Influence of Mixing and Standing Times on the Rheological Properties and Performance of Coatings for Lost Foam Shell Casting
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Analysis
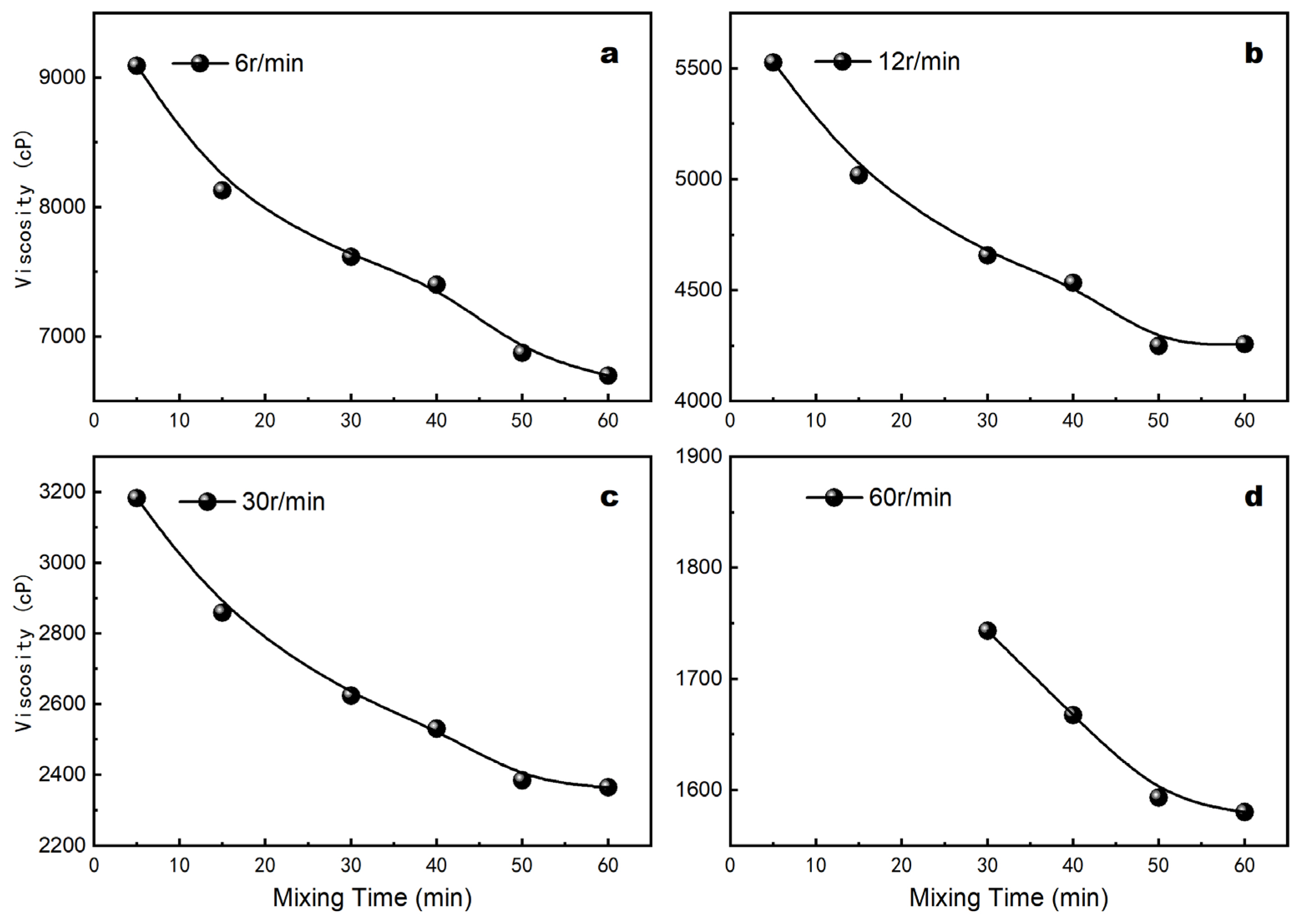
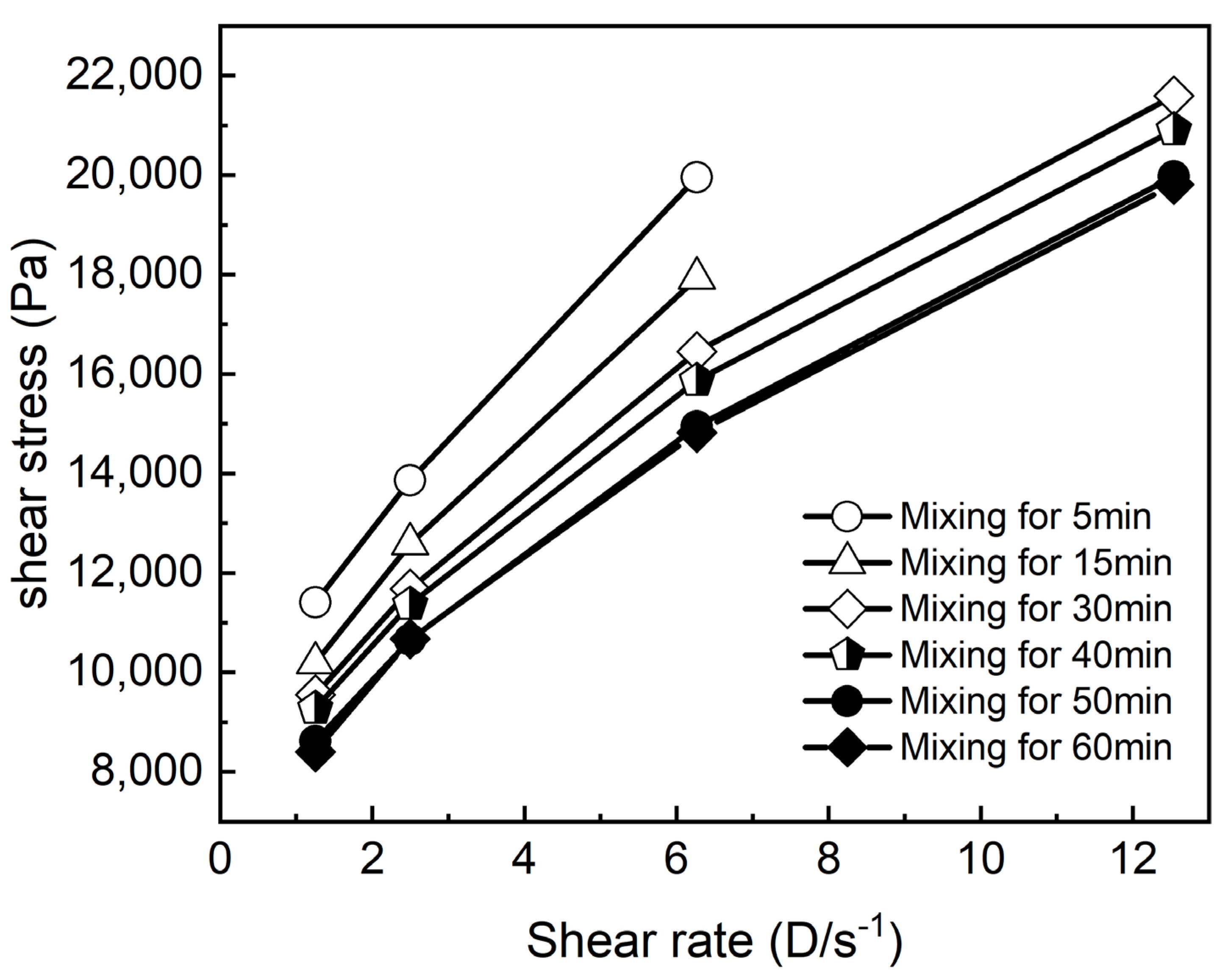
3.1. Effect of Mixing Time on the Coating
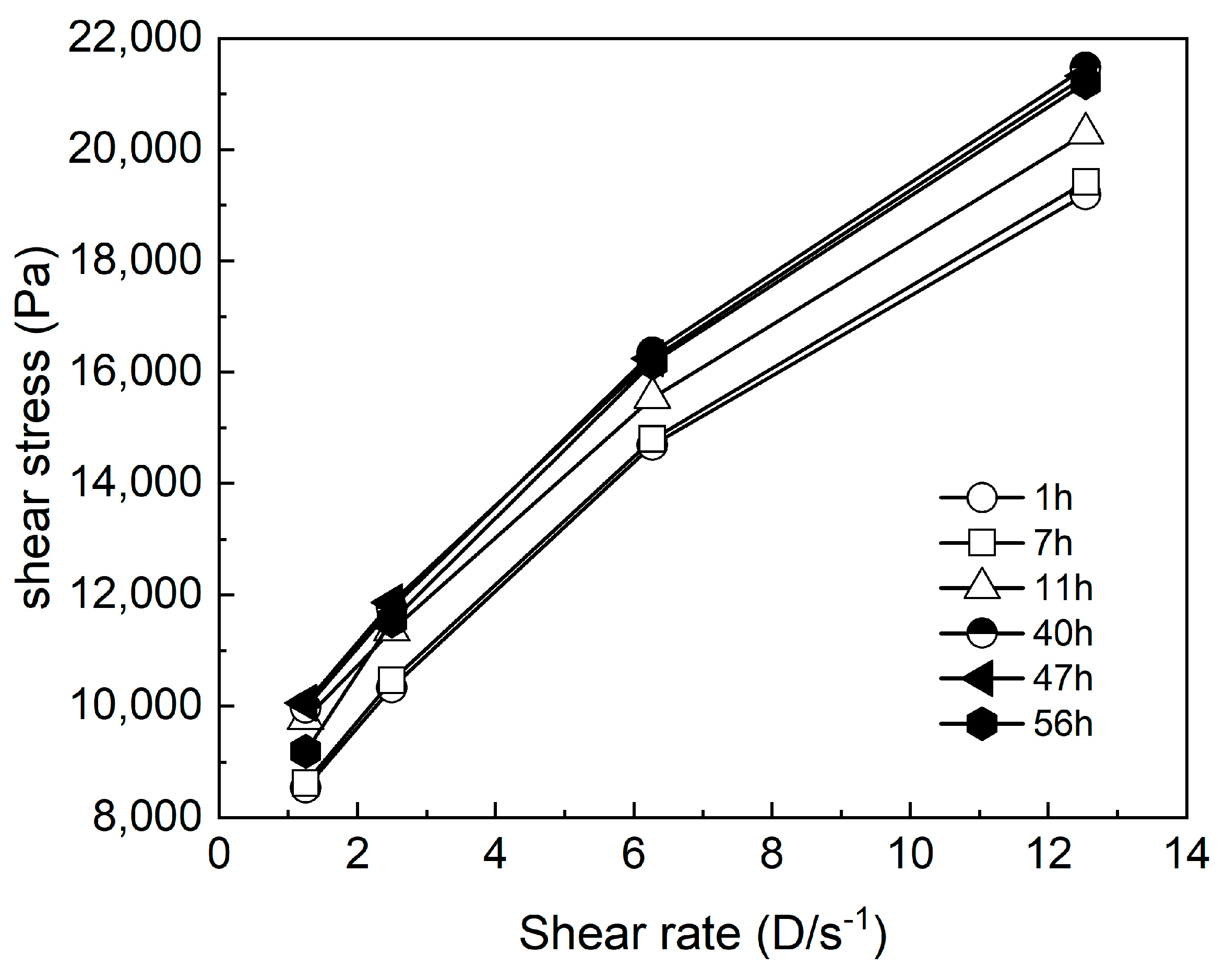
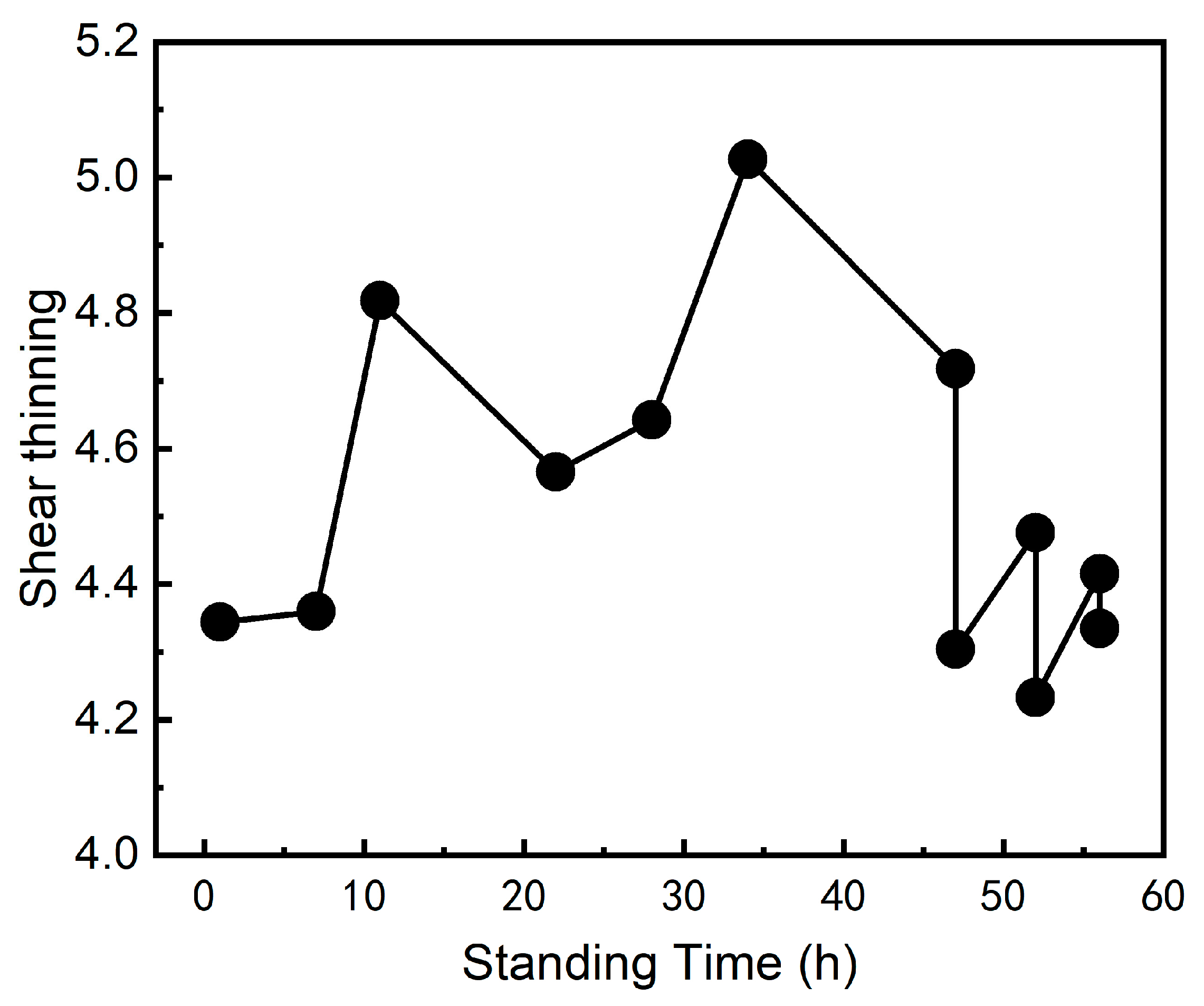
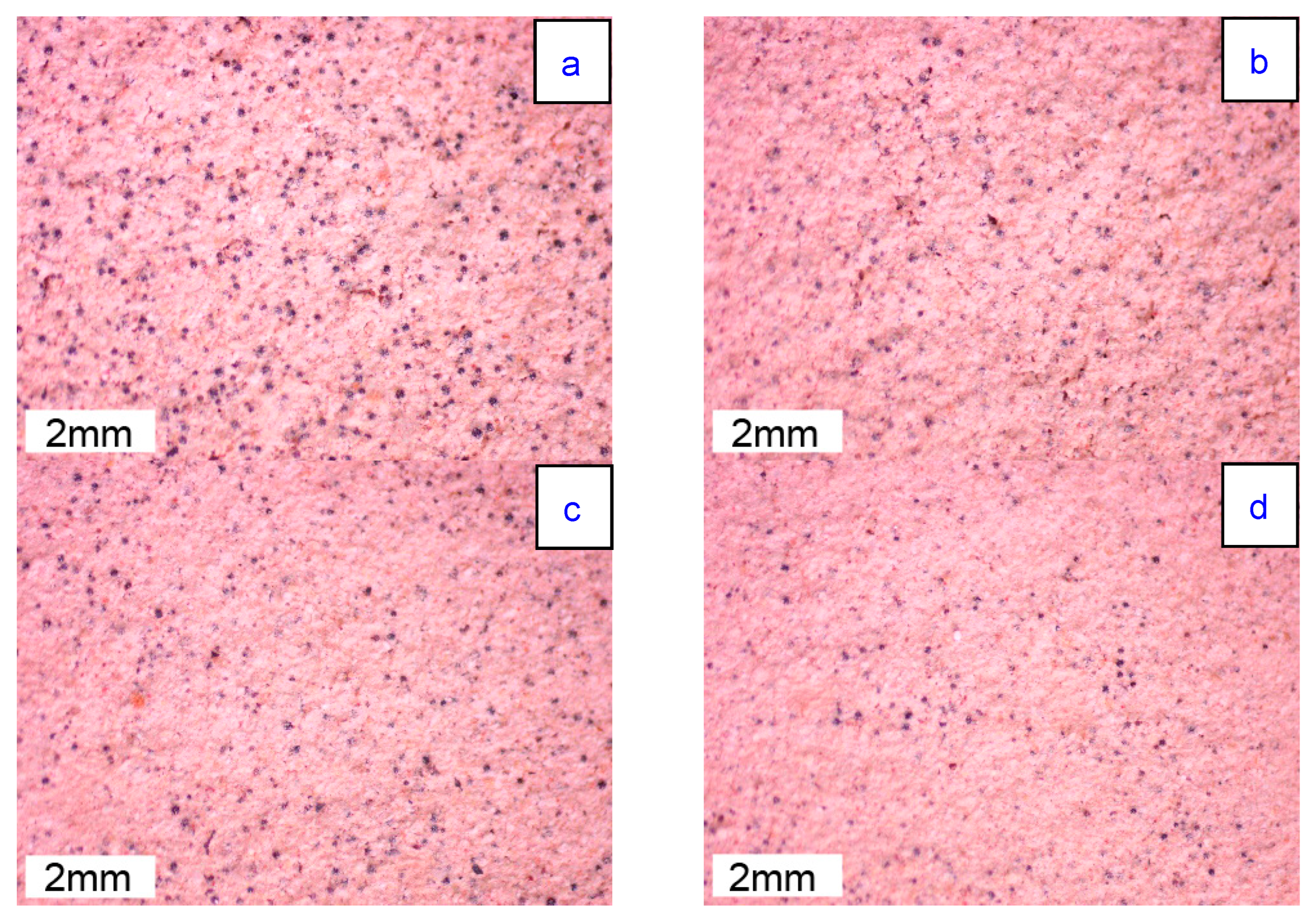
3.2. Effect of Standing Time on the Coating
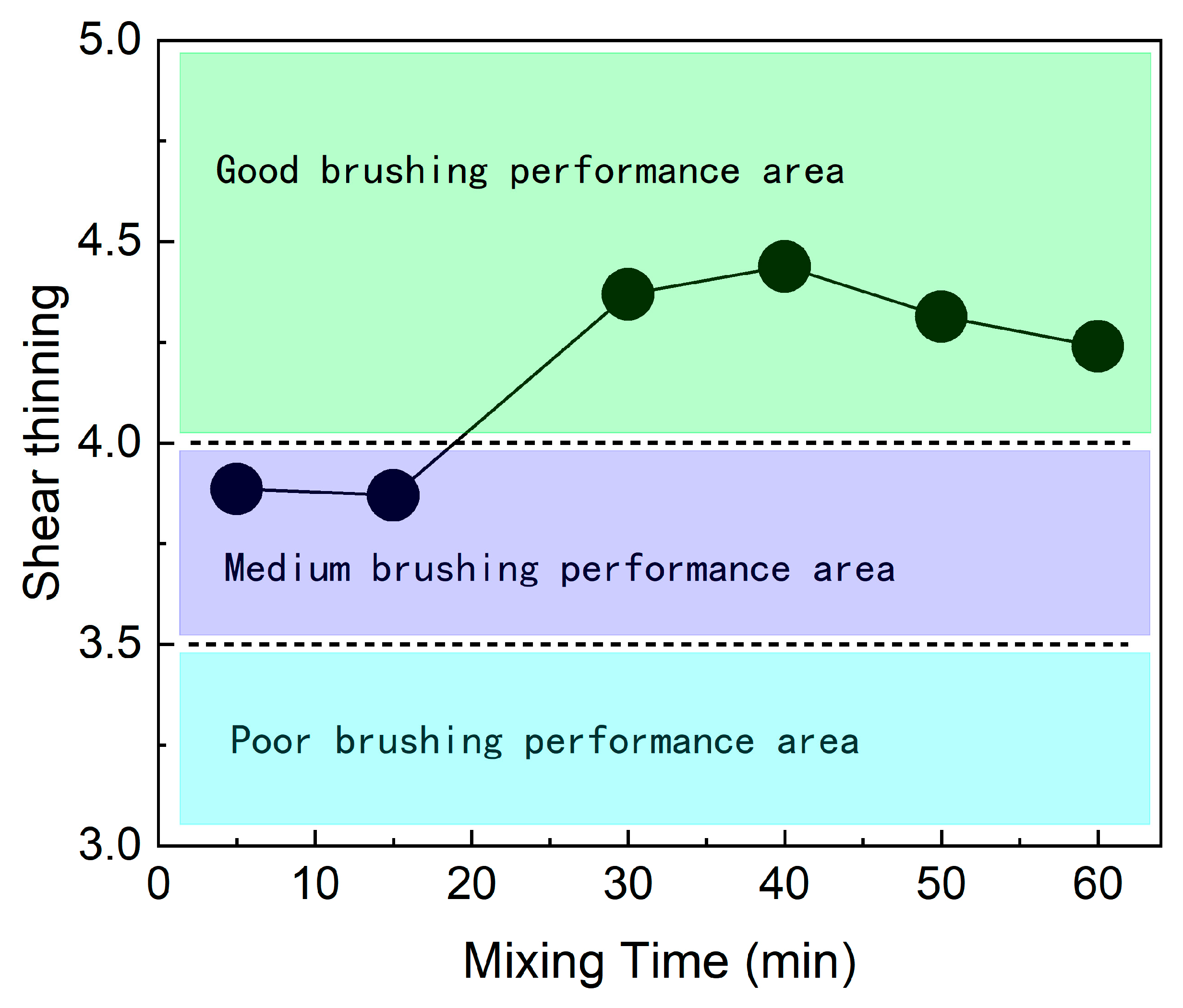
3.3. Discussion of Coating Performance Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajagukguk, K. Effect of Pattern Coating Thickness on Surface Roughness and Porosity of Nodular Cast Iron (FCD) 450 Using Lost Foam Casting Method. 2020. Available online: https://www.researchgate.net/publication/344519006_Effect_of_Pattern_Coating_Thickness_on_Surface_Roughness_and_Porosity_of_Nodular_Cast_Iron_FCD_450_Using_Lost_Foam_Casting_Method (accessed on 1 October 2018).
- Shivkumar, S.; Wang, L.; Apelian, D. The lost-foam casting of aluminum alloy components. JOM 1990, 42, 38–44. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, L.; Li, H. Microstructure, Hardness, Wear Resistance, and Corrosion Resistance of As-Cast and Laser-Deposited FeCoNiCrAl0.8Cu0.5Si0.5 High Entropy Alloy. Coatings 2024, 14, 663. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, Y.; Wang, D.; Li, J.; Ji, X.; Li, W.; Shen, X.; Fu, W.; Zhou, J. High ductility of Be–Al alloy fabricated by investment casting. J. Mater. Res. Technol. 2024, 29, 789–802. [Google Scholar] [CrossRef]
- Chekmyshev, K.E.; Ovcharenko, P.G. Numerical simulation of bimetallic casting cooling during the process of lost foam casting. J. Cryst. Growth 2019, 527, 125243. [Google Scholar] [CrossRef]
- Karimian, M.; Idris, M.H.; Ourdjini, A.; Muthu, K.; Chinesta, F.; Chastel, Y.; El Mansori, M. Effect of flask vibration time on casting integrity, Surface Penetration and Coating Inclusion in lost foam casting of Al-Si Alloy. Eur. J. Pharmacol. 2011, 1315, 633–638. [Google Scholar]
- Lohar, A.K.; Mukherjee, D.; Roy, H.; Panigrahi, S.C. Enhancing mechanical properties of Al-6Mg-0.3Sc-0.15Zr alloy through high cooling rate oil quench investment casting. Mater. Today Commun. 2024, 39, 108565. [Google Scholar] [CrossRef]
