Effect of Heat Treatment Temperature on the Microstructure and Mechanical Properties of Cu0.3Cr2Fe2Ni3Mn2Nbx High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
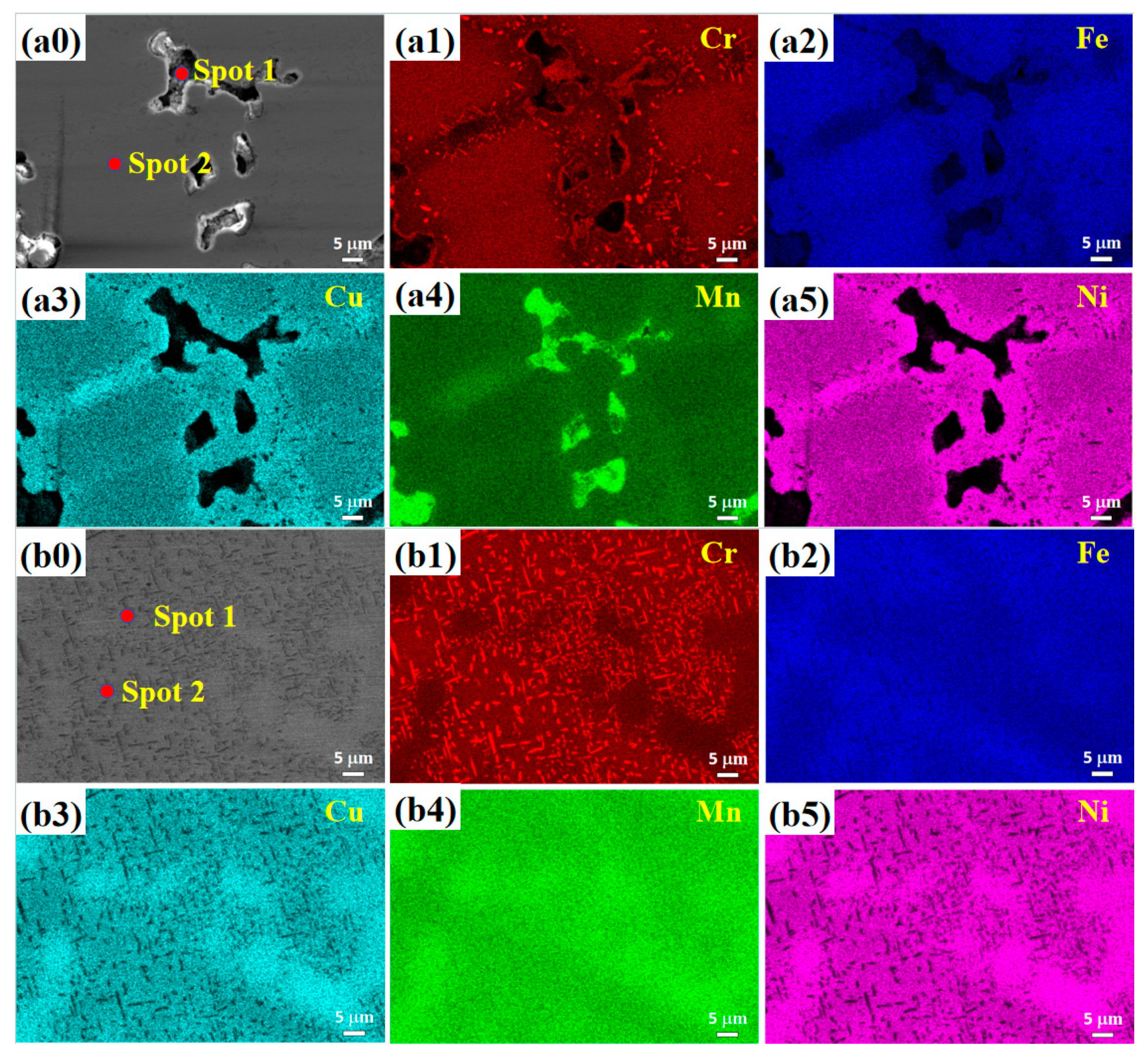
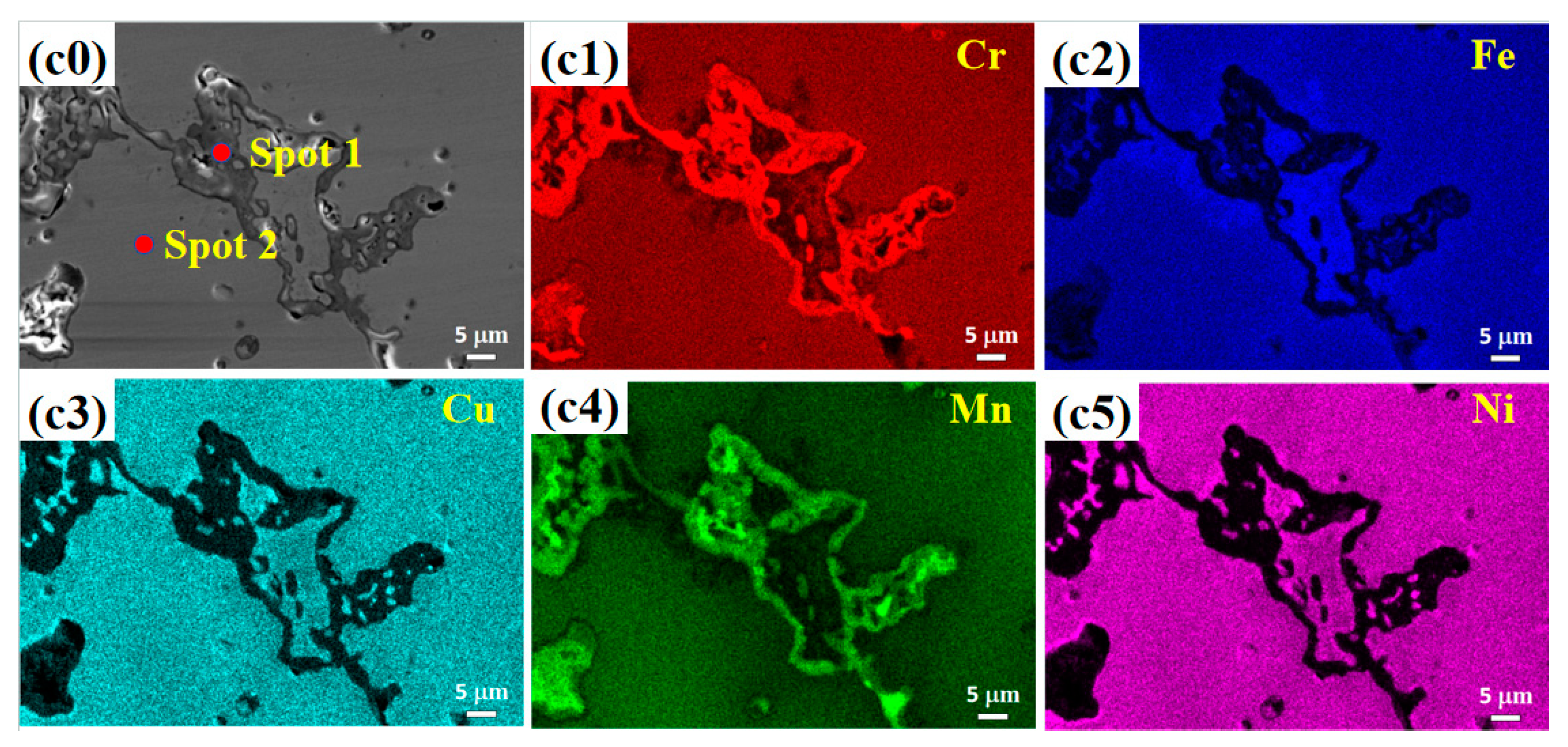
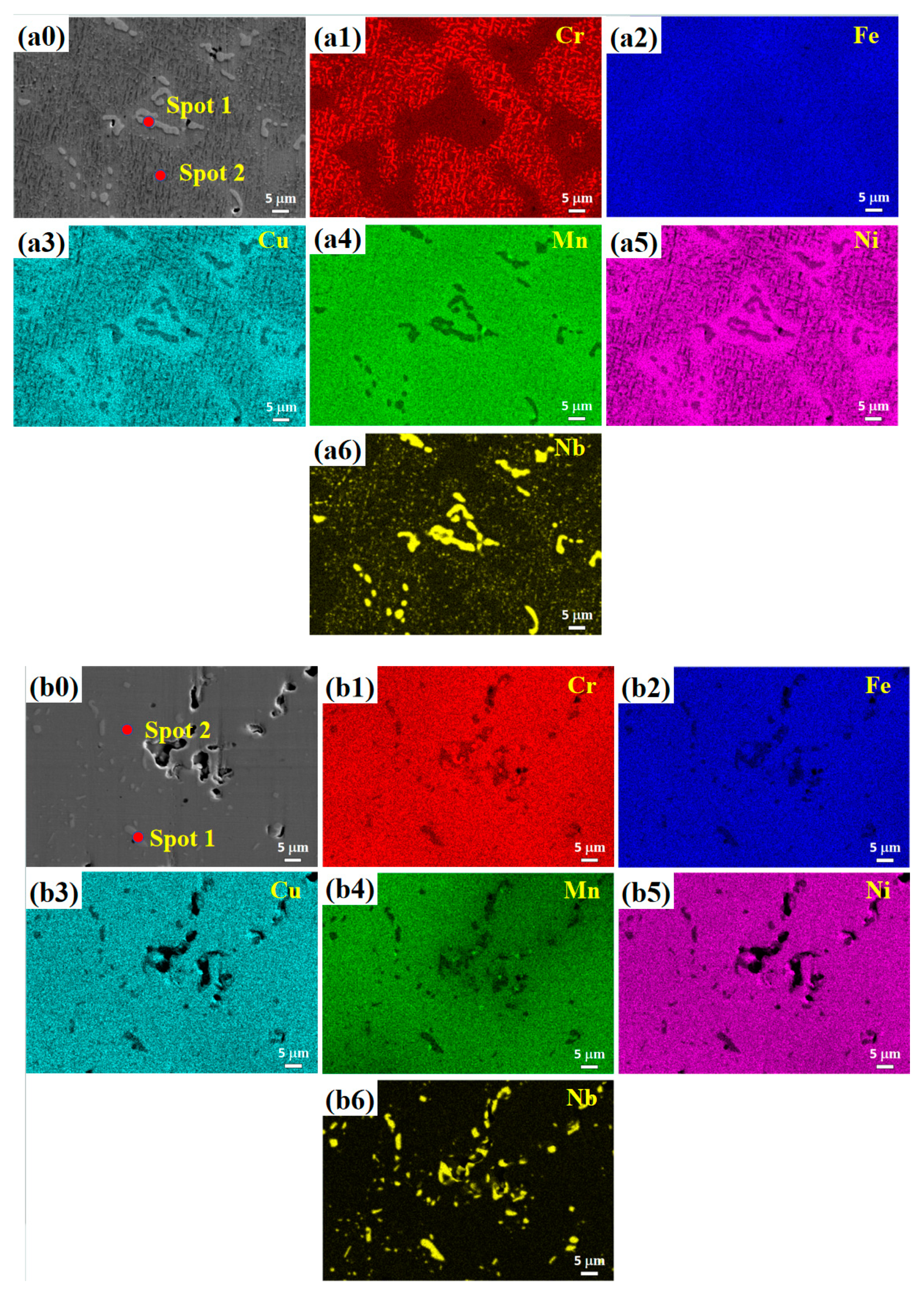
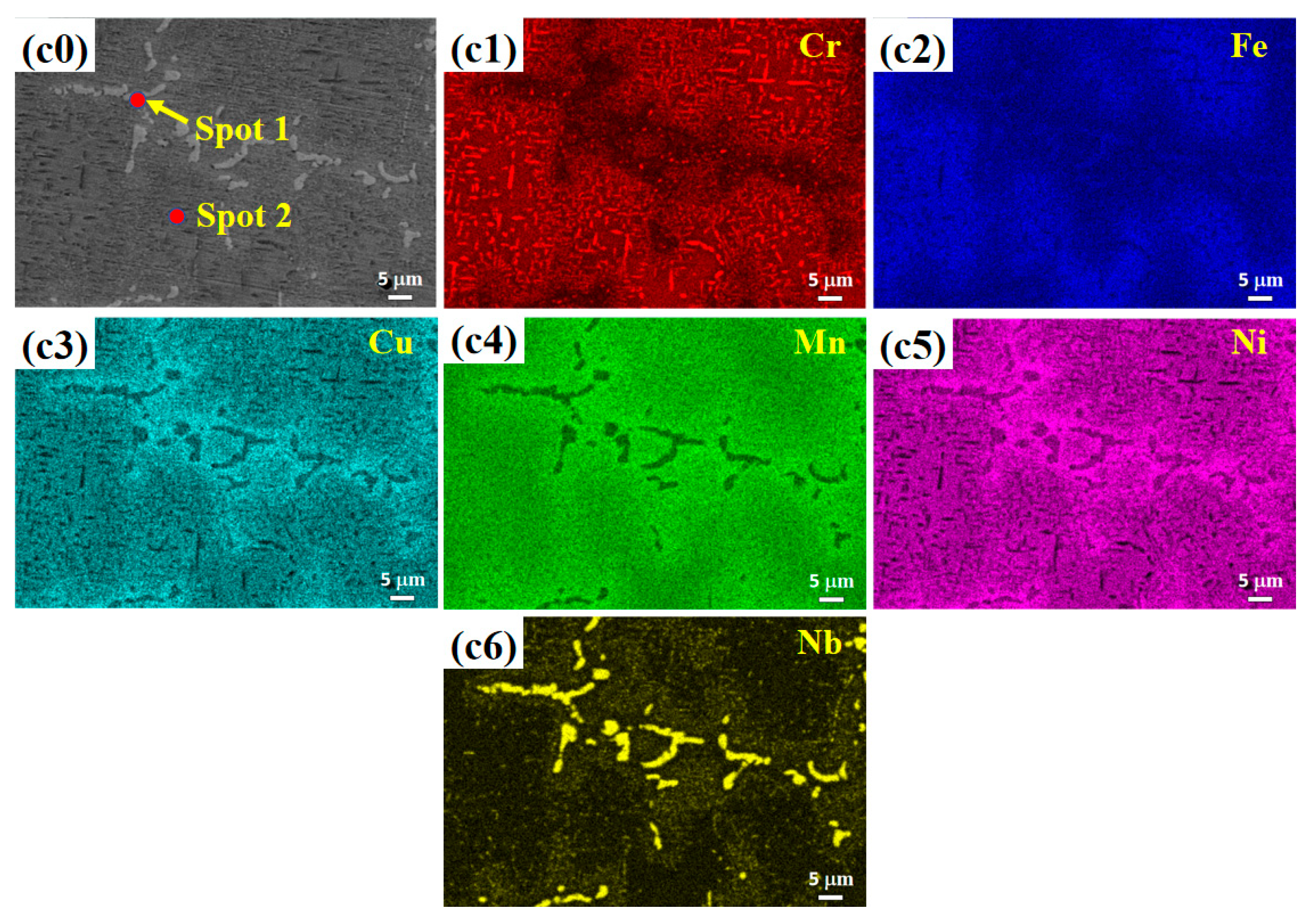
3.2. Microstructure
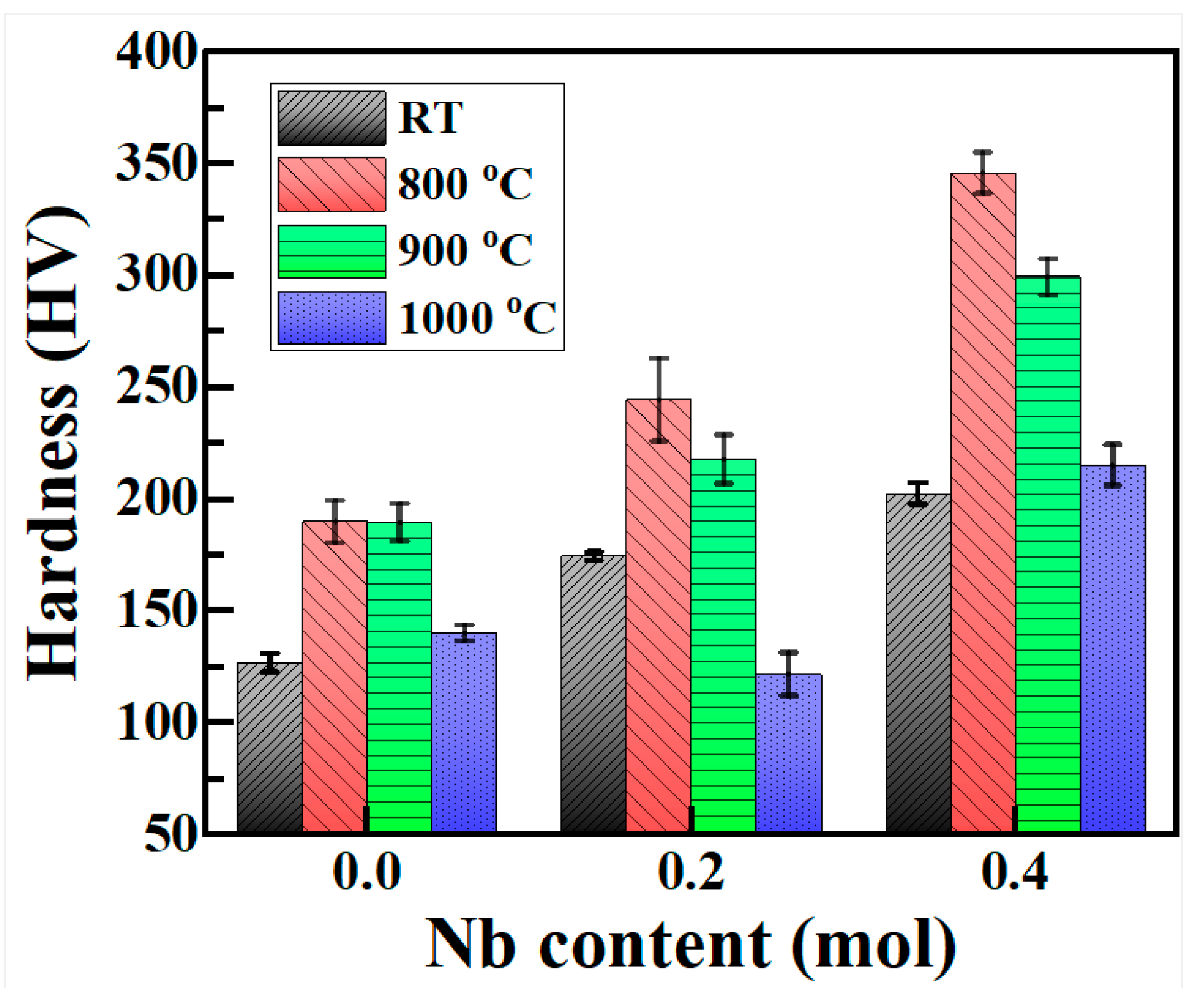
3.3. Microhardness
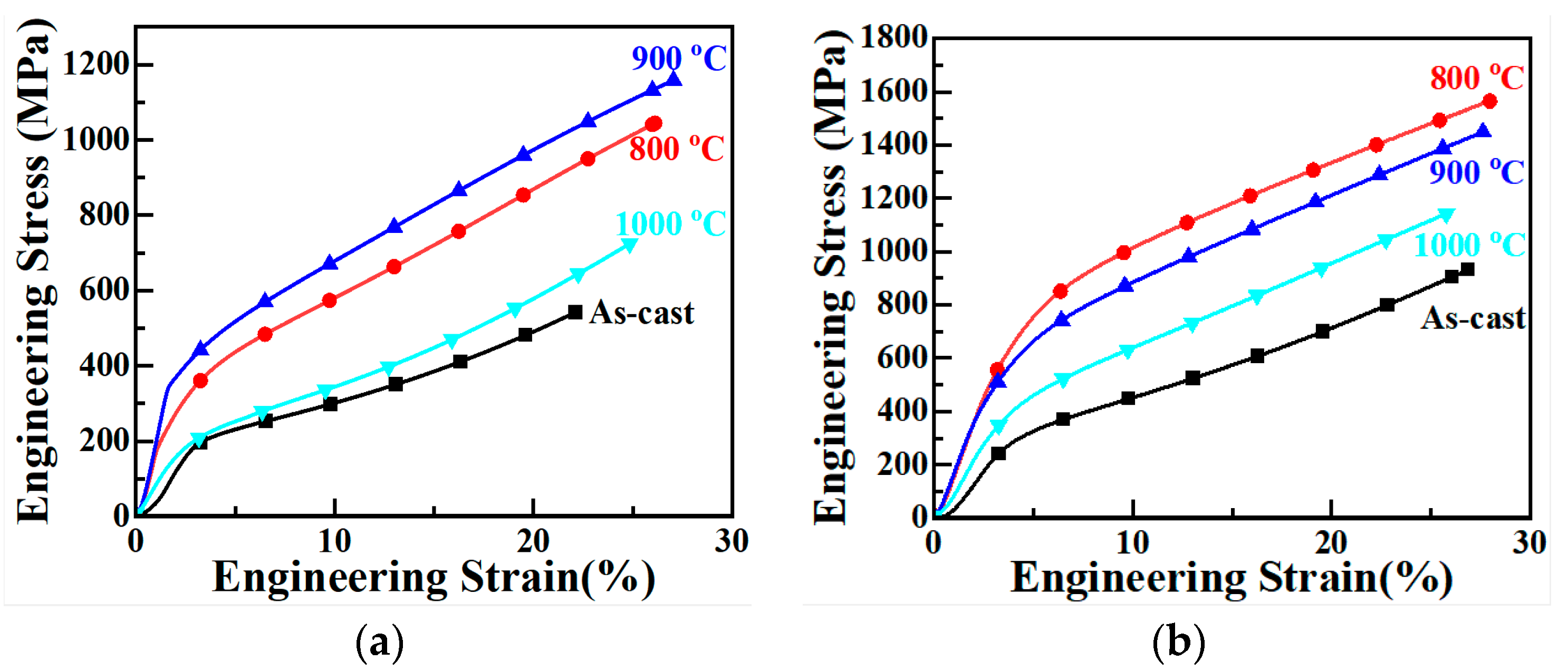
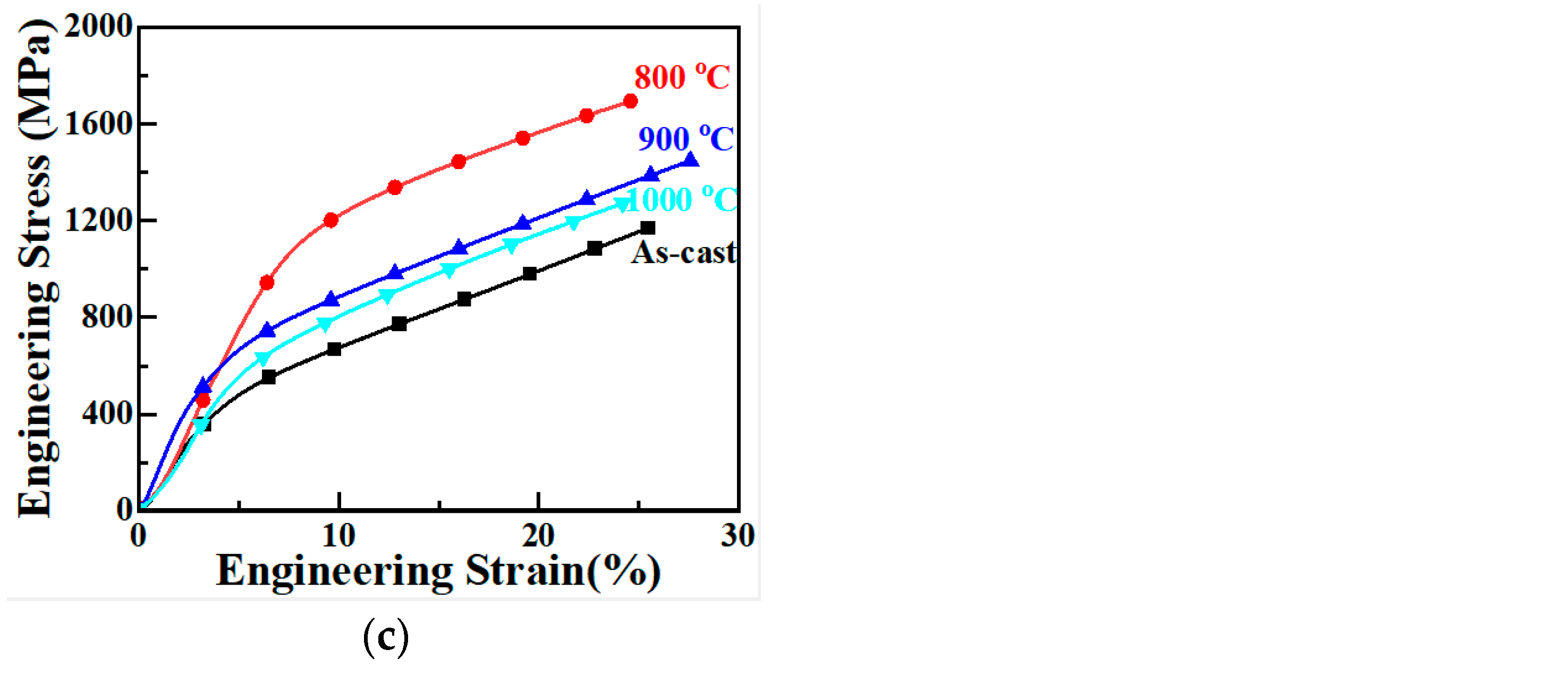
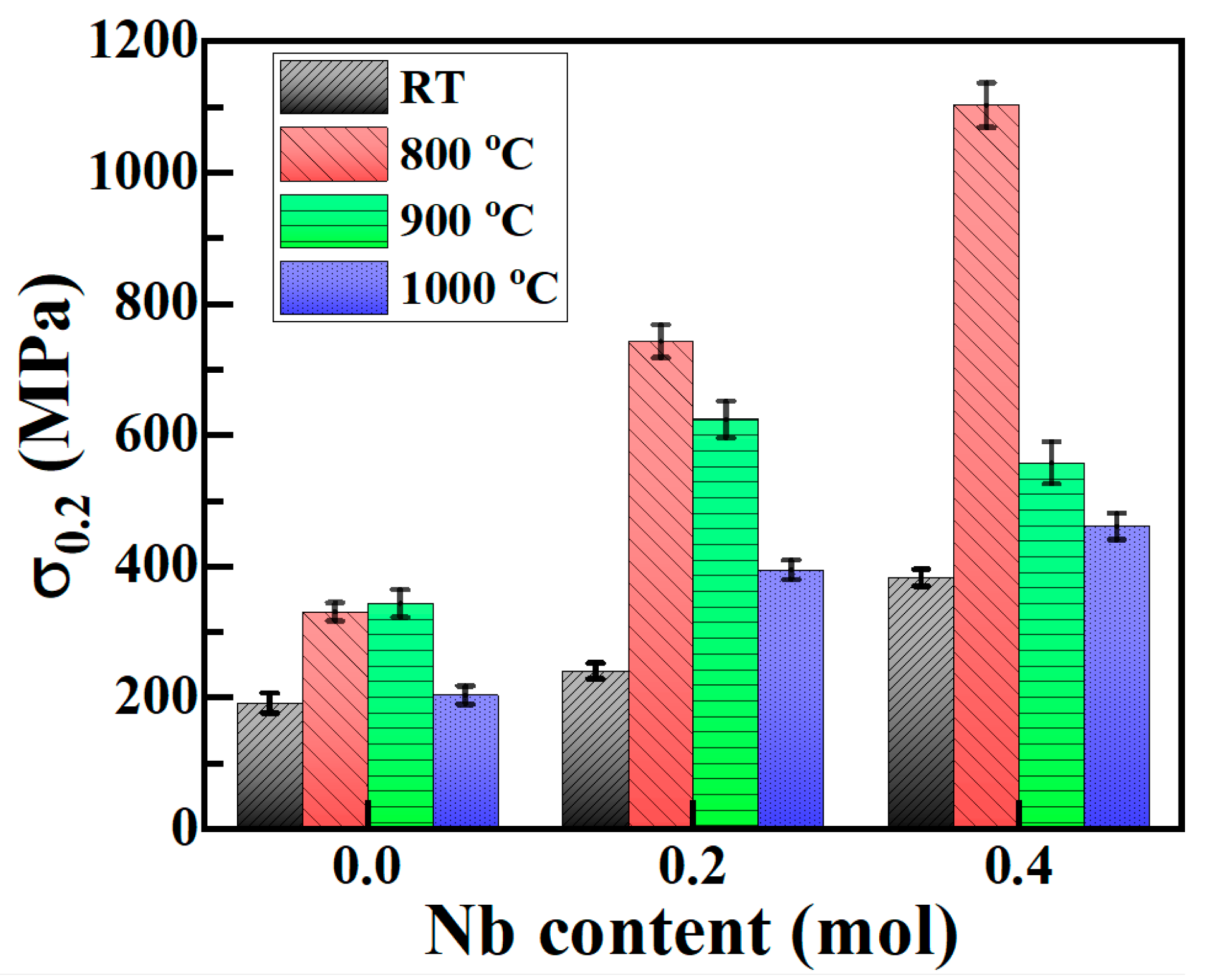
3.4. Compression Performance
4. Conclusions
- (1) In the as-cast state, the Nb0 alloy is composed of a single FCC solid solution phase. As the Nb content increases, the Laves phase gradually forms, which increases in content as the Nb content further increases. After heat treatment at 800 °C, all three alloys form BCC solid solution phases rich in Cr, Fe, and Mn. The BCC phases in the Nb0.2 and Nb0.4 alloys decompose after heat treatment at 900 and 1000 °C, respectively.
- (2) The microstructure of the Cu0.3Cr2Fe2Ni3Mn2Nbx HEAs is basically dendritic matrix and interdendritic morphology. After heat treatment, the Laves phase is distributed in the interdendritic region in the form of rods or particles, whereas the Cr-, Fe-, and Mn-rich BCC phase and Cu-, Mn-, Ni-, and Fe-rich FCC phase are distributed in the matrix. As the heat treatment temperature increases, the Laves phase aggregates and grows, whereas the BCC phase coarsens or decomposes.
