Microorganisms in Red Ceramic Building Materials—A Review
Abstract
1. Introduction
- (a)
- a resident fungus;, i.e., a mycobiont;
- (b)
- an extracellular arrangement of one or more photosynthetic partners, i.e., green algae and/or photobionts;
- (c)
- an unspecified number of other microscopic organisms [12], i.e., the lichen microbiome, comprising mainly bacteria and additional fungi.
2. Factors Determining Microbial Growth
- Photolithotrophic autotrophy (photolithoautotrophy):
- Sources of energy: Sources of energy;
- Hydrogen/electrons: Inorganic hydrogen/electron (H/e−) donor;
- Sources of carbon: CO2;
- Microorganisms: Algae, purple and green sulphur bacteria, Cyanobacteria.
- Photoorganotrophic heterotrophy (photoorganoheterotrophy):
- Sources of energy: Sources of energy;
- Hydrogen/electrons: Organic H/e− donor;
- Sources of carbon: Organic carbon or CO2;
- Microorganisms: Purple nonsulphur bacteria, green nonsulphur bacteria.
- Chemolithotrophic autotrophy (chemolithoautotrophy):
- Sources of energy: Chemical energy source (inorganic);
- Hydrogen/electrons: Inorganic H/e− donor;
- Sources of carbon: CO2;
- Microorganisms: Sulphur-oxidizing bacteria, hydrogen bacteria, nitrifying bacteria, iron-oxidizing bacteria.