- Romazanov, Z.; Silayeva, O.; Tatieva, M.; Latypova, M.; Petrovskaya, A. The Feasibility Study for the Creation of Production Based on Technology of Lost-Foam Casting. Metalurgija 2023, 62, 103–106. [Google Scholar]
- Qiao, F.L.; Yin, Y.M.; Zhi, X.H. Study on the Casting Process of the Large-Scale High-Chromium Cast Iron Impeller. Adv. Mater. Res. 2010, 139–141, 622–625. [Google Scholar] [CrossRef]
- Su, Y.; Li, D.; Zhang, X. Optimising hardenability of high chromium white cast iron. China Foundry 2006, 3, 284–287. [Google Scholar]
- Yoo, S.M.; Cho, Y.S.; Lee, C.C. Optimization of casting conditions for heat and abrasion resistant large grey iron castings. China Foundry 2007, 4, 124–127. [Google Scholar]
- Sands, M.; Shivkumar, S. Influence of coating thickness and sand fineness on mold filling in the lost foam casting process. J. Mater. Sci. 2003, 38, 667–673. [Google Scholar] [CrossRef]
- Shi, T.; Guo, Z.; Gao, L. Wear-Resistant Coatings Prepared by Combination of SHS and Lost Foam Casting. Mater. Sci. Forum 2013, 749, 595–599. [Google Scholar] [CrossRef]
- Yang, S.H.; Du, X.M. The Lost Foam Casting Simulation of the Gray Cast Iron Linner. Adv. Mater. Res. 2013, 834–836, 1580–1583. [Google Scholar] [CrossRef]
- Johnson, C.K.; Penumadu, D.; Murshed, M. Methods for Measuring Rheological Properties of Lost Foam Coatings. Trans. Am. Foundry Soc. 2005, 1059–1070. [Google Scholar]
- Ovcharenko, P.G.; Kuz’Minykh, E.V.; Lad’Yanov, V.I. Interaction of a Nonstick Corundum Coating with Iron–Carbon Melts under Lost-Foam Casting Conditions. Russ. Metall. (Met.) 2020, 2020, 115–120. [Google Scholar] [CrossRef]
- Zhang, L.; He, H.Q.; Kwek, W.R.; Ma, J.; Tang, E.H.; Jiang, S.P. Fabrication and Characterization of Anode-Supported Tubular Solid-Oxide Fuel Cells by Slip Casting and Dip Coating Techniques. J. Am. Ceram. Soc. 2010, 92, 302–310. [Google Scholar] [CrossRef]
- Kerber, F.; Zienert, T.; Neumann, M.; Malczyk, P.; Schemmel, T.; Jansen, H.; Aneziris, C.G. Insulating refractories based on rice husk ashes functionalized by flame-sprayed alumina coatings for steel ingot casting. J. Eur. Ceram. Soc. 2024, 44, 7296–7309. [Google Scholar] [CrossRef]
- Nayak, R.K.; Sadarang, J. Development of A356 Alloy Green Sand Mold Casting Process Using Narmada Riverbed Sand in India: Design of Experiment and Optimization. Int. J. Met. 2022, 17, 1296–1307. [Google Scholar] [CrossRef]