- (3) As the Nb content increases, the microhardness of the as-cast Cu0.3Cr2Fe2Ni3Mn2Nbx HEAs increases from 127 to 203 HV. After heat treatment, the microhardness of the alloys substantially improves, with the Nb0.4 alloy having the highest microhardness after 800 °C heat treatment, approximately 346 HV. After heat treatment at 900 and 1000 °C, the microhardness of the three alloys decreases. The increase in Nb content in the as-cast hardness is mainly attributed to the precipitation strengthening of the Laves phase. After heat treatment, the hardness of the alloys first increases and then decreases mainly due to the formation and decomposition of the BCC phase and the coarsening of the Laves phase.
- (4) The yield strength of the as-cast Cu0.3Cr2Fe2Ni3Mn2Nbx HEAs increases with Nb content and first increases and then decreases with increasing heat treatment temperature. When cast, the yield strength of the Nb0.4 alloy is the highest (approximately 383 MPa). After heat treatment at 800 °C, its yield strength increases to 1103 MPa. The mechanism underlying the improvement in the compressive yield strength of these alloys is the same as the hardness-enhancing mechanism owing to the second-phase strengthening of the Laves phase and the solid solution strengthening of the BCC phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Can, J.Y.; Chin, T.S.; Shun, T.T. Nanostructured high entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructure development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Pang, J.Y.; Xiong, T.; Yang, W.F.; Ge, H.L.; Zheng, X.D.; Song, M.; Zhang, H.W.; Zheng, S.J. Atomic scale structure dominated FCC and B2 responses to He ion irradiation in eutectic high-entropy alloy AlCoCrFeNi2.1. J. Mater. Sci. Technol. 2022, 129, 87–95. [Google Scholar] [CrossRef]
- Tripathy, B.; Saha, R.; Bhattacharjee, P.P. Microstructure and unusually strong recrystallization texture of the FCC phase of a cost-effective high-strength dual-phase AlCrFe2Ni2 high entropy alloy. Intermetallics 2022, 145, 107559. [Google Scholar] [CrossRef]
- Shun, T.T.; Hung, C.H.; Lee, C.F. Formation of ordered/disordered nanoparticles in FCC high entropy alloys. J. Alloys Compd. 2010, 493, 105–109. [Google Scholar] [CrossRef]
- Qiao, D.X.; Liang, H.; Wu, S.Y.; He, J.Y.; Cao, Z.Q.; Lu, Y.P.; Li, T.J. The mechanical and oxidation properties of novel B2-ordered Ti2ZrHf0.5VNb0.5Alx refractory high-entropy alloys. Mater. Charact. 2021, 178, 111287. [Google Scholar] [CrossRef]
- Jin, D.M.; Wang, Z.H.; Yuan, J.H.; Jiang, B.B.; Yu, F.Y.; Li, J.F.; Wang, Q. High-strength and energetic Al2Ti6Zr2Nb3Ta3 high entropy alloy containing a cuboidal BCC/B2 coherent microstructure. J. Alloys Compd. 2023, 931, 167546. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.R.; Niu, H.W.; Yang, G.J.; Yang, L.; Xu, M.Q.; Yi, J.J. BCC+L21 dual-phase Cu40Al20Ti20V20 near-eutectic high-entropy alloy with a combination of strength and plasticity. J. Alloys Compd. 