- Chemoorganotrophic heterotrophy (chemoorganoheterotrophy):
- Sources of energy: Chemical energy source (inorganic);
- Hydrogen/electrons: Inorganic H/e− donor;
- Sources of carbon: Organic carbon;
- Microorganisms: Protozoa, Fungi, most nonphotosynthetic bacteria (including most pathogens).
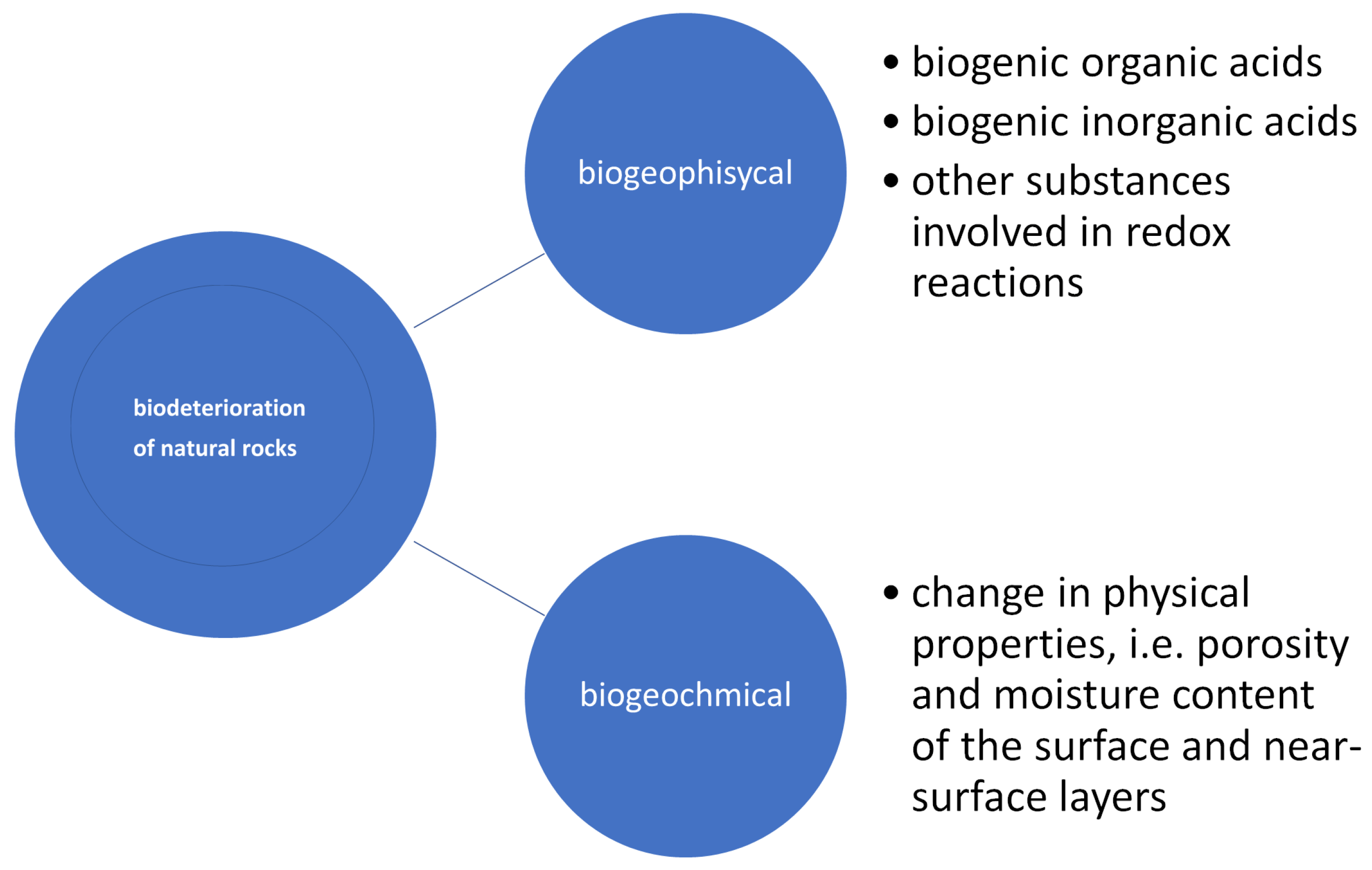
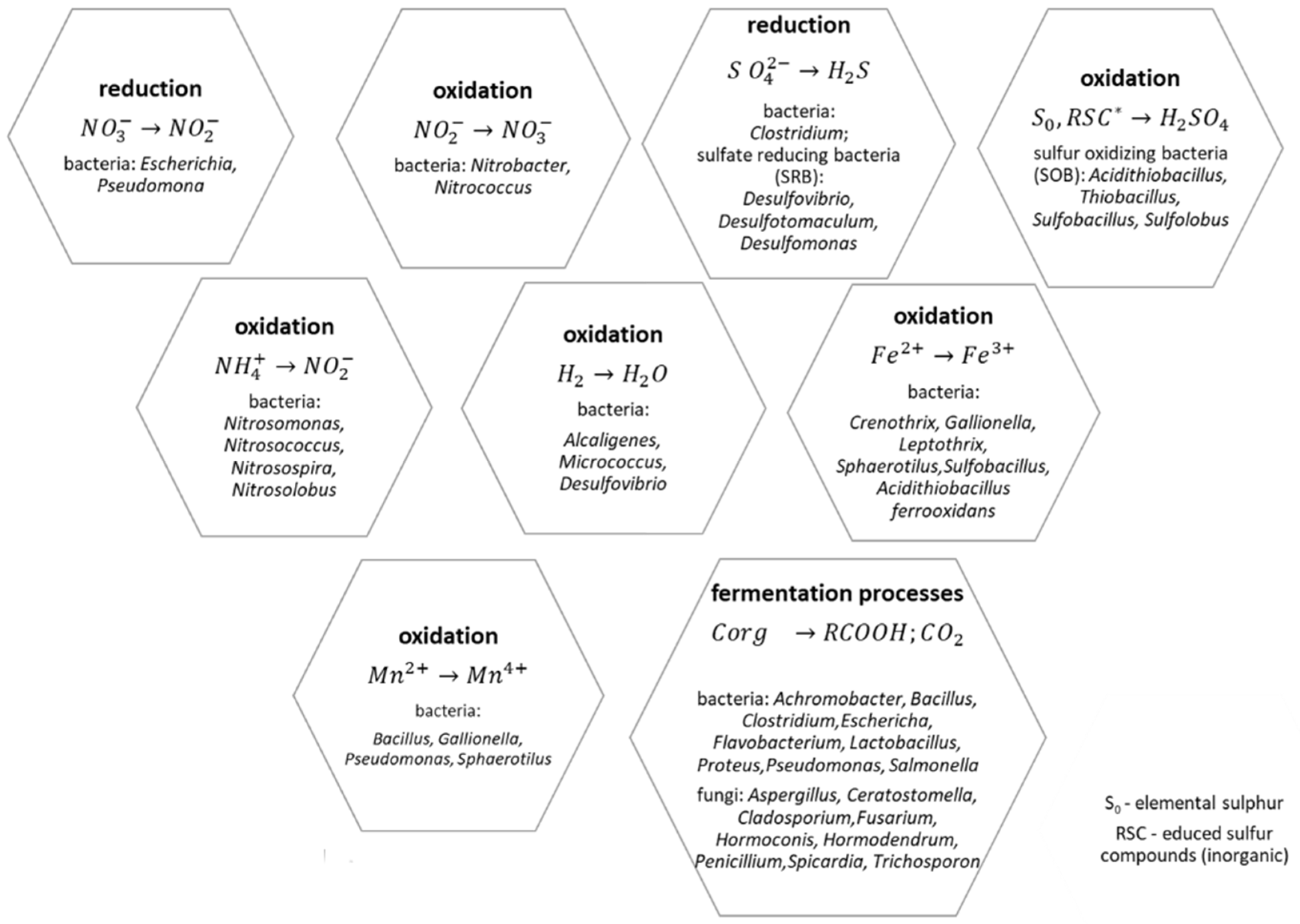
- acid hydrolysis, which is coupled with chemolithotrophic processes that release sulphuric and nitric acids into the environment;
- oxidation-reduction reactions involving ions, e.g., oxidation of iron and manganese;
- a change in stone porosity or pore size, caused by the formation of a biological membrane on the stone surface;
- alteration of gas diffusion within the material, caused by extracellular polymeric substances of EPS or compounds that reduce surface tension;
- formation of discolouration due to the release of biogenic pigments, i.e., melamine, chlorophyll, and changes in thermal and moisture properties;
- use of biofilm as an absorbent to trap atmospheric pollutants and as a precursor to crust formation;
- facilitating the migration of salts deep into the stone;
- alternation of aerobic and anaerobic conditions [49].