- Song, X.; Baghoolizadeh, M.; Alizadeh, A.; Jasim, D.J.; Basem, A.; Sultan, A.J.; Salahshour, S.; Piromradian, M. Utilizing machine learning algorithms for prediction of the rheological behavior of ZnO (50%)-MWCNTs (50%)/Ethylene glycol (20%)-water (80%) nano-refrigerant. Int. Commun. Heat Mass Transf. 2024, 156, 107634. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barnes, C. Rapid Temperature-Dependent Rheological Measurements of Non-Newtonian Solutions Using a Machine-Learning Aided Microfluidic Rheometer. Anal. Chem. 2022, 94, 3617–3628. [Google Scholar] [CrossRef]
- Romanelli Vicente Bertolo, M.; da Conceição Amaro Martins, V.; de Guzzi Plepis, A.M.; Bogusz Junior, S. Rheological study of the incorporation of grape seed extract in chitosan and gelatin coatings. J. Appl. Polym. Sci. 2021, 138, 50052. [Google Scholar] [CrossRef]
- Kimura, H.; Kosemura, T.; Ando, C. Temperature Control Design for Coating Fluid Circulatory Systems. J. Qual. Eng. Soc. 2015, 23, 41–46. [Google Scholar]
- Chen, X.; Penumadu, D. Characterizing microstructure of refractory porous materials. J. Mater. Sci. 2006, 41, 3403–3415. [Google Scholar] [CrossRef]