2022, 908, 164683. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Panina, E.S.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A.; Stepanov, N.D. Microstructure evolution of a novel low-density Ti-Cr-Nb-V refractory high entropy alloy during cold rolling and subsequent annealing. Mater. Charact. 2019, 158, 109980. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T. High-entropy alloys with a hexagonal close-packed structure designed by equi-atomic alloy strategy and binary phase diagrams. JOM 2014, 66, 1984–1992. [Google Scholar] [CrossRef]
- Lužnik, J.; Koželj, P.; Vrtnik, S.; Jelen, A.; Jagličić, Z.; Meden, A.; Feuerbacher, M.; Dolinšek, J. Complex magnetism of Ho-Dy-Y-Gd-Tb hexagonal high-entropy alloy. Phys. Rev. B 2015, 92, 22420122. [Google Scholar] [CrossRef]
- Ren, H.; Chen, R.R.; Gao, X.F.; Liu, T.; Qin, G.; Wu, S.P.; Guo, J.J. Phase formation and mechanical features in (AlCoCrFeNi)100-xHfx high-entropy alloys: The role of Hf. Mater. Sci. Eng. A 2022, 858, 144156. [Google Scholar] [CrossRef]
- Zhi, Q.; Tan, X.R.; Xie, J.L.; Liu, Y.; Yang, K.; Zhang, Q.; Liu, W.H.; Liu, Z.X. Microstructure and properties of lightweight Al2NbTixV2Zr high entropy alloy. J. Mater. Eng. Perform. 2022, 31, 4934–4944. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.P.; Jiang, L.; Wang, T.M.; Li, T.J. Effects of electro-negativity on the stability of topologically close-packed phase in high entropy alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Huang, Y.B.; Hu, Y.L.; Zhang, M.J.; Mao, C.; Tong, Y.G.; Zhang, J.; Li, K.W.; Wang, K.M. On the enhanced wear resistance of laser-clad CoCrCuFeNiTix high-entropy alloy coatings at elevated temperature. Tribol. Int. 2022, 174, 107767. [Google Scholar] [CrossRef]
- Liang, H.; Hou, J.X.; Jiang, L.; Cao, Z.Q. Microstructure and dry-sliding wear resistance of CoCrFeNiMoTix high entropy alloy coatings produced by laser cladding. Coatings 2024, 14, 221. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Li, D.M.; Shi, L.; Cai, B.; Wang, M.X. Corrosion behavior of CuCrFeNiMn high entropy alloy system in 1M sulfuric acid solution. Mater. Corros. 2012, 63, 828–834. [Google Scholar] [CrossRef]
- Ren, B.; Liang, Y.C.; Zhang, X.F.; Yu, Y.; Zhao, R.F.; Jiang, A.Y.; Liu, J.X.; Zhou, Y.J.; Zhang, B.F.; Liu, Z.X. Corrosion behavior of NbxCu0.3Cr2Fe2Ni3Mn2 high-entropy alloys in HNO3 solution. Results Phys. 2023, 51, 106780. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zhang, L.J.; Fan, J.T.; Yu, P.F.; Li, G. Novel Co-free CrFeNiNb0.1Tix high-entropy alloys with ultra high hardness and strength. Mater. Sci. Eng. A 2019, 764, 138212. [Google Scholar] [CrossRef]
- Nguyen, T.; Huang, M.; Li, H.J.; Hong, L.; Yang, S. Effect of Al content on microstructure and mechanical properties of as-cast AlxFeMnNiCrCu0.5 high-entropy alloys. Mater. Sci. Eng. A 2022, 832, 142495. [Google Scholar] [CrossRef]