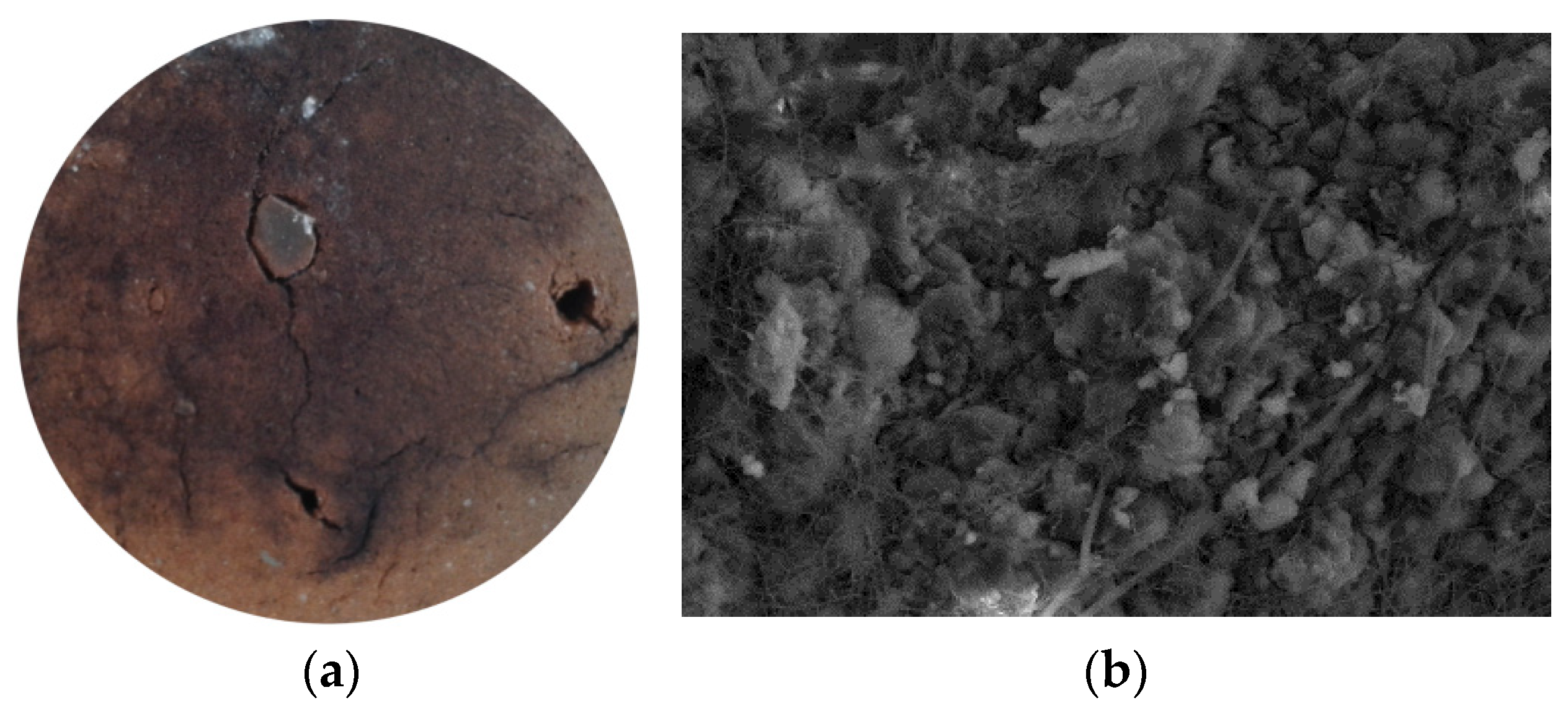
3. Biodeterioration of Ceramics
3.1. Brick
- -
- -
- -
- lichens, bryophytes, and plants [62].
