Zirconia Implants: A Brief Review and Surface Analysis of a Lost Implant
Abstract
:1. Introduction
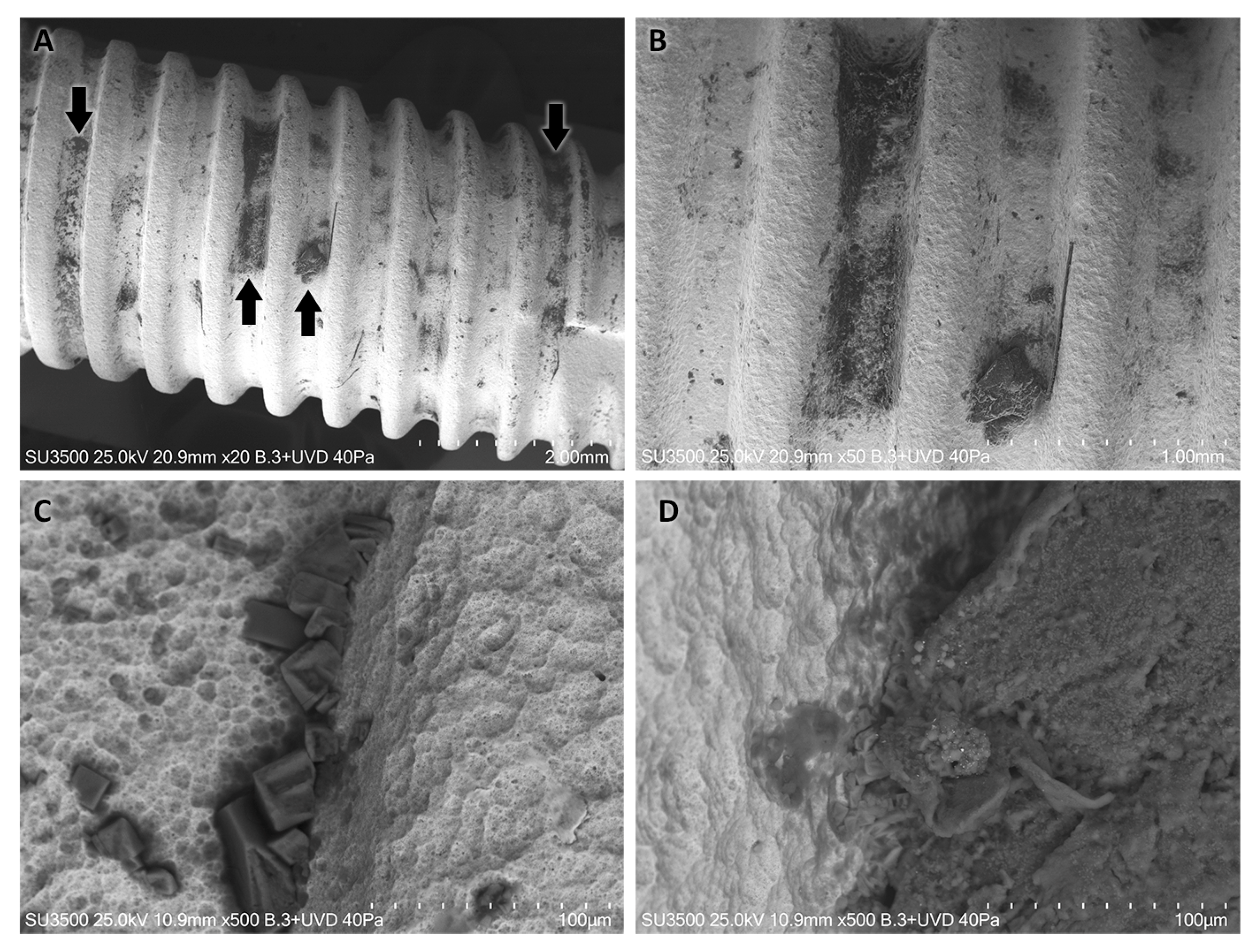
2. Case Report—Surface Characterization and Elemental Analysis of a Lost Zirconia Implant
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stadlinger, B.; Hennig, M.; Eckelt, U.; Kuhlisch, E.; Mai, R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int. J. Oral Maxillofac. Surg. 2010, 39, 585–592. [Google Scholar] [CrossRef]
- Kohal, R.J.; Spies, B.C.; Bauer, A.; Butz, F. One-piece zirconia oral implants for single-tooth replacement: Three-year results from a long-term prospective cohort study. J. Clin. Periodontol. 2018, 45, 114–124. [Google Scholar] [CrossRef] [PubMed]
- van Brakel, R.; Noordmans, H.J.; Frenken, J.; de Roode, R.; de Wit, G.C.; Cune, M.S. The effect of zirconia and titanium implant abutments on light reflection of the supporting soft tissues. Clin. Oral Implants Res. 2011, 22, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Afrashtehfar, K.I.; Del Fabbro, M. Clinical performance of zirconia implants: A meta-review. J. Prosthet. Dