Effect of Isothermal Oxidation on the Structural Properties of (Ni,Pt)Al Coatings Doped with Zr at 1150 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Coating Preparation
2.2. Isothermal Oxidation Test
2.3. Characterization
3. Results and Discussion
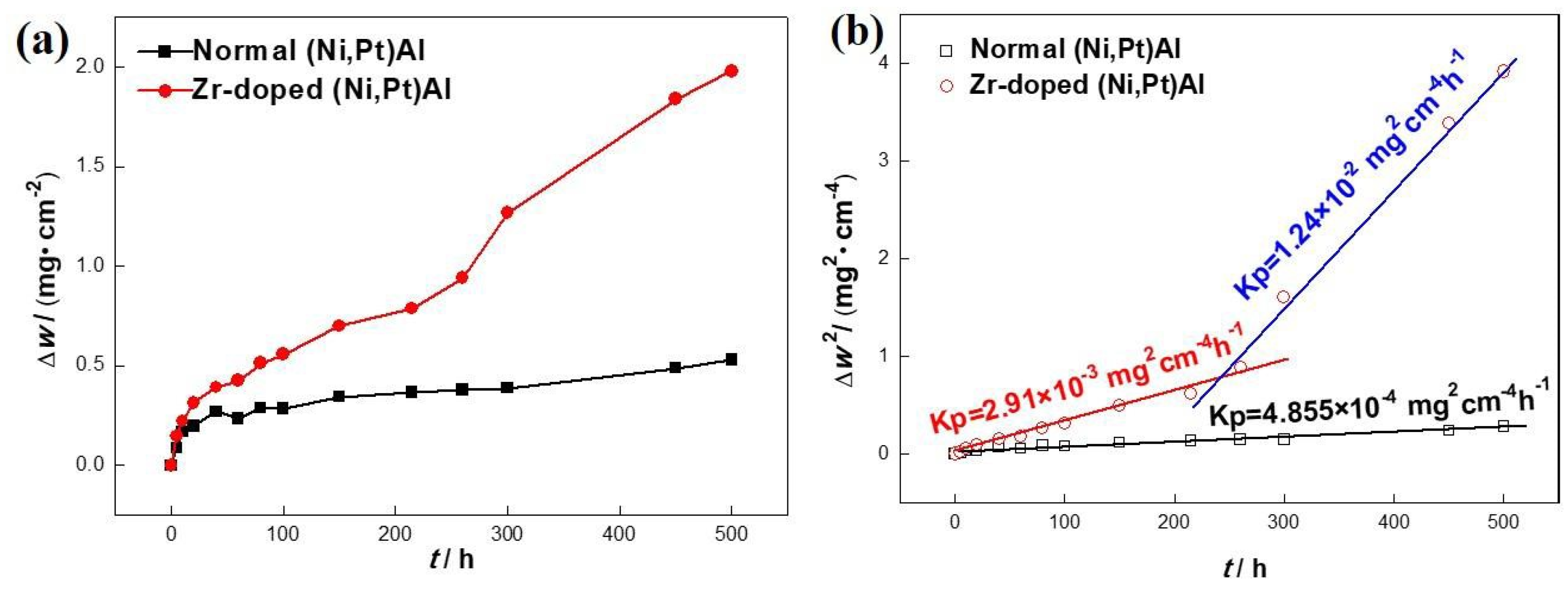
3.1. Oxidation Kinetics
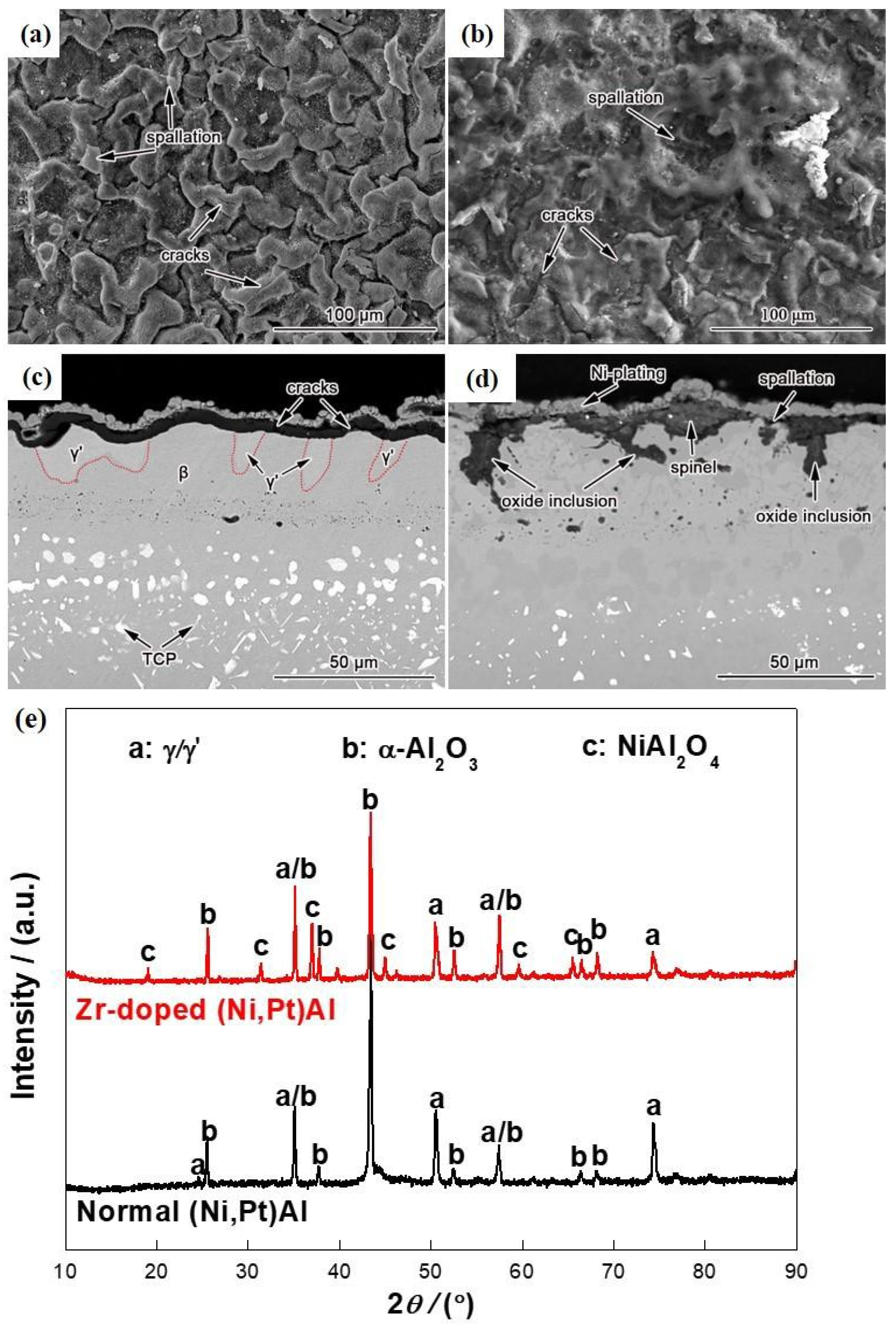
3.2. Microstructure and Phase Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Perepezko, J.H. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Liu, Y.; Su, H.; Qu, W.W.; Zhang, H.; Pei, Y.L.; Li, S.S.; Gong, S.K. Effects of coating growth mode on the oxidation behavior of a hybrid Pt/Ru-modified aluminide coating at 1200 °C. Corros. Sci. 2023, 225, 111608. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, C.; Niu, X.Y.; Zhang, C.Y.; Li, S.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Chlorine-induced high-temperature corrosion of the Pt-modified aluminide coating in simulated marine environment. Corros. Sci. 2023, 224, 111493. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Q.Y.; Yang, L.L.; Ren, P.; Yang, Y.F.; Wang, Q.W.; Li, W.; Zhu, S.L.; Wang, F.H. The cyclic oxidation behavior of a Pt modified γ’ nanocrystalline coating at 1150 °C. Corros. Sci. 2022, 208, 110638. [Google Scholar] [CrossRef]
- Hou, P.Y.; Izumi, T.; Gleeson, B. Sulfur segregation at Al2O3/γ-Ni+γ’-Ni3Al interfaces: Effects of Pt, Cr and Hf additions. Oxid. Met. 2009, 72, 109–124. [Google Scholar] [CrossRef]
- Yang, L.Y.; Zheng, L.; Guo, H.B. The residual stress of oxide scales grown on Ni-Al alloys doped with minor Dy and Y. Corros. Sci. 2016, 112, 542–551. [Google Scholar] [CrossRef]