3.2. Clay Roof Tiles
4. Nature of Corrosion
4.1. Physical
4.2. Chemical
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carlos, M.L.; Lanzara, E.; Capone, M. Innovative Uses of Conventional Ceramic Materials and Climate Awareness. Contemporary Critical Regionalism Challenging the Modern Canon. Dearq 2023, 37, 54–67. [Google Scholar] [CrossRef]
- Farid, S.B.H. Overview. In Woodhead Publishing Series in Biomaterials, Bioceramics: For Materials Science and Engineering; Saad, B.H.F., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–37. [Google Scholar] [CrossRef]
- Allen, E.; Iano, J. Fundamentals of Building Construction: Materials and Methods, 7th ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Jacobson, N.; Fox, D.; Smialek, J.; Deliacorte, C.; Lee, K. Performance of Ceramics in Severe Environments. In Corrosion Materials; Cramer, S., Covino, B., Eds.; ASM International: Almere, The Netherlands, 2005. [Google Scholar]
- Huang, Z.; Gou, Z.; Cheng, B. An investigation of outdoor thermal environments with different ground surfaces in the hot summer-cold winter climate region. J. Build. Eng. 2020, 27, 100994. [Google Scholar] [CrossRef]
- Santa, A.C.; Gómez, M.A.; Castaño, J.G.; Tamayo, J.A.; Baena, L.M. Atmospheric deterioration of ceramic building materials and future trends in the field: A review. Heliyon 2023, 9, e15028. [Google Scholar] [CrossRef]
- Wang, D.; Guan, F.; Feng, C.; Mathivanan, K.; Zhang, R.; Sand, W. Review on Microbially Influenced Concrete Corrosion. Microorganisms 2023, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Naleway, S.E. Hyphal systems and their effect on the mechanical properties of fungal sporocarps. Acta Biomater. 2022, 145, 272–282. [Google Scholar] [CrossRef]
- Brambilla, A.; Sangiorgio, A. Moisture and buildings. In Woodhead Publishing Series in Civil and Structural Engineering, Moisture and Buildings; Brambilla, A., Sangiorgio, A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- McConnaughey, M. Physical and Chemical Properties of Fungi. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Asmone, A.S.; Lin Chew, M.Y. A study on the effectiveness of biological growth resistant coatings on external building façade systems in the tropics. J. Build. Eng. 2020, 31, 101377. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Lendemer, J.C. A call to reconceptualize lichen symbioses. Trends Ecol. Evol. 2022, 37, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Mahto, K.U.; Vandana; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Monds, R.D.; O’Tool, G.A. The developmental model of microbial biofilms: Ten years of a paradigm up for review. Trends Microbiol. 2009, 17, 73–87. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.-I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Guiamet, P.; Crespo, M.; Lavin, P.; Ponce, B.; Gaylarde, C.; de Saravia, S.G. Biodeterioration of funeral sculptures in La recoleta cemetery, buenos aires, Argentina: Pre- and post-intervention studies. Colloids Surf. B Biointerfaces 2013, 101, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Stanaszek-Tomal, E. Influence of pore structure on humidity parameters of cement-polymer mortars contaminated with filamentous fungi. PLoS ONE 2020, 15, e0231347. [Google Scholar] [CrossRef]
- Coutinho, M.; Miller, A.; Gutierrez-Patricio, S.; Hernandez-Marine, M.; Gomez-Bolea, A.; Rogerio-Candelera, M.; Philips, A.; Jurado, V.; Saiz-Jimenez, C.; Macedo, M. Microbial communities on deteriorated artistic tiles from pena national palace (sintra, Portugal). Int. Biodeterior. Biodegrad. 2013, 84, 322–332. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, H.; Wu, M.; Li, Y.; Cao, H.; Rong, R. Seasonal dynamics of airborne culturable fungi and its year-round diversity monitoring in Dahuting Han Dynasty Tomb of China. Sci. Total Environ. 2022, 838, 155990. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Yang, X.; Ma, K.; Guo, P.; Sun, Q.; Jia, S.; Pan, J. Analysis and control of fungal deterioration on the surface of pottery figurines unearthed from the tombs of the Western Han Dynasty. Front. Microbiol. 2022, 13, 956774. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; Abdel-Nasser, M.; Sultan, M.H.; Eid, A.M.; Alotaibi, S.H.; Hassan, S.E.; Fouda, A. Fungal Biodeterioration of a Historical Manuscript Dating Back to the 14th Century: An Insight into Various Fungal Strains and Their Enzymatic Activities. Life 2022, 12, 1821. [Google Scholar] [CrossRef]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Palm Bay, FL, USA, 2017. [Google Scholar]
- Piontek, M.; Lechów, H. Biodeterioration Due to Improper Exploitation of External Facades. Civ. Environ. Eng. Rep. 2018, 28, 145–154. [Google Scholar] [CrossRef]
- Adams, R.I.; Sylvain, I.; Spilak, M.P.; Taylor, J.W.; Waring, M.S.; Mendell, M.J. Fungal Signature of Moisture Damage in Buildings: Identification by Targeted and Untargeted Approaches with Mycobiome Data. Appl. Environ. Microbiol. 2020, 86, e01047-20. [Google Scholar] [CrossRef]
- Valle, M.; Van Long, N.N.; Jany, J.-L.; Bregier, T.; Pawtowski, A.; Barbier, G.; Rigalma, K.; Vasseur, V.; Huchet, V.; Coroller, L. Impact of water activity on the radial growth of fungi in a dairy environment. Food Res. Int. 2022, 157, 111247. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Environmental Factors Causing the Development of Microorganisms on the Surfaces of National Cultural Monuments Made of Mineral Building Materials—Review. Coatings 2020, 10, 1203. [Google Scholar] [CrossRef]
- Bruslind, L. General Microbiology. 2019. Available online: https://open.oregonstate.education/generalmicrobiology/ (accessed on 23 June 2024).
