Natural Tannin Layers for the Corrosion Protection of Steel in Contact with Water-Based Media
Abstract
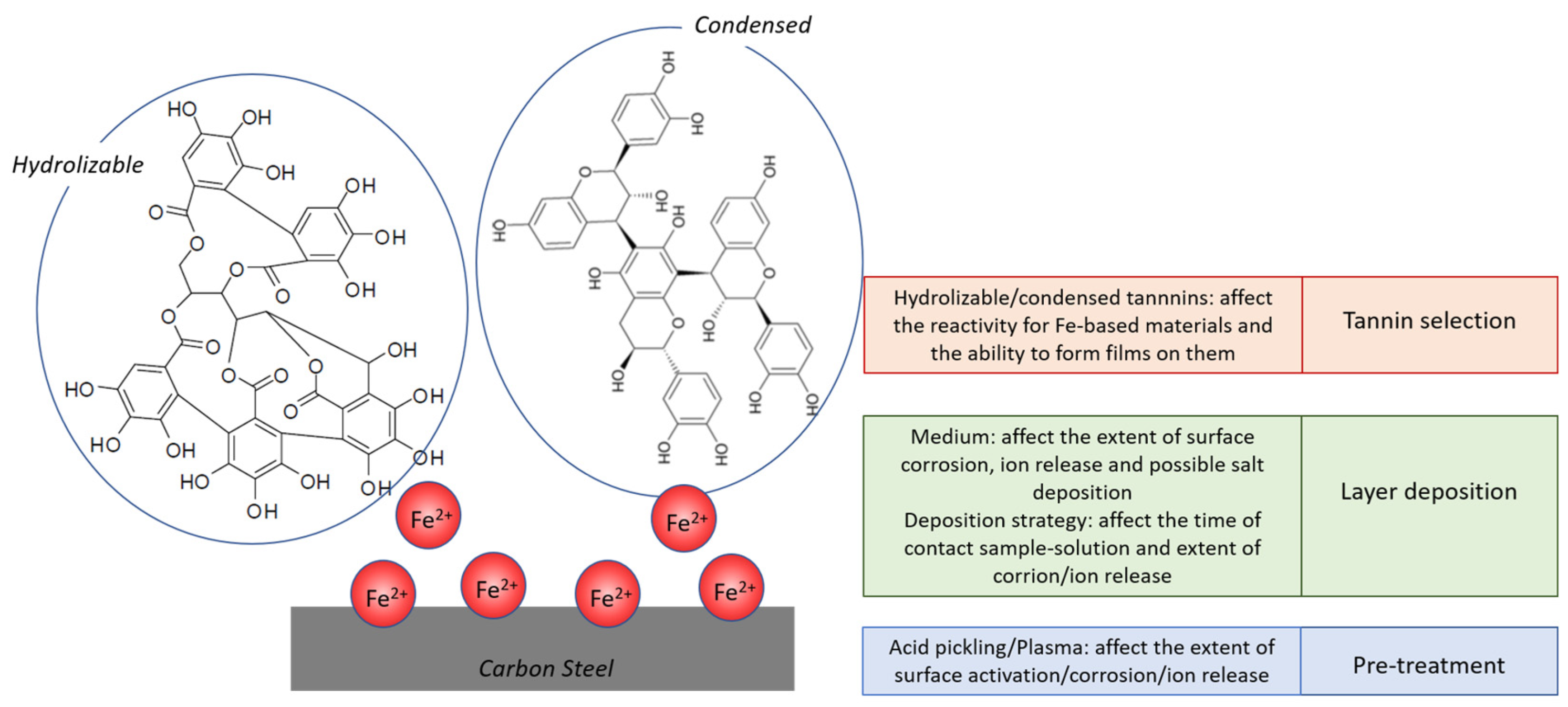
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Characterization
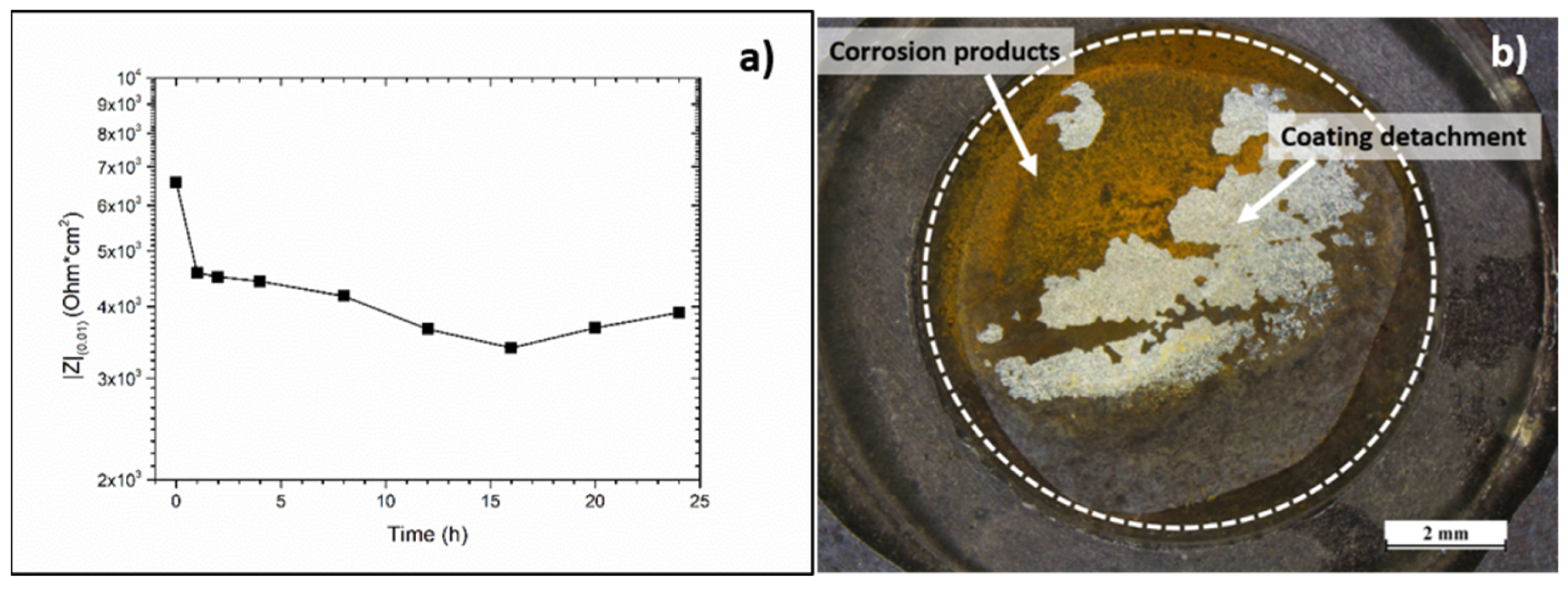
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.-S.; Li, J.-L.; Guo, W.-J.; Xu, P.-F.; Zhang, S.-H.; Zhang, Y.-Z. Progress in corrosion-resistant coatings on surface of low alloy steel. J. Iron Steel Res. Int. 2023, 30, 193–215. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef]
- Mizuno, D. Automotive Corrosion and Accelerated Corrosion Tests for Zinc Coated Steels. ISIJ Int. 2018, 58, 1562–1568. [Google Scholar] [CrossRef]
- Gerengi, H.; Sen, N.; Uygur, I.; Solomon, M.M. Corrosion response of ultra-high strength steels used for automotive applications. Mater. Res. Express 2019, 6, 0865a6. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Nazdracheva, T. Anti-corrosion coatings for protection of steel railway structures exposed to atmospheric environments: A review. Constr. Build. Mater. 2021, 288, 123115. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W.G. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Angst, U.M. A Critical Review of the Science and Engineering of Cathodic Protection of Steel in Soil and Concrete. Corrosion 2019, 75, 1420–1433. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Frankel, G.S. The carbon footprint of steel corrosion. NPJ Mater. Degrad. 2022, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, Z.; Li, M.; Han, B.; Liang, Y.; Hayat, M.D.; Sun, Y.; Jin, D.; Li, J.; Wang, B.; et al. Synergistic effect on corrosion behavior of X80 steel influenced by Pseudomonas aeruginosa and Acetobacter aceti. Sep. Purif. Technol. 2024, 351, 128135. [Google Scholar] [CrossRef]
- Hussein Farh, H.M.; El Amine Ben Seghier, M.; Zayed, T. A comprehensive review of corrosion protection and control techniques for metallic pipelines. Eng. Fail. Anal. 2023, 143, 106885. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Azzawi, W.K. Organic Synthesized Inhibitors for Corrosion Protection of Carbon Steel: A Comprehensive Review. J. Bio. Tribo. Corros. 2023, 9, 74. [Google Scholar] [CrossRef]
