Influence of Ce3+ Doping on Photoluminescence Properties and Stability of Cs4SnBr6 Zero-Dimensional Perovskite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cs4SnBr6 and Ce3+-Doped Cs4SnBr6
2.3. Characterizations
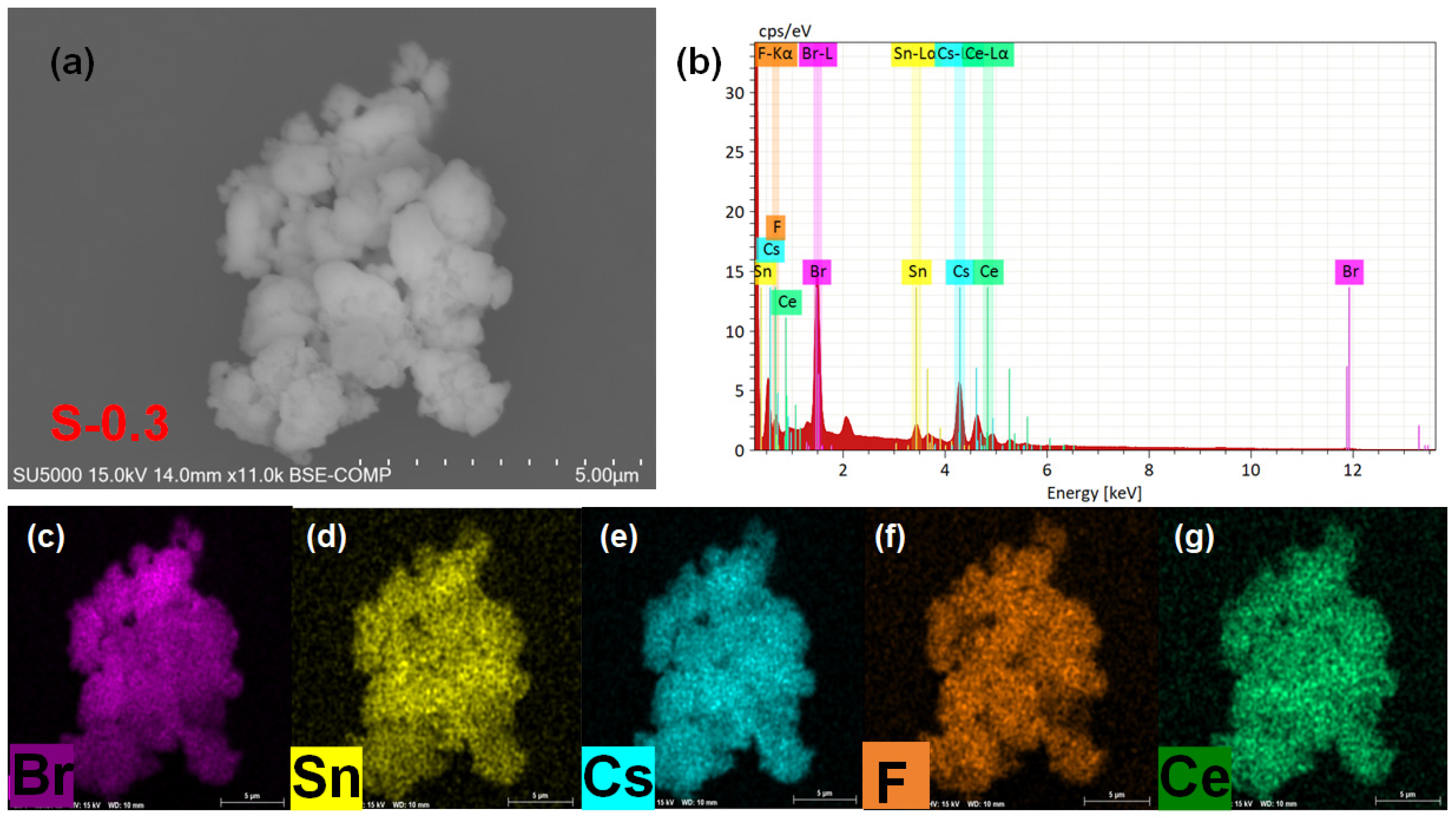
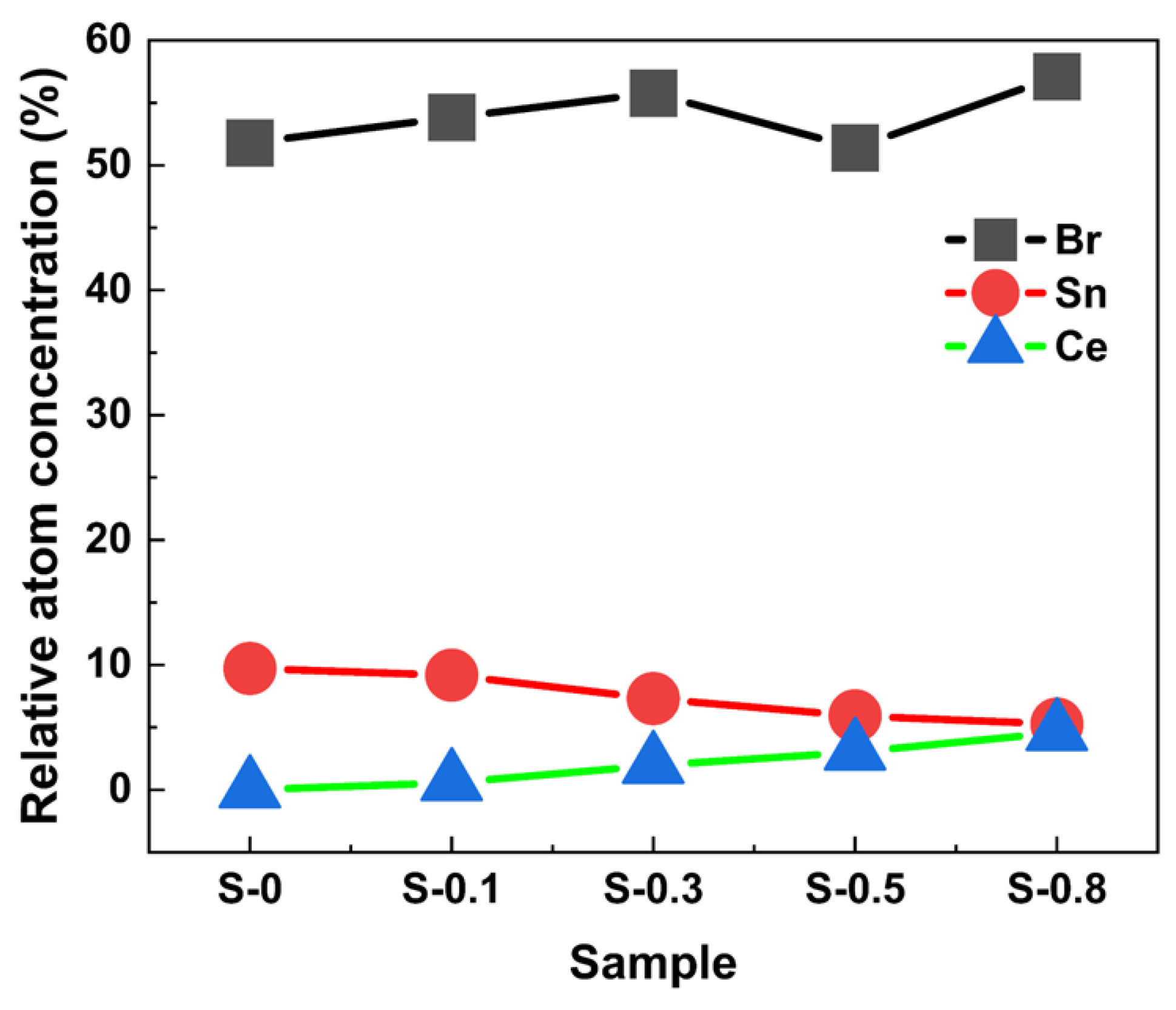
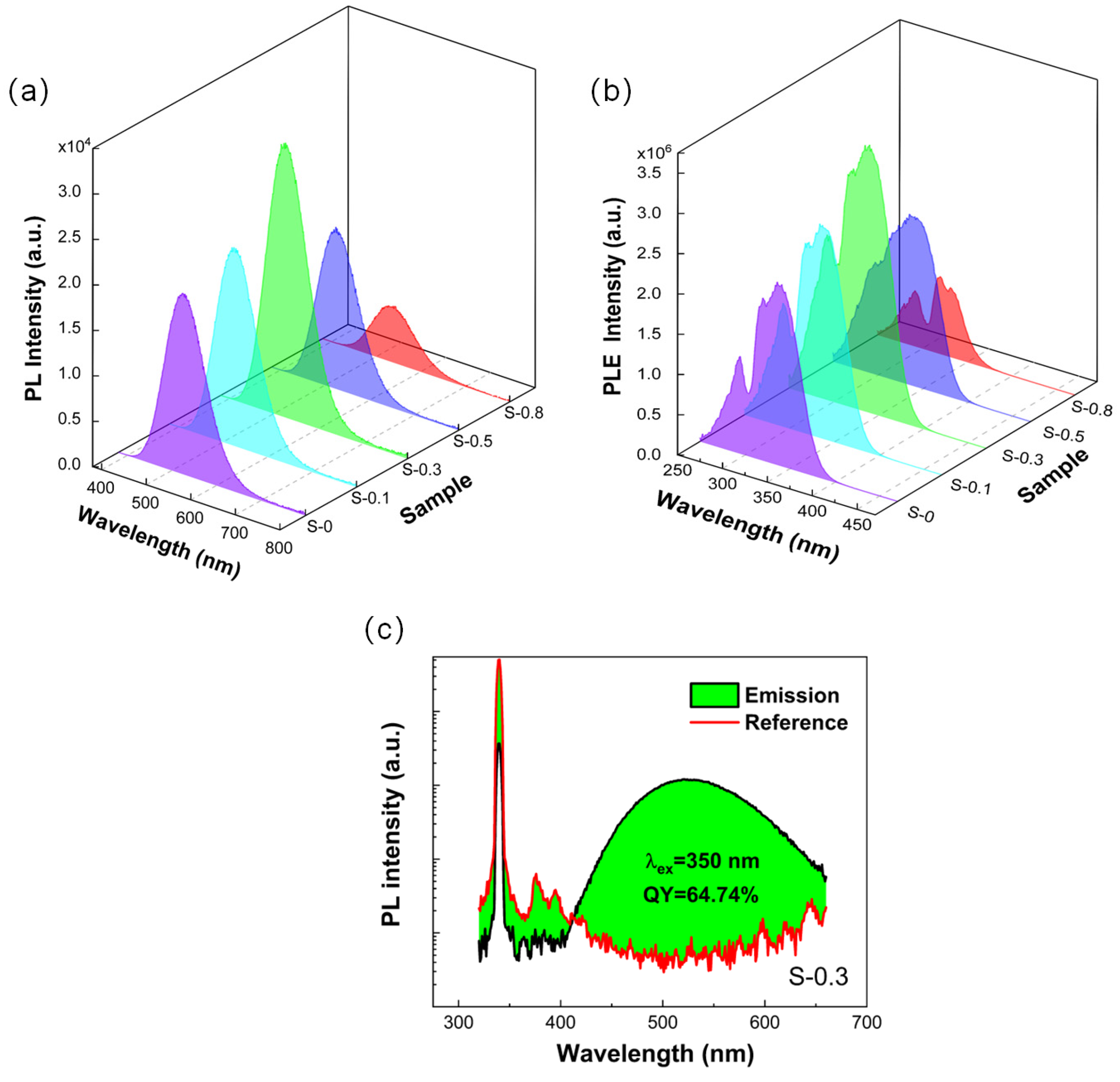
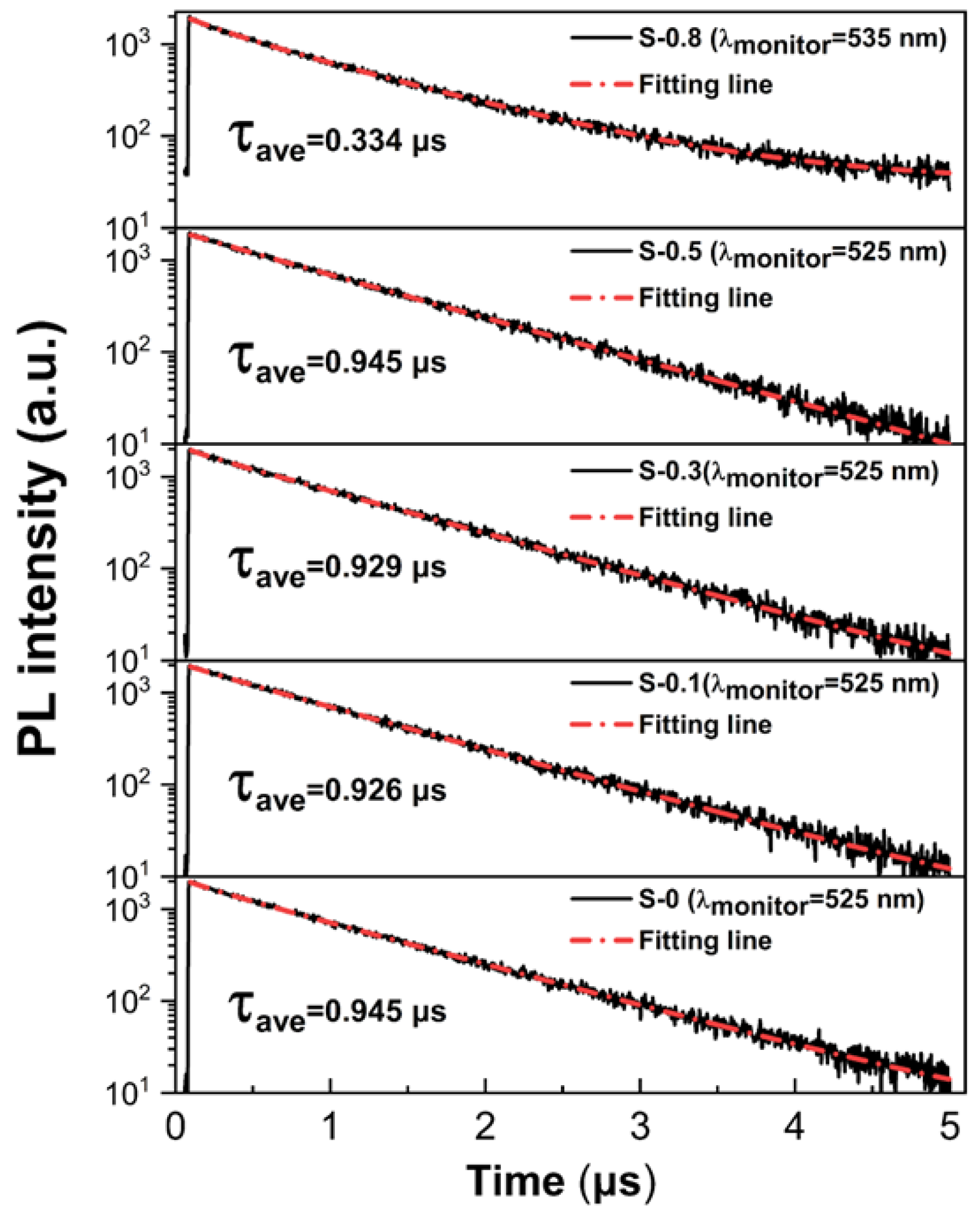
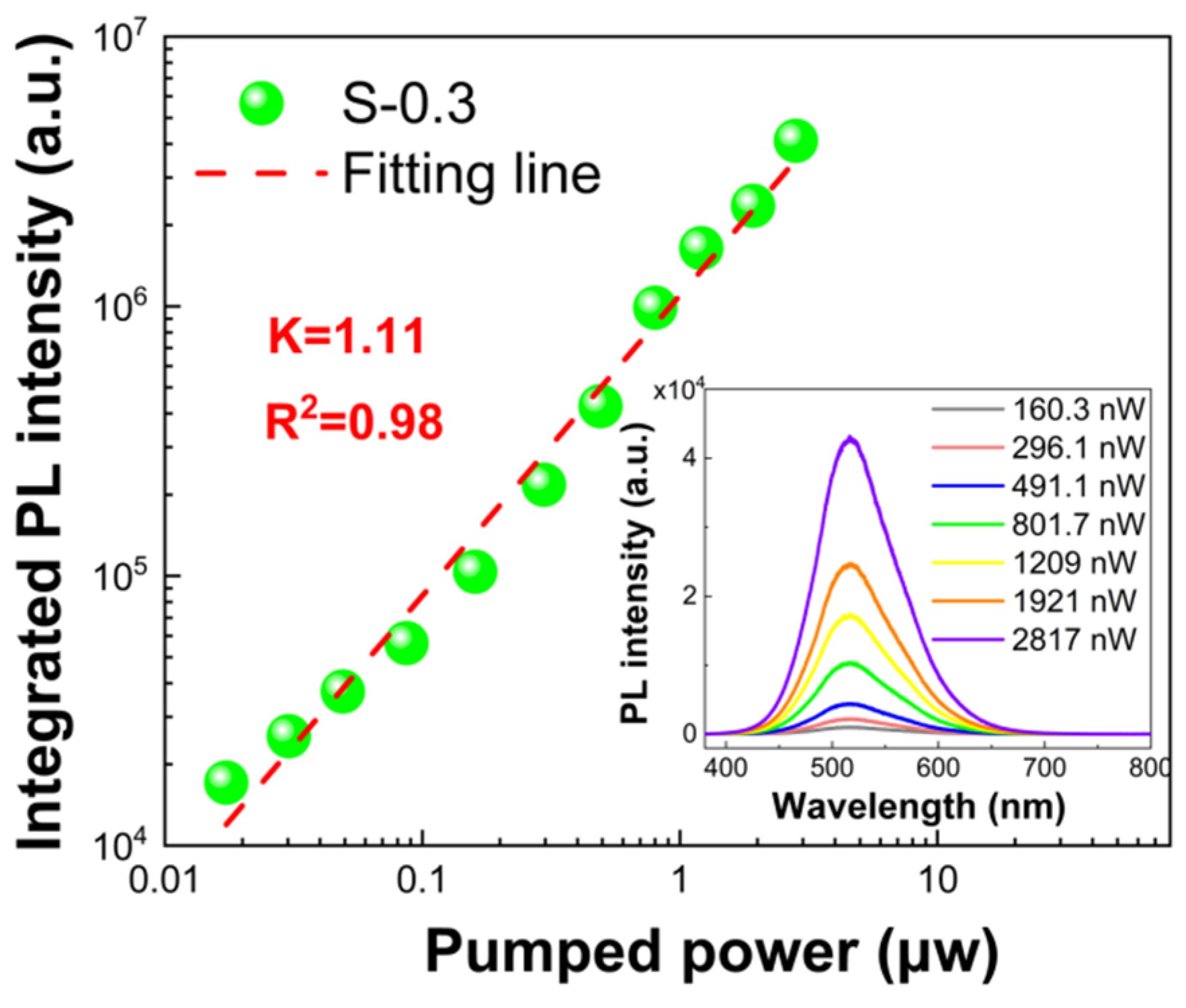
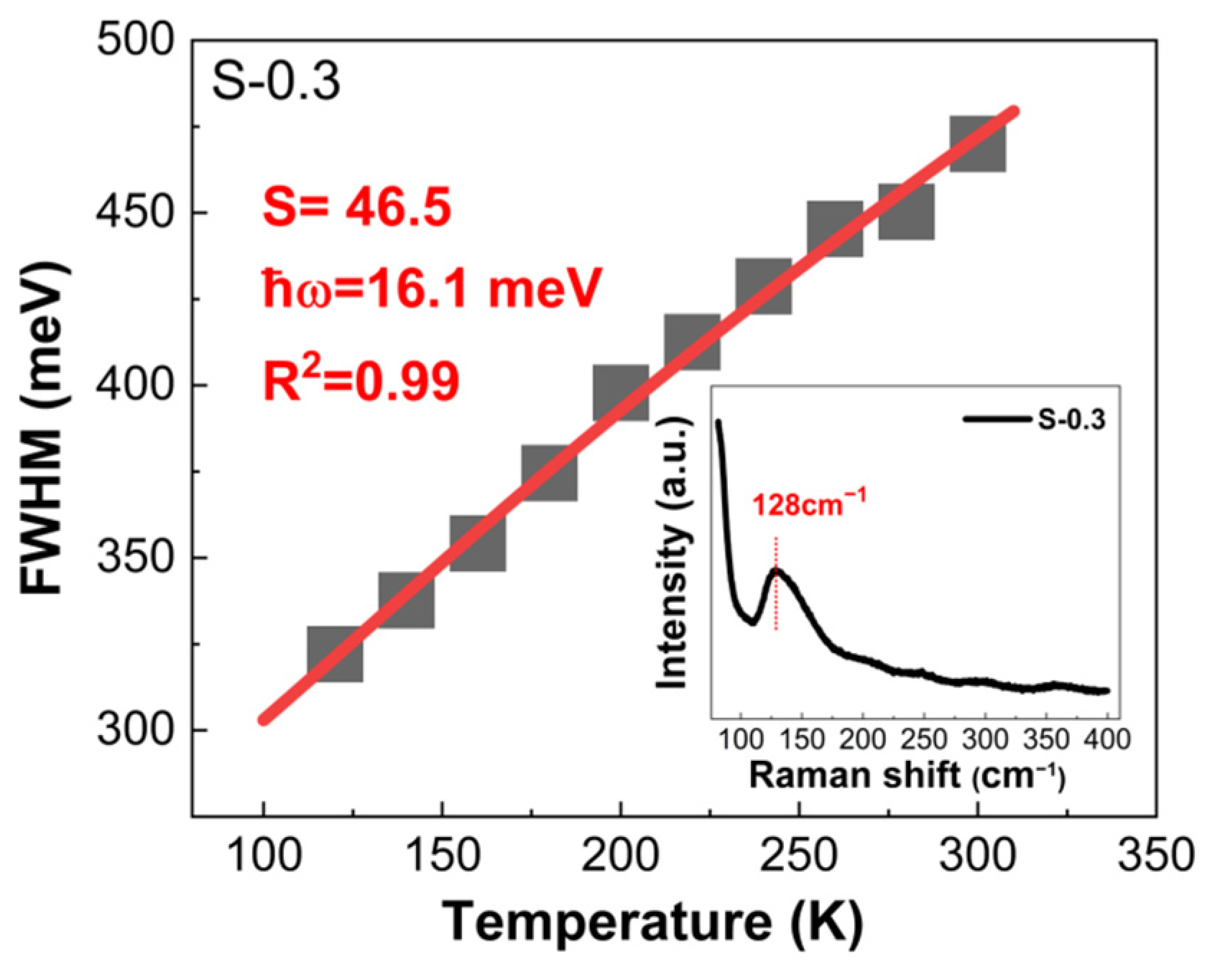
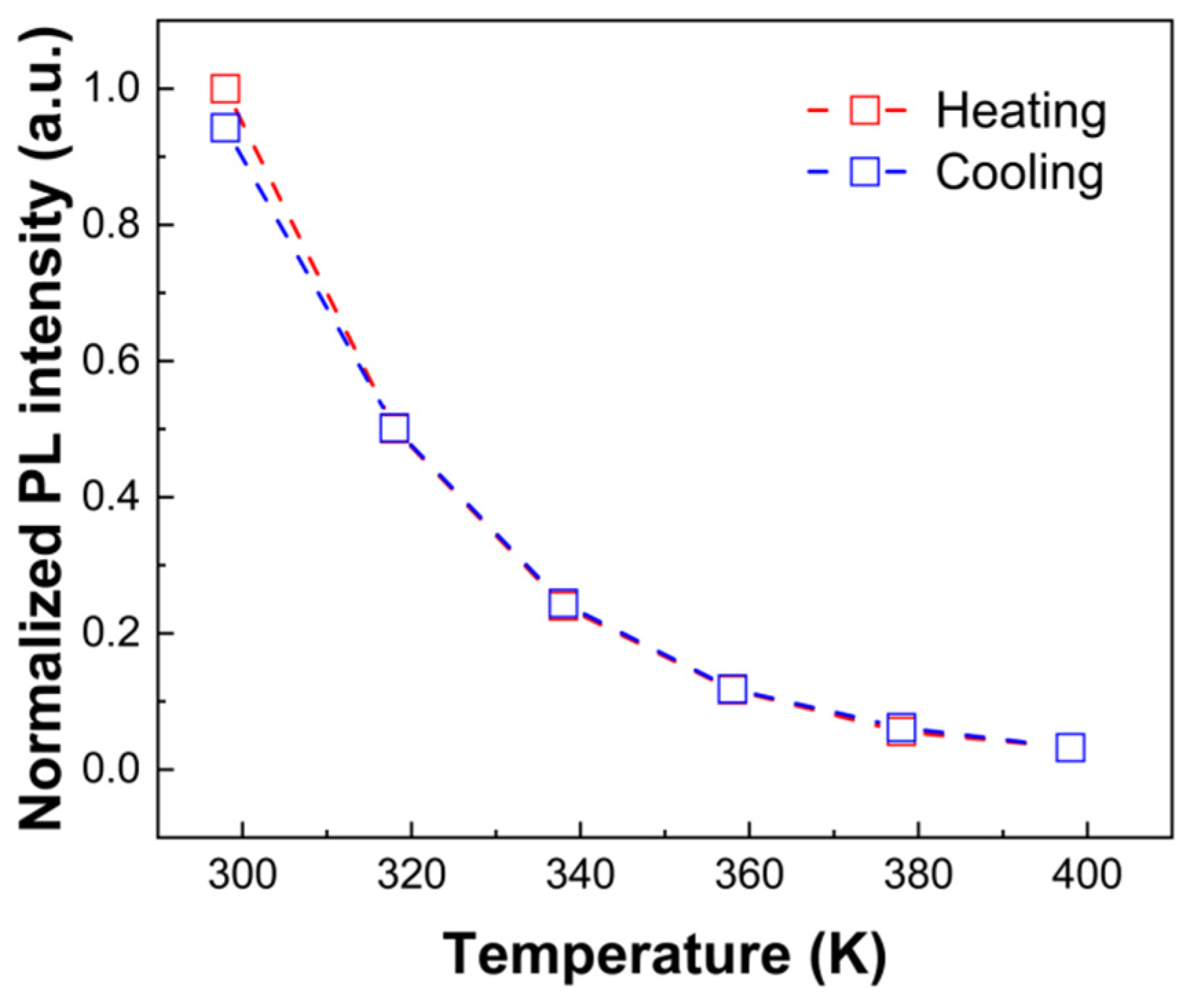
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-J.; Pettit, E.C.; Swartwout, R.; Zhitomirsky Kadosh, T.; Srinivasan, S.; Wassweiler, E.L.; Haugstad, G.; Bulović, V.; Holmes, R.J. Efficient Metal-Halide Perovskite Photovoltaic Cells Deposited via Vapor Transport Deposition. Sol. RRL 2024, 8, 2300758. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, L.; Chen, G.; Peng, C.; Jiang, Y. All-inorganic Mn2+-doped metal halide perovskite crystals for the late-time detection of X-ray afterglow imaging. Nanoscale 2023, 15, 13628–13634. [Google Scholar] [CrossRef]
- Girolami, M.; Matteocci, F.; Pettinato, S.; Serpente, V.; Bolli, E.; Paci, B.; Generosi, A.; Salvatori, S.; Di Carlo, A.; Trucchi, D.M. Metal-halide perovskite submicrometer-thick films for ultra-stable self-powered direct X-ray detectors. Nano-Micro Lett. 2024, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeong, S.H.; Park, M.H.; Kim, Y.H.; Wolf, C.; Lee, C.L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S.; et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Bai, W.; Xuan, T.; Zhao, H.; Dong, H.; Cheng, X.; Wang, L.; He, R.J. Perovskite light-emitting diodes with an external quantum efficiency exceeding 30%. Adv. Mater. 2023, 35, 2302283. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Biesold-McGee, G.V.; Ma, J.; Xu, Q.; Pan, S.; Peng, J.; Lin, Z. Lead-free halide perovskite nanocrystals: Crystal structures, synthesis, stabilities, and optical properties. Angew. Chem. Int. Ed. 2020, 59, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Arfin, H.; Nag, A. Origin of luminescence in Sb3+- and Bi3+-doped Cs2SnCl6 perovskites: Excited state relaxation and spin–orbit coupling. J. Phys. Chem. Lett. 2021, 12, 10002–10008. [Google Scholar] [CrossRef]
- Infante, I.; Manna, L. Are there good alternatives to lead halide perovskite nanocrystals? Nano Lett. 2020, 21, 6–9. [Google Scholar] [CrossRef]
- Xu, K.; Wei, Q.; Wang, H.; Yao, B.; Zhou, W.; Gao, R.; Chen, H.; Li, H.; Wang, T.; Ning, Z. The 3D-structure-mediated growth of zero-dimensional Cs4SnX6 nanocrystals. Nanoscale 2022, 14, 2248–2255. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yin, J.; Zhang, B.B.; Chen, J.K.; Zhou, Y.; Zhang, L.M.; Wang, L.M.; Zhao, Q.; Hou, J.; Shu, J.; et al. Theory-guided synthesis of highly luminescent colloidal cesium tin halide perovskite nanocrystals. J. Am. Chem. Soc. 2021, 143, 5470–5480. [Google Scholar] [CrossRef]
- Kang, C.; Rao, H.; Fang, Y.; Zeng, J.; Pan, Z.; Zhong, H. Antioxidative stannous oxalate derived lead-free stable CsSnX3 (X = Cl, Br, and I) perovskite nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 660–665. [Google Scholar] [CrossRef]
- Zhang, B.B.; Chen, J.K.; Zhang, C.; Shirahata, N.; Sun, H.T. Mechanistic Insight into the Precursor Chemistry of Cesium Tin Iodide Perovskite Nanocrystals. ACS Mater. Lett. 2023, 5, 1954–1961. [Google Scholar] [CrossRef]
- Benin, B.M.; Dirin, D.N.; Morad, V.; Wörle, M.; Yakunin, S.; Rainò, G.; Nazarenko, O.; Fischer, M.; Infante, I.; Kovalenko, M.V. Highly emissive self-trapped excitons in fully inorganic zero-dimensional tin halides. Angew. Chem. Int. Ed. 2018, 57, 11329–11333. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, W.; Li, Q.; Luo, Z.; Zou, C.; Tang, M.; Zhang, L.; He, J.; Quan, Z. Colloidal syntheses of zero-dimensional Cs4SnX6 (X = Br, I) nanocrystals with high emission efficiencies. Chem. Commun. 2020, 56, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; He, M.; Zheng, W.; Wan, Q.; Liu, M.; Liao, X.; Zhan, W.; Yuan, C.; Liu, J.; et al. Stable Lead-Free Tin Halide Perovskite with Operational Stability > 1200 h by Suppressing Tin (II) Oxidation. Angew. Chem. 2022, 134, e202205463. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Lu, S.; Wang, L.; Feng, X.; Yang, D.; Wang, K.; Xiao, G.; Zhang, L.; Redfern, S.A.; et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals. Nat. Commun. 2018, 9, 4506. [Google Scholar] [CrossRef]
- Li, K.; Ye, Y.; Zhang, W.; Zhou, Y.; Zhang, Y.; Lin, S.; Lin, H.; Ruan, J.; Liu, C. Ultra-stable and color-tunable manganese ions doped lead-free cesium zinc halides nanocrystals in glasses for light-emitting applications. Nano Res. 2022, 15, 9368–9376. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, A.; Huang, R.; Wu, H.; Song, J.; Lin, Z.; Hou, D.; Zhang, Z.; Guo, Y.; Lan, S. Manipulating the sublattice distortion induced by Mn2+ doping for boosting the emission characteristics of self-trapped excitons in Cs4SnBr6. J. Mater. Chem. C 2023, 11, 5680–5687. [Google Scholar] [CrossRef]
- Wu, H.; Lin, Z.; Song, J.; Zhang, Y.; Guo, Y.; Zhang, W.; Huang, R. Boosting the Self-Trapped Exciton Emission in Cs4SnBr6 Zero-Dimensional Perovskite via Rapid Heat Treatment. Nanomaterials 2023, 13, 2259. [Google Scholar] [CrossRef] [PubMed]