- Qian, L.Y.; Xu, F.; Voisey, K.T.; Nekouie, V.; Zhou, Z.X.; Silberschmidt, V.V.; Hou, X.H. Incorporation and evolution of ZrO2 nano-particles in Pt-modified aluminide coating for high temperature applications. Surf. Coat. Technol. 2017, 311, 238–247. [Google Scholar] [CrossRef]
- Liang, J.J.; Wei, H.; Zhu, Y.L.; Sun, X.F.; Hu, Z.Q.; Dargusch, M.S.; Yao, X.D. Influence Re on the properties of a NiCoCrAlY coating alloy. J. Mater. Sci. Technol. 2011, 27, 408–414. [Google Scholar] [CrossRef]
- Allam, I.M.; Whittle, D.P.; Stringer, J. The oxidation behavior of CoCrAl systems containing active element additions. Oxid. Met. 1978, 12, 35–66. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, G.H.; Han, W.K.; Lee, K.S.; Kang, S.G. Effect of zirconium addition on cyclic oxidation behavior of platinum-modified aluminide coating on nickel-based superalloy. Intermetallics 2010, 18, 864–870. [Google Scholar] [CrossRef]
- Haynes, J.A.; Pint, B.A.; More, K.L.; Zhang, Y.; Wright, I.G. Influence of sulfur, platinum, and hafnium on the oxidation behavior of CVD NiAl bond coatings. Oxid. Met. 2002, 58, 513–544. [Google Scholar] [CrossRef]
- Wei, F.I.; Stott, F.H. The development of Cr2O3 scales on iron-chromium alloys containing reactive elements. Corros. Sci. 1989, 29, 839–861. [Google Scholar] [CrossRef]
- Thanneeru, R.; Patil, S.; Deshpande, S.; Seal, S. Effect of trivalent rare earth dopants in nanocrystalline ceria coatings for high-temperature oxidation resistance. Acta Mater. 2007, 55, 3457–3466. [Google Scholar] [CrossRef]
- Sánchez, L.; Bolívar, F.J.; Hierro, M.P.; Pérez, F.J. Effect of Ce and La additions in low temperature aluminisation process by CVD–FBR on 12%Cr ferritic/martensitic steel and behaviour in steam oxidation. Corros. Sci. 2008, 50, 2318–2326. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Yang, Y.F.; Zhang, Z.Y.; Bao, Z.B.; Chen, M.H.; Zhu, S.L.; Wang, F.H. A Zr-doped single-phase Pt-modified aluminide coating and the enhanced hot corrosion resistance. Corros. Sci. 2018, 133, 406–416. [Google Scholar] [CrossRef]