- Javid, A.A.S.; Ghoddousi, P.; Aghajani, S.; Naseri, H.; Pour, S.H. Investigating the Effects of Mixing Time and Mixing Speed on Rheological Properties, Workability, and Mechanical Properties of Self-Consolidating Concretes. Int. J. Civ. Eng. 2021, 19, 339–355. [Google Scholar] [CrossRef]
- Vyas, A.V.; Pandya, M.P.; Sutaria, M.P. Effect of mixing proportion and mixing time on primary slurry retention and surface roughness of investment casting shells. IOP Conf. Ser. Mater. Sci. Eng. 2020, 872, 012094. [Google Scholar] [CrossRef]
- Bambauer, R.A.; Lee, T.; Delong, T. Effect of Continuous Mixing on Viscosity and Permeability of an Iron Lost Foam Coating: A Joint Study. Trans. Am. Foundrymens Soc. 1996, 104, 329–333. [Google Scholar]
- Alter, H. The gelation of plastisols: An automatic method for the determination of plastisol temperature-rheology characteristics. J. Appl. Polym. Sci. 2003, 2, 312–317. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Chen, M.; Liu, Z.; Jiang, S.; Wang, L. Preparation of Durable Superhydrophobic Coatings Based on Discrete Adhesives. Coatings 2024, 14, 463. [Google Scholar] [CrossRef]
- Gulizia, S.; Jahedi, M.Z.; Doyle, E.D. Performance evaluation of PVD coatings for high pressure die casting. Surf. Coat. Technol. 2001, 140, 200–205. [Google Scholar] [CrossRef]