- Qin, G.; Wang, S.; Chen, R.R.; Gong, X.; Wang, L.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructures and mechanical properties of Nb-alloyed CoCrCuFeNi high-entropy alloys. J. Mater. Sci. Technol. 2018, 34, 365–369. [Google Scholar] [CrossRef]
- Wu, J.; Qiu, H.; Zhu, H.G.; Xie, Z.H.; Cheng, J.L. Influences of Si and C addition on microstructure and mechanical properties of Fe2.5CoNiCu high-entropy alloy. Trans. Nonferrous Met. Soc. China 2023, 33, 3406–3417. [Google Scholar] [CrossRef]
- Sun, H.T.; Liu, T.; Oka, H.; Hashimoto, N.; Cao, Y.; Luo, R. Role of aging temperature on thermal stability of Co-free Cr0.8FeMn1.3Ni1.3 high-entropy alloy: Decomposition and embrittlement at intermediate temperatures. Mater. Charact. 2024, 210, 113804. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, Z.H.; Mo, N.; Kent, D.; Candella, A.R.; Koey, K.E.; Tan, Q.; Bermingham, M.; Zhang, M.X. High-temperature age-hardening of a novel cost-effective Fe45Ni25Cr25Mo5 high entropy alloy. Mater. Sci. Eng. A 2020, 788, 139580. [Google Scholar] [CrossRef]
- Chen, S.T.; Tang, W.Y.; Kuo, Y.F.; Chen, S.Y.; Tsau, C.H.; Shun, T.T.; Yeh, J.W. Microstructure and properties of age-hardenable AlxCrFe1.5MnNi0.5 alloys. Mater. Sci. Eng. A 2010, 527, 5818–5825. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Cai, B.; Wang, M.X.; Shi, L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater. Des. 2012, 33, 121–126. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]