- Aisha, M.A.; Maznah, W.O. Effect of ultraviolet radiation on the growth of microorganisms developing on cave wall paintings. AIP Conf. Proc. 2018, 1994, 070006. [Google Scholar] [CrossRef]
- Bhunia, P. 3.4—Fundamentals of Biological Treatment. In Comprehensive Water Quality and Purification; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 47–73. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-thermal Processing of Liquid Foods. Food Bioproc. Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Daba, H.G.; Delele, M.A.; Fanta, S.W.; Habtu, N.G.; Abera, M.K. Mycotoxigenic fungal growth within stored maize (Zea mays L.) at different storage altitudes and durations. J. Stored Prod. Res. 2024, 108, 102383. [Google Scholar] [CrossRef]
- Drewello, R.; Weissmann, R. Microbially influenced corrosion of glass. Appl. Microbiol. Biotechnol. 1997, 47, 337–346. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Zilles, R.; Mocelin, A.; Gaylarde, C.C.; Gorbushina, A.; Heidrich, G.; Giudice, M.C.; Del Negro, G.M.B.; John, V.M. Microbial colonization affects the efficiency of photovoltaic panels in a tropical environment. J. Environ. Manag. 2015, 157, 160–167. [Google Scholar] [CrossRef]
- Lu, R.; Pørneki, A.D.; Lindgreen, J.N.; Li, Y.; Madsen, A.M. Species of Fungi and Pollen in the PM1 and the Inhalable Fraction of Indoor Air in Homes. Atmosphere 2021, 12, 404. [Google Scholar] [CrossRef]
- Gutarowska, B.; Żakowska, Z. Elaboration and application of mathematical model for estimation of mould contamination of some building materials based on ergosterol content determination. Int. Biodeterior. Biodegrad. 2002, 49, 299–305. [Google Scholar] [CrossRef]
- Prussin, A.J.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef]
- Orlik-Kożdoń, B. Microclimate Conditions in Rooms: Their Impact on Mold Development in Buildings. Energies 2020, 13, 4492. [Google Scholar] [CrossRef]
- Cao, D.; Lou, Y.; Jiang, X.; Zhang, D.; Liu, J. Fungal Diversity in Barley Under Different Storage Conditions. Front. Microbiol. 2022, 13, 895975. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos-Soares, I.; Papazoglou, E.L.; Karmiris-Obratański, P.; Karkalos, N.K.; Markopoulos, A.P. Surface antibacterial properties enhanced through engineered textures and surface roughness: A review. Colloids Surf. B Biointerfaces 2023, 231, 113584. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic acid production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture and Medicine, Kluwer Academic; Tkacz, J.S., Lange, L., Eds.; Plenum Publisher: New York, NY, USA, 2004; pp. 307–339. [Google Scholar]
- Berthelin, J. Microbial weathering processes. In Microbial Geochemistry; Krumbein, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1983. [Google Scholar]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Mandl, J.; Grauer, A.; Neuberg, G. Solubilization of insoluble matter in nature. II. The part played by salts of organic and inorganic acids occurring in nature. Biochem. Biophys. Acta 1953, 10, 540–569. [Google Scholar] [CrossRef]
- Hueck-van der Plas, E.H. The Microbiological Deterioration of Porous Building Materials. Int. Biodeterior. Bull. 1968, 4, 11–28. [Google Scholar]
- Küzel, H. Mechanismen der Steinschädigung bei Krustenbildung. Bautenschutz Bautensanierung 1988, 11, 61. [Google Scholar]
- Stanaszek-Tomal, E. Biological Corrosion of Mineral Matrix Composites; Cracow University of Technology Publishing House: Cracow, Poland, 2020. [Google Scholar]
- Fomina, M.; Skorochod, I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiol. Soc. 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Chen, J.; Teng, H.H. Cellular dissolution at hypha- and spore-mineral interfaces revealing unrecognized mechanisms and scales of fungal weathering. Geology 2016, 44, 319–322. [Google Scholar] [CrossRef]