- Iyer, R.R.; Bousfield, D.W. The leveling of coating defects with shear thinning rheology. Chem. Eng. Sci. 1996, 51, 4611–4617. [Google Scholar] [CrossRef]
- Gilbert, D.; Valette, R.; Lemaire, E. Impact of particle stiffness on shear-thinning of non-Brownian suspensions. J. Rheol. 2022, 66, 161–176. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Pang, Y.; Wang, J.; Lu, Y.; Ren, Y.; Zhao, S.; Gao, S. Flow characteristics inside shear thinning xanthan gum non-Newtonian droplets moving in rectangular microchannels. Exp. Fluids 2021, 62, 203. [Google Scholar] [CrossRef]
- Kimura, M.; Hanafi, Z.A.; Takagi, T.; Sawara, R.; Fujii, S. Shear-Thinning Characteristics of Nematic Liquid Crystals Doped with Nanoparticles. Crystals 2016, 6, 145. [Google Scholar] [CrossRef]
- Noda, K.; Yamagami, M.; Yamamoto, M.; Sato, H.; Itaya, H.; Okajima, A.; Yamada, M. Thin Film Formation by Direct Reverse Roll-Coating on Plastic Web. Jpn. J. Appl. Phys. 2003, 42, 5722–5725. [Google Scholar] [CrossRef]
- Sun, C.; Cao, Z. Effects of the Wettability Between the Coating and the Liquid EPS on the Filling Process of Lost Foam Casting. Inter. Met. 2024, 18, 1318–1328. [Google Scholar] [CrossRef]