- Galedari, S.A.; Mahdavi, A.; Azarmi, F.; Huang, Y.; McDonald, A. A Comprehensive Review of Corrosion Resistance of Thermally-Sprayed and Thermally-Diffused Protective Coatings on Steel Structures. J. Therm. Spray. Technol. 2019, 28, 645–677. [Google Scholar] [CrossRef]
- Medupin, R.O.; Ukoba, K.O.; Yoro, K.O.; Jen, T.-C. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: A review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Al Shibli, F.S.Z.S.; Bose, S.; Kumar, P.S.; Rajasimman, M.; Rajamohan, N.; Vo, D.-V.N. Green technology for sustainable surface protection of steel from corrosion: A review. Environ. Chem. Lett. 2022, 20, 929–947. [Google Scholar] [CrossRef]
- Sharma, S.; Ganjoo, R.; Kumar, A. Polyphenols for Anticorrosion Application. In Science and Engineering of Polyphenols: Fundamentals and Industrial Scale Applications; Verma, C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Pan, Y.; Qin, R.; Hou, M.; Xue, J.; Zhou, M.; Xu, L.; Zhang, Y. The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 2022, 300, 121831. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant Polyphenols: Chemical Properties, biological Activities, and Synthesis. Angew. Chem. Int. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant extracts as sustainable and green corrosion inhibitors for protection of ferrous metals in corrosive media: A mini review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Lopez de Lacalle, J.; Minudri, D.; Picchio, M.L.; Gallastegui, A.; Mantione, D.; Forsyth, M.; Mecerreyes, D. Protection coatings for corrosion control of mild steel using phenolic polymeric deep eutectic solvents. RSC Sustain. 2024, 2, 1809–1818. [Google Scholar] [CrossRef]
- Sesia, R.; Spriano, S.; Sangermano, M.; Ferraris, S. Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals 2023, 13, 1070. [Google Scholar] [CrossRef]
- Faris, A.H.; Najib, A.M.; Hamid, K.J.; Yousif, R.H.; Ibrahim, M.N.M. Using Lignin and Tannin as Green Corrosion Inhibitors for Carbon Steel Petrolum Tanks. BIO Web Conf. 2024, 97, 00026. [Google Scholar] [CrossRef]