- Wessel, E.; Kochubey, V.; Naumenko, D.; Niewolak, L.; Singheiser, L.; Quadakkers, W.J. Effect of Zr addition on the microstructure of the alumina scales on FeCrAlY-alloys. Scr. Mater. 2004, 51, 987–992. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Qian, L.Y.; Feng, M.; Liu, H.; Bao, Z.B.; Chen, M.H.; Zhu, S.L.; Wang, F.H. Benefits of Zr addition to oxidation resistance of a single-phase (Ni,Pt)Al coating at 1373 K. J. Mater. Sci. Technol. 2019, 35, 1334–1344. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.X.; Yang, R.; Li, D. First principles study of site substitution of ternary elements in NiAl. Acta Mater. 2001, 49, 1647–1654. [Google Scholar] [CrossRef]



| Area | O | Al | Ni | Cr | Co | Zr |
|---|---|---|---|---|---|---|
| 1 | 56.3 | 27.9 | 7.9 | 0.6 | 0.7 | 6.6 |
| 2 | 64.6 | 32.5 | 0.5 | / | / | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Yang, L.; Jiang, C. Effect of Isothermal Oxidation on the Structural Properties of (Ni,Pt)Al Coatings Doped with Zr at 1150 °C. Coatings 2024, 14, 927. https://doi.org/10.3390/coatings14080927
Feng M, Yang L, Jiang C. Effect of Isothermal Oxidation on the Structural Properties of (Ni,Pt)Al Coatings Doped with Zr at 1150 °C. Coatings. 2024; 14(8):927. https://doi.org/10.3390/coatings14080927
Chicago/Turabian StyleFeng, Min, Linlin Yang, and Chengyang Jiang. 2024. "Effect of Isothermal Oxidation on the Structural Properties of (Ni,Pt)Al Coatings Doped with Zr at 1150 °C" Coatings 14, no. 8: 927. https://doi.org/10.3390/coatings14080927