- Stohl, L.; Manninger, T.; von Werder, J.; Dehn, F.; Gorbushina, A.; Meng, B. Bioreceptivity of concrete: A review. J. Build. Eng. 2023, 76, 107201. [Google Scholar] [CrossRef]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Garcia-Diaz, E.; Damidot, D.; Devès, O.; Ruot, B. Influence of the intrinsic characteristics of mortars on biofouling by Klebsormidium flaccidum. Int. Biodeterior. Biodegrad. 2012, 70, 31–39. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Huang, L.; Zhao, L.; Zeng, Q.; Liu, X.; Sheng, Y.; Shi, L.; Wu, G.; Jiang, H.; Li, F.; et al. A critical review of mineral-microbe interaction and co-evolution: Mechanisms and applications. Natl. Sci. Rev. 2022, 9, 128. [Google Scholar] [CrossRef]
- Wijesooriya, M.M.; Wijesekara, H.; Bolan, N.; Rajapaksha, A.U.; Vithanage, M. Clays and Clay Minerals: Long-Lasting Applications in Environmental Remediation. In Clay Composites Environmental Applications; Vithanage, M., Lazzara, G., Rajapaksha, A.U., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Romani, M.; Carrion, C.; Fernandez, F.; Intertaglia, L.; Pecqueur, D.; Lebaron, P.; Lami, R. High bacterial diversity in pioneer biofilms colonizing ceramic roof tiles. Int. Biodeterior. Biodegrad. 2019, 144, 104745. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Otlewska, A.; Koziróg, A.; Rajkowska, K.; Piotrowska, M.; Gutarowska, B.; Żydzik-Białek, A. Diversity of an aerial phototrophic coating of historic buildings in the former Auschwitz II-Birkenau concentration camp. Sci. Total Environ. 2014, 493, 116–123. [Google Scholar] [CrossRef]
- Mandal, S.; Rath, J. Algal colonization and its ecophysiology on the fine sculptures of terracotta monuments of Bishnupur, West Bengal, India. Int. Biodeterior. Biodegrad. 2013, 84, 291–299. [Google Scholar] [CrossRef]
- Bastos, B.F. Primo Licenciada em Conservação e Restauro Biodeterioração por Fungos de Cerâmicas sem Revestimento Dissertação para Obtenção do Grau de Mestre em Conservação e Restauro. 2019. Available online: https://run.unl.pt/bitstream/10362/95072/1/Primo_2019.pdf (accessed on 23 June 2024).
- Cozzolino, A.; Adamo, P.; Bonanomi, G.; Motti, R. The Role of Lichens, Mosses, and Vascular Plants in the Biodeterioration of Historic Buildings: A Review. Plants 2022, 11, 3429. [Google Scholar] [CrossRef] [PubMed]
- Stryszewska, T.; Kańka, S. Characteristics of Factors Determining the Durability of Brick Masonry. In Brick and Block Masonry—From Historical to Sustainable Masonry: Proceedings of the 17th International Brick/Block Masonry Conference (17thIB2MaC 2020), Kraków, Poland, 5–8 July 2020, 1st ed.; Kubica, J., Kwiecień, A., Bednarz, Ł., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E.; Stryszewska, T. Influence of salting mineral materials on the development of fungi. Procedia Eng. 2015, 108, 301–308. [Google Scholar] [CrossRef]
- Kiurski, J.S.; Ranogajec, J.G.; Ujhelji, A.L.; Radeka, M.M.; Bokorov, M.T. Evaluation of the effect of lichens on ceramic roofing tiles by scanning electronmicroscopy and energy-dispersive spectroscopy analyses. Scanning 2005, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Berdahl, P.; Akbari, H.; Levinson, R.; Miller, W.A. Weathering of roofing materials—An overview. Constr. Build. Mater. 2008, 22, 423–433. [Google Scholar] [CrossRef]
- Sedighi, M.; Pourmoghaddam Qhazvini, P.; Amidpour, M. Algae-Powered Buildings: A Review of an Innovative, Sustainable Approach in the Built Environment. Sustainability 2023, 15, 3729. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Romani, M.; Adouane, E.; Carrion, C.; Veckerlé, C.; Boeuf, D.; Fernandez, F.; Lefèvre, M.; Intertaglia, L.; Rodrigues, A.M.S.; Lebaron, P.; et al. Diversity and activities of pioneer bacteria, algae, and fungi colonizing ceramic roof tiles during the first year of outdoor exposure. Int. Biodeterior. Biodegrad. 2021, 162, 105230. [Google Scholar] [CrossRef]