- Zheng, K.-K.; Lin, Y.-X.; Yan, S.-Q.; Chen, W.-P. Research on Surface Coating Treatment of Casting Die for Copper Alloy Water-Meter Shell. Inter. Met. 2023, 17, 922–934. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, Q.; Zhang, Y.; Ding, Y.; Chen, S.; Qiao, Y.; Shen, L. Preparation and coating properties of alkyd polyol-based autoxidizable waterborne polyurethane dispersions with high fatty acid content, long storage stability, and low viscosity. J. Coat. Technol. Res. 2024. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Wang, Q.; Luan, S. Influence of Mixing and Standing Times on the Rheological Properties and Performance of Coatings for Lost Foam Shell Casting. Coatings 2024, 14, 954. https://doi.org/10.3390/coatings14080954
Sun G, Wang Q, Luan S. Influence of Mixing and Standing Times on the Rheological Properties and Performance of Coatings for Lost Foam Shell Casting. Coatings. 2024; 14(8):954. https://doi.org/10.3390/coatings14080954
Chicago/Turabian StyleSun, Guojin, Qi Wang, and Shiyu Luan. 2024. "Influence of Mixing and Standing Times on the Rheological Properties and Performance of Coatings for Lost Foam Shell Casting" Coatings 14, no. 8: 954. https://doi.org/10.3390/coatings14080954