- Ciriminna, R.; Meneguzzo, F.; Li Petri, G.; Meneguzzo, C.; Angellotti, G.; Pagliaro, M. Chestnut tannin: New use, research and bioeconomy. J. Bioresour. Bioprod. 2024, 9, 246–252. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. South Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Tüken, T.; Yazıcı, B.; Erbil, M. A new multilayer coating for mild steel protection. Prog. Org. Coat. 2004, 50, 115–122. [Google Scholar] [CrossRef]
- Tüken, T.; Arslan, G.; Yazıcı, B.; Erbil, M. The corrosion protection of mild steel by polypyrrole/polyphenol multilayer coating. Corros. Sci. 2004, 46, 2743–2754. [Google Scholar] [CrossRef]
- Koerner, C.M.; Hopkinson, D.P.; Ziomek-Moroz, M.E.; Rodriguez, A.; Xiang, F. Environmentally Friendly Tannic Acid Multilayer Coating for Reducing Corrosion of Carbon Steel. Ind. Eng. Chem. Res. 2021, 60, 243–250. [Google Scholar] [CrossRef]
- Cheng, X.; Lu, R.; Zhang, X.; Zhu, Y.; Wei, S.; Zhang, Y.; Zan, X.; Geng, W.; Zhang, L. Silanization of a Metal–Polyphenol Coating onto Diverse Substrates as a Strategy for Controllable Wettability with Enhanced Performance to Resist Acid Corrosion. Langmuir 2021, 37, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Bhagawati, B.; Khandagiri, P.; Shamshoddin, S.; Bhadu, M.K.; Rout, T.K.; Das, S. Anticorrosive and lubricating polyphenol coatings on galvanneal steel. Surf. Eng. 2017, 33, 410–427. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Li, P.; Xiao, B.; Liu, G.; Chen, X.; Hu, Y.; Yang, Z. Tannin-based modified graphene oxide anti-corrosion composite coating with favourable corrosion inhibition, self-healing and photothermal conversion properties. Corros. Sci. 2024, 231, 111956. [Google Scholar] [CrossRef]
- Lamprakou, Z.; Bi, H.; Weinell, C.E.; Dam-Johansen, K. Tannin-based inhibitive pigment for sustainable epoxy coatings formulation. Prog. Org. Coat. 2022, 167, 106841. [Google Scholar] [CrossRef]
- ASTM D 3359-97; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2000.