| Alloy | Cu | Cr | Fe | Ni | Mn | Nb |
|---|---|---|---|---|---|---|
| Cu0.3Cr2Fe2Ni3Mn2 (Nb0) | 0.3 | 2 | 2 | 3 | 2 | 0 |
| Cu0.3Cr2Fe2Ni3Mn2Nb0.2 (Nb0.2) | 0.3 | 2 | 2 | 3 | 2 | 0.2 |
| Cu0.3Cr2Fe2Ni3Mn2Nb0.4 (Nb0.4) | 0.3 | 2 | 2 | 3 | 2 | 0.4 |
| Temperature | Spot | Cu | Cr | Fe | Ni | Mn |
|---|---|---|---|---|---|---|
| 800 °C | Nominal | 3.2 | 21.5 | 21.5 | 32.3 | 21.5 |
| 1 | 2.7 | 36.0 | 11.2 | 20.2 | 29.9 | |
| 2 | 3.7 | 25.1 | 25.8 | 28.8 | 16.7 | |
| 900 °C | Nominal | 3.2 | 21.5 | 21.5 | 32.3 | 21.5 |
| 1 | 3.3 | 56.6 | 16.3 | 11.2 | 12.5 | |
| 2 | 5.8 | 19.6 | 22.2 | 31.4 | 21.1 | |
| 1000 °C | Nominal | 3.2 | 21.5 | 21.5 | 32.3 | 21.5 |
| 1 | 1.4 | 37.8 | 21.4 | 1.8 | 37.5 | |
| 2 | 6.6 | 24.1 | 26.8 | 35.3 | 7.3 |
| Temperature | Spot | Cu | Cr | Fe | Ni | Mn | Nb |
|---|---|---|---|---|---|---|---|
| 800 °C | Nominal | 3.2 | 21.1 | 21.1 | 31.6 | 21.1 | 2.1 |
| 1 | 1.9 | 17.2 | 23.7 | 20.9 | 12.2 | 24.1 | |
| 2 | 7.2 | 16.8 | 19.8 | 32.9 | 22.3 | 0.9 | |
| 900 °C | Nominal | 3.2 | 21.1 | 21.1 | 31.6 | 21.1 | 2.1 |
| 1 | 0.6 | 17.3 | 15.9 | 14.0 | 5.8 | 46.4 | |
| 2 | 6.6 | 22.0 | 22.6 | 33.6 | 14.3 | 0.9 | |
| 1000 °C | Nominal | 3.2 | 21.1 | 21.1 | 31.6 | 21.1 | 2.1 |
| 1 | 1.8 | 14.7 | 23.3 | 24.1 | 14.5 | 21.5 | |
| 2 | 4.7 | 23.4 | 25.7 | 27.8 | 17.7 | 0.7 |
| Temperature | Spot | Cu | Cr | Fe | Ni | Mn | Nb |
|---|---|---|---|---|---|---|---|