- Chiara, R.; Ciftci, Y.O.; Queloz, V.I.; Nazeeruddin, M.K.; Grancini, G.; Malavasi, L. Green-emitting lead-free Cs4SnBr6 zero-dimensional perovskite nanocrystals with improved air stability. J. Phys. Chem. Lett. 2020, 11, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Liou, S.C.; Chen, W.L.; Wu, C.L.; Lin, G.R.; Chang, Y.M. Tunable and stable UV-NIR photoluminescence from annealed SiOx with Si nanoparticles. Opt. Express 2013, 21, 23416–23424. [Google Scholar] [CrossRef] [PubMed]
- Bergman, L.; Chen, X.B.; Morrison, J.L.; Huso, J.; Purdy, A.P. Photoluminescence dynamics in ensembles of wide-band-gap nanocrystallites and powders. J. Appl. Phys. 2004, 96, 675–682. [Google Scholar] [CrossRef]
- Stadler, W.; Hofmann, D.M.; Alt, H.C.; Muschik, T.; Meyer, B.K.; Weigel, E.; Müller-Vogt, G.; Salk, M.; Rupp, E.; Benz, K.W. Optical investigations of defects in Cd1−xZnxTe. Phys. Rev. B 1995, 51, 10619. [Google Scholar] [CrossRef]