- Sesia, R.; Ferraris, S.; Spriano, S.; Sangermano, M. UV-Cured Chitosan-Based Hydrogels Strengthened by Tannic Acid for the Removal of Copper Ions from Water. Polymers 2022, 14, 4645. [Google Scholar] [CrossRef] [PubMed]
- Cesprini, E.; De Iseppi, A.; Giovando, S.; Tarabra, E.; Zanetti, M.; Šket, P.; Marangon, M.; Tondi, G. Chemical characterization of cherry (Prunus avium) extract in comparison with commercial mimosa and chestnut tannins. Wood Sci. Technol. 2022, 56, 1455–1473. [Google Scholar] [CrossRef]
- Wahyono, T.; Astuti, D.A.; Wiryawan, I.K.G.; Sugoro, I.; Jayanegara, A. Fourier Transform Mid-Infrared (FTIR) Spectroscopy to Identify Tannin Compounds in The Panicle of Sorghum Mutant Lines. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042045. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier Transform Infrared (FTIR) Spectroscopy in the Characterization of Tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Pardini, O.R.; Amalvy, J.I.; Di Sarli, A.R.; Romagnoli, R.; Vetere, V.F. Formulation and testing of a waterborne primer containing chestnut tannin. J. Coat. Technol. 2001, 73, 99–106. [Google Scholar] [CrossRef]
- Vetere, V.F.; Romagnoli, R. Chemical and electrochemical assessment of tannins and aqueous primers containing tannins. Surf. Coat. Int. 1998, 81, 385–391. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodríguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Hüsing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Aaron Lau, K.H.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Gust, J.; Suwalski, J. Use of Mössbauer Spectroscopy to Study Reaction Products of Polyphenols and Iron Compounds. Corrosion 1994, 50, 355–365. [Google Scholar] [CrossRef]
- Saji, V.S. Progress in rust converters. Prog. Org. Coat. 2019, 127, 88–99. [Google Scholar] [CrossRef]
- Sack, S.H.; Romagnoli, R.; Vetere, V.F.; Elsner, C.I.; Pardini, O.; Amalvy, J.I.; Di Sarli, A.R. Evaluation of steel/primer based on chestnut tannin/paint film systems by EIS. J. Coat. Technol. 2002, 74, 63–69. [Google Scholar] [CrossRef]
- Afidah, R.A.; Jain, K. Recent Development of Vegetal Tannins in Corrosion Protection of Iron and Steel. Recent Pat. Mater. Sci. 2008, 1, 223–231. [Google Scholar] [CrossRef]
- Byrne, C.; D’Alessandro, O.; Selmi, G.J.; Romagnoli, R.; Deyá, C. Primers based on tara and quebracho tannins for poorly prepared steel surfaces. Prog. Org. Coat. 2019, 130, 244–250. [Google Scholar] [CrossRef]
- Gust, J.; Wawer, I. Relationship Between Radical Scavenging Effects and Anticorrosive Properties of Polyphenols. Corrosion 1995, 51, 37–44. [Google Scholar] [CrossRef]
- Stewart, R.; Goodship, V.; Guild, F.; Green, M.; Farrow, J. Investigation and demonstration of the durability of air plasma pre-treatment on polypropylene automotive bumpers. Int. J. Adhes. Adhes. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Sundriyal, P.; Pandey, M.; Bhattacharya, S. Plasma-assisted surface alteration of industrial polymers for improved adhesive bonding. Int. J. Adhes. Adhes. 2020, 101, 102626. [Google Scholar] [CrossRef]
- Ciardiello, R.; D’Angelo, D.; Cagna, L.; Croce, A.; Paolino, D.S. Effects of plasma treatments of polypropylene adhesive joints used in the automotive industry. Proc. I Mech. E Part C 2022, 236, 6204–6218. [Google Scholar] [CrossRef]
- De Zanet, A.; Salvo, M.; Casalegno, V. Surface modification of SiC to improve joint strength via a Corona plasma treatment. Ceram. Int. 2022, 48, 23492–23497. [Google Scholar] [CrossRef]
- Akram, M.; Jansen, K.M.B.; Ernst, L.J.; Bhowmik, S. Atmospheric plasma modification of polyimide sheet for joining to titanium with high temperature adhesive. Int. J. Adhes. Adhes. 2016, 65, 63–69. [Google Scholar] [CrossRef]
- Zhai, Z.; He, Q.; Fu, L.; Liu, N.; Liu, X.; Jiang, B.; Zhou, M. Effect of plasma treatment parameters on the interfacial joining strength of overmolded hybrid fiber reinforced thermoplastic composites. J. Appl. Polym. Sci. 2022, 139, e52166. [Google Scholar] [CrossRef]
- Ferraris, S.; Prato, M.; Vineis, C.; Varesano, A.; Gautier di Confiengo, G.; Spriano, S. Coupling of keratin with titanium: A physico-chemical characterization of functionalized or coated surfaces. Surf. Coat. Technol. 2020, 397, 126057. [Google Scholar] [CrossRef]
- Spriano, S.; Dmitruk, A.; Naplocha, K.; Ferraris, S. Tannic Acid Coatings to Control the Degradation of AZ91 Mg Alloy Porous Structures. Metals 2023, 13, 200. [Google Scholar] [CrossRef]
- ISO 8504-4:2022; Preparation of Steel Substrates before Application of Paints and Related Products—Surface Preparation Methods, Part 4: Acid Pickling. International Organization for Standardization: Geneva, Switzerland, 2022.