- Coutinho, M.; Miller, A.Z.; Macedo, M.F. Biological colonization and biodeterioration of architectural ceramic materials: An overview. J. Cult. Herit. 2015, 16, 759–777. [Google Scholar] [CrossRef]
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm colonization of metamorphic lithotypes of a renaissance cathedral exposed to urban atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- May, E.; Papida, S.; Abdulla, H.; Tayler, S.; Dewedar, A. Comparative Studies of Microbial Communities on Stone Monuments in Temperate and Semi-Arid Climates, of Microbes and Art; Springer: Berlin/Heidelberg, Germany, 2000; pp. 49–62. [Google Scholar]
- Watanabe, K.; Ohfuji, H.; Kitagawa, R.; Matsui, Y. Nanoscale pseudo brookite layer in the surface glaze of a Japanese sekishu roof tile. Clay Miner. 2009, 44, 177–180. [Google Scholar] [CrossRef]
- Hupa, L.; Bergman, R.; Fröberg, L.; Vane-Tempest, S.; Hupa, M.; Kronberg, T.; Pesonen-Leinonen, E.; Sjöberg, A.M. Chemical resistance and cleanability of glazed surfaces. Surf. Sci. 2005, 584, 113–118. [Google Scholar] [CrossRef]
- Roeselers, G.; Zippel, B.; Staal, M.; van Loosdrecht, M.; Muyzer, G. On the reproducibility of microcosm experiments—Different community composition in parallel phototrophic biofilm microcosms. FEMS Microbiol. Ecol. 2006, 58, 169–179. [Google Scholar] [CrossRef]
- Silva, T.P.; Figueiredo, M.O.; Barreiros, M.A.; Prudêncio, M.I. Diagnosis of pathologies in ancient (seventeenth-eighteenth centuries) decorative blue-and-white ceramic tiles: Green stains in the glazes of a panel depicting Lisbonprior to the 1755 earthquake. Stud. Conserv. 2014, 59, 63–68. [Google Scholar] [CrossRef]
- McCauley, R.A. Corrosion of Ceramic Materials, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Shirakawa, M.A.; Werle, A.P.; Gaylarde, C.C.; Loh, K.; John, V.M. Fungal and phototroph growth on fiber cement roofs and its influence on solar reflectance in a tropical climate. Int. Biodeterior. Biodegrad. 2014, 95, 332–337. [Google Scholar] [CrossRef]
- Carter, N.E.A.; Viles, H.A. Lichen hotspots: Raised rock temperatures beneath Verrucaria nigrescens on limestone. Geomorphology 2004, 62, 1–16. [Google Scholar] [CrossRef]
- Ranogajec, J.; Radosavljevic, S.; Marinkovic-Neducin, R.; Zivanovic, B. Chemical corrosion phenomena of roofing tiles. Ceram. Int. 1997, 23, 99–103. [Google Scholar] [CrossRef]
- Giacomucci, L.; Bertoncello, R.; Salvadori, O.; Martini, I.; Favaro, M.; Villa, F.; Sorlini, C.; Cappitelli, F. Microbial deterioration of artistic tiles from the facade of the Grande Albergo Ausonia & Hungaria (Venice, Italy). Microb. Ecol. 2011, 62, 287–298. [Google Scholar] [CrossRef]
- Gorbushina, A.A. Life on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Miller, A.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Sáiz-Jiménez, C.; Macedo, M.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, M.; Cursio, G.; Graziani, L.; Aquilanti, L.; Osimani, A.; Clementi, F.; Yéprémian, C.; Lariccia, V.; Amoroso, S. Effects of water absorption and surface roughness on the bioreceptivity of ETICS compared to clay bricks. Build. Environ. 2014, 77, 20–28. [Google Scholar] [CrossRef]
- Ranogajec, J.; Markov, S.; Kiurski, J.; Radeka, M.; Ducman, V. Microbial deterioration of clay roofing tiles as a function of the firing temperature. J. Am. Ceram. Soc. 2008, 91, 3762–3767. [Google Scholar] [CrossRef]
- Huang, C.; Clark, G.G.; Zaki, F.R.; Won, J.; Ning, R.; Boppart, S.A.; Elbanna, A.E.; Nguyen, T.H. Effects of phosphate and silicate on stiffness and viscoelasticity of mature biofilms developed with simulated drinking water. Biofouling 2023, 39, 36–46. [Google Scholar] [CrossRef]
