi.org/10.3390/coatings14080921