- Anderez, A.; Alguacil, F.J.; López, F.A. Acid pickling of carbon steel. Rev. Metal 2022, 58, e226. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Sesia, R.; Cardone, A.G.; Ferraris, S.; Spriano, S.; Sangermano, M. Exploitation of tannic acid as additive for the adhesion enhancement of UV-curable bio-based coating. Prog. Org. Coat. 2024, 189, 108311. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Akbarinezhad, E.; Bahremandi, M.; Faridi, H.; Rezaei, F. Another approach for ranking and evaluating organic paint coatings via electrochemical impedance spectroscopy. Corros. Sci. 2009, 51, 356–363. [Google Scholar] [CrossRef]
- Truncali, A.; Laxminarayan, T.; Rajagopalan, N.; Weinell, C.E.; Kiil, S.; Johansson, M. Epoxidized technical Kraft lignin as a particulate resin component for high-performance anticorrosive coatings. J. Coat. Technol. Res. 2024, 1–17. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Liu, J.; Liu, R.; Luo, J. Synthesis of acrylated tannic acid as bio-based adhesion promoter in UV-curable coating with improved corrosion resistance. Colloids Surf. A 2022, 644, 128834. [Google Scholar] [CrossRef]
- Xu, L.Q.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Thiol Reactive Maleimido-Containing Tannic Acid for the Bioinspired Surface Anchoring and Post-Functionalization of Antifouling Coatings. ACS Sustain. Chem. Eng. 2016, 4, 4264–4272. [Google Scholar] [CrossRef]
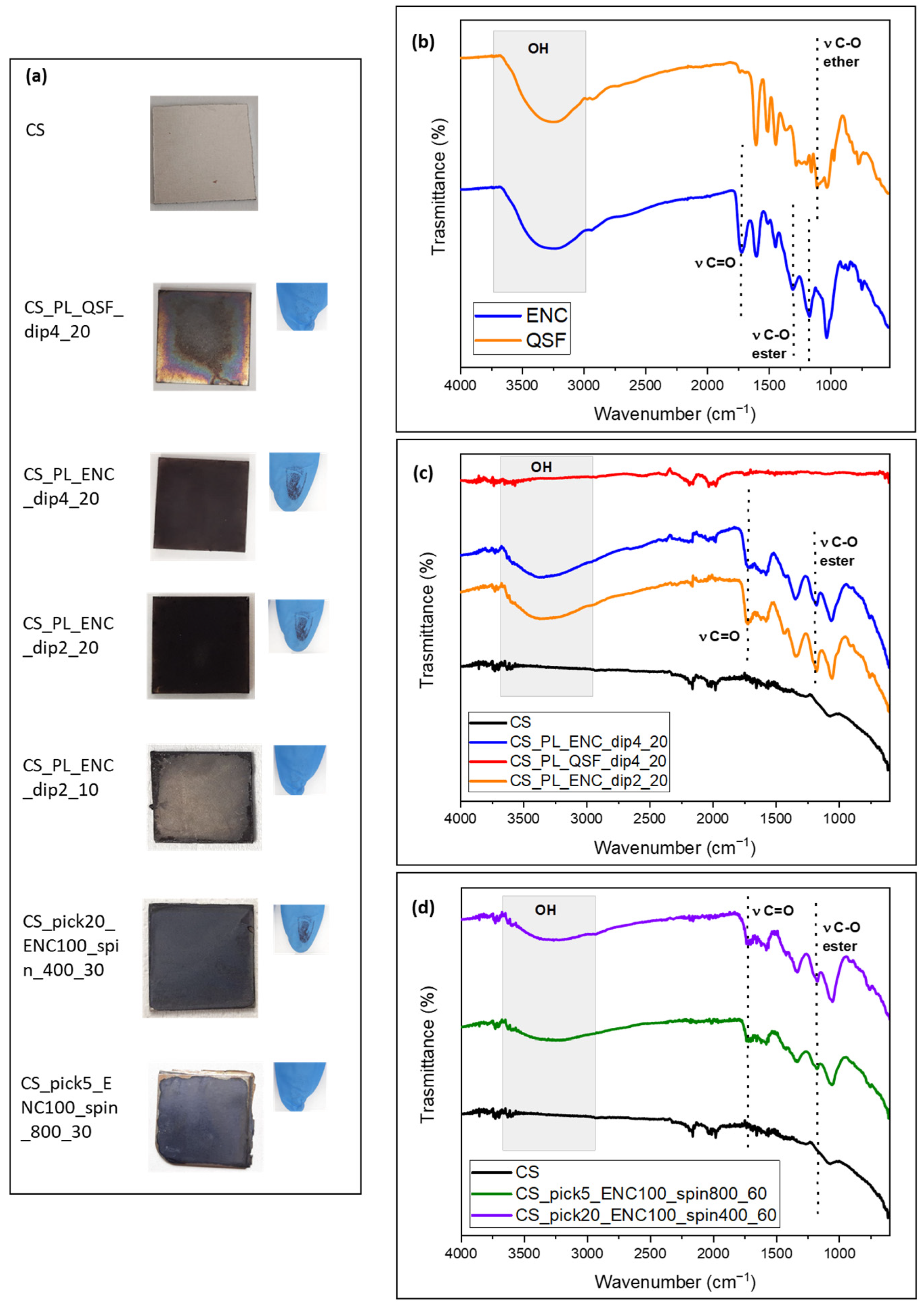



| Acronym | Substrate | Pre-Treatment | Tannin (Type, Solution, Concentration) | Deposition Strategy and Parameters |