- Gu, J.-D.; Katayama, Y. Microbiota and Biochemical Processes Involved. In Biodeterioration of Cultural Heritage and Protection; Joseph, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; D’Orazio, M. The influence of clay brick substratum on the inhibitory efficiency of TiO2 nanocoating against biofouling. Build. Environ. 2014, 82, 128–134. [Google Scholar] [CrossRef]
- Abin, L.; Coto, O.; Gomez, Y.; Bosecker, K. Screening of fungi with capacity for organic acid production. Biol. Rev. 2002, 16, 69–71. [Google Scholar]
- Pinna, D. Microbial Growth and its Effects on Inorganic Heritage Materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gómez-Alarcón, G.; Muñoz, M.L.; Flores, M. Excretion of organic acids by fungal strains isolated from decayed sandstone. Int. Biodeterior. Biodegrad. 1994, 34, 169–180. [Google Scholar] [CrossRef]
- Warscheid, T.H. Microbial impact on building materials: An overview. Mater. Struct. 2003, 36, 342–352. [Google Scholar]
- Doherty, B.; Pamplona, M.; Costanza, M.; Matteini, M.; Sgamellotti, A.; Brunetti, B. Durability of the Artificial Calcium Oxalate Protective on Two Florentine Monuments. J. Cult. Herit. 2007, 8, 186. [Google Scholar] [CrossRef]
- Cwalina, B. Biodeterioration of concrete, brick and other mineral-based building materials. In Understanding Biocorrosion: Fundamentals and Applications, European Federation of Corrosion Publications; Liengen, T., Feron, D., Basseguy, R., Beech, I.B., Eds.; Woodhead Publ. Limited: Cambridge, UK, 2014; Volume 66. [Google Scholar]
- Wang, Q.; Ma, G.Y.; He, L.Y.; Sheng, X.F. Characterization of bacterial community inhabiting the surfaces of weathered bricks of Nanjing Ming city walls. Sci. Total Environ. 2011, 409, 756–762. [Google Scholar] [CrossRef]
- Otlewska, A.; Adamiak, J.; Gutarowska, B. Clone-based comparative sequence analysis of 16S rRNA genes retrieved from biodeteriorating brick buildings of the former Auschwitz II-Birkenau concentration and extermination camp. Syst. Appl. Microbiol. 2014, 38, 48–55. [Google Scholar] [CrossRef]
- Sylvestre, M.; Urzl, C.; Videla, H.A.; Herrera, L.K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Kong, L.; Liu, X.; Cao, M.; Fang, J. Mechanism study of the role of biofilm played in sewage corrosion of mortar. Constr. Build. Mater. 2018, 164, 44–56. [Google Scholar] [CrossRef]
- Cwalina, B. Biodeterioration of concrete. Archit. Civ. Eng. Environ. 2008, 1, 133–140. [Google Scholar]
- Gutarowska, B. Niszczenie Materiałów Technicznych Przez Drobnoustroje. LAB Lab. Apar. Badania 2013, 18, 10–14. [Google Scholar]
- Pinna, D.; Mazzotti, V.; Gualtieri, S.; Voyron, S.; Andreotti, A.; Favero-Longo, S.E. Damaging and protective interactions of lichens and biofilms on ceramic dolia and sculptures of the International Museum of Ceramics, Faenza, Italy. Sci. Total Environ. 2023, 877, 162607. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.P.M.; Warke, P.A.; Smith, B.J. Lichen-induced biomodification of calcareous surfaces: Bioprotection versus biodeterioration. Prog. Phys. Geogr. Earth Environ. 2013, 37, 325–351. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Bacterial Concrete as a Sustainable Building Material? Sustainability 2020, 12, 696. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanaszek-Tomal, E. Microorganisms in Red Ceramic Building Materials—A Review. Coatings 2024, 14, 985. https://doi.org/10.3390/coatings14080985
Stanaszek-Tomal E. Microorganisms in Red Ceramic Building Materials—A Review. Coatings. 2024; 14(8):985. https://doi.org/10.3390/coatings14080985
Chicago/Turabian StyleStanaszek-Tomal, Elżbieta. 2024. "Microorganisms in Red Ceramic Building Materials—A Review" Coatings 14, no. 8: 985. https://doi.org/10.3390/coatings14080985
APA StyleStanaszek-Tomal, E. (2024). Microorganisms in Red Ceramic Building Materials—A Review. Coatings, 14(8), 985. https://doi.org/10.3390/coatings14080985