| 800 °C | Nominal | 3.1 | 20.6 | 20.6 | 30.9 | 20.6 | 4.1 |
| 1 | 1.9 | 14.9 | 24.0 | 23.8 | 13.2 | 22.3 | |
| 2 | 9.0 | 11.3 | 16.4 | 35.6 | 25.5 | 2.2 | |
| 900 °C | Nominal | 3.1 | 20.6 | 20.6 | 30.9 | 20.6 | 4.1 |
| 1 | 1.6 | 17.9 | 23.8 | 21.2 | 11.8 | 23.6 | |
| 2 | 6.6 | 18.0 | 20.7 | 32.5 | 21.2 | 0.9 | |
| 1000 °C | Nominal | 3.1 | 20.6 | 20.6 | 30.9 | 20.6 | 4.1 |
| 1 | 2.0 | 18.4 | 22.1 | 24.4 | 8.7 | 24.4 | |
| 2 | 6.7 | 20.3 | 20.7 | 33.9 | 17.1 | 1.3 |
| Element | Cr | Fe | Cu | Ni | Mn | Nb |
|---|---|---|---|---|---|---|
| Cr | - | −1 | 12 | −7 | 2 | −7 |
| Fe | - | 13 | −2 | 0 | −16 | |
| Cu | - | 4 | 4 | 3 | ||
| Ni | - | −8 | −30 | |||
| Mn | - | −4 | ||||
| Nb | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, F.; Wang, C.; Ren, B. Effect of Heat Treatment Temperature on the Microstructure and Mechanical Properties of Cu0.3Cr2Fe2Ni3Mn2Nbx High-Entropy Alloys. Coatings 2024, 14, 950. https://doi.org/10.3390/coatings14080950
Guo F, Wang C, Ren B. Effect of Heat Treatment Temperature on the Microstructure and Mechanical Properties of Cu0.3Cr2Fe2Ni3Mn2Nbx High-Entropy Alloys. Coatings. 2024; 14(8):950. https://doi.org/10.3390/coatings14080950
Chicago/Turabian StyleGuo, Fuqiang, Chunyan Wang, and Bo Ren. 2024. "Effect of Heat Treatment Temperature on the Microstructure and Mechanical Properties of Cu0.3Cr2Fe2Ni3Mn2Nbx High-Entropy Alloys" Coatings 14, no. 8: 950. https://doi.org/10.3390/coatings14080950