- Kuok, M.H.; Tan, L.S.; Shen, Z.X.; Huan, C.H.; Mok, K.F. A Raman study of RbSnBr3. Solid State Commun. 1996, 97, 497–501. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wu, H.; Xu, J.; Chen, J.; Huang, Y.; Li, H.; Song, J.; Huang, R. Influence of Ce3+ Doping on Photoluminescence Properties and Stability of Cs4SnBr6 Zero-Dimensional Perovskite. Coatings 2024, 14, 945. https://doi.org/10.3390/coatings14080945
Lu X, Wu H, Xu J, Chen J, Huang Y, Li H, Song J, Huang R. Influence of Ce3+ Doping on Photoluminescence Properties and Stability of Cs4SnBr6 Zero-Dimensional Perovskite. Coatings. 2024; 14(8):945. https://doi.org/10.3390/coatings14080945
Chicago/Turabian StyleLu, Xinye, Haixia Wu, Jisheng Xu, Jianni Chen, Yaqian Huang, Hongliang Li, Jie Song, and Rui Huang. 2024. "Influence of Ce3+ Doping on Photoluminescence Properties and Stability of Cs4SnBr6 Zero-Dimensional Perovskite" Coatings 14, no. 8: 945. https://doi.org/10.3390/coatings14080945