|---|---|---|---|---|
| CS | Carbon Steel | - | - | - |
| CS_PL_QSF_dip4_20 | Carbon Steel | Plasma, air 10 min@100 W | QSF 4 mg/mL in PBS | Soaking 20 min |
| CS_PL_ENC_dip4_20 | Carbon Steel | Plasma, air 10 min@100 W | ENC 4 mg/mL in PBS | Soaking 20 min |
| CS_PL_ENC_dip2_20 | Carbon Steel | Plasma, air 10 min@100 W | ENC 2 mg/mL in PBS | Soaking 20 min |
| CS_PL_ENC_dip2_20 | Carbon Steel | Plasma, air 10 min@100 W | ENC 2 mg/mL in PBS | Soaking 10 min |
| CS_pick20_ENC100_spin400_30 | Carbon Steel | HCl 2M pickling 20 min | ENC 100 mg/mL in water | Spin coating 400 rpm 30 s |
| CS_pick5_ENC100_spin800_60 | Carbon Steel | HCl 2M pickling 5 min | ENC 100 mg/mL in water | Spin coating 800 rpm 60 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesia, R.; Spriano, S.; Sangermano, M.; Calovi, M.; Rossi, S.; Ferraris, S. Natural Tannin Layers for the Corrosion Protection of Steel in Contact with Water-Based Media. Coatings 2024, 14, 965. https://doi.org/10.3390/coatings14080965
Sesia R, Spriano S, Sangermano M, Calovi M, Rossi S, Ferraris S. Natural Tannin Layers for the Corrosion Protection of Steel in Contact with Water-Based Media. Coatings. 2024; 14(8):965. https://doi.org/10.3390/coatings14080965
Chicago/Turabian StyleSesia, Rossella, Silvia Spriano, Marco Sangermano, Massimo Calovi, Stefano Rossi, and Sara Ferraris. 2024. "Natural Tannin Layers for the Corrosion Protection of Steel in Contact with Water-Based Media" Coatings 14, no. 8: 965. https://doi.org/10.3390/coatings14080965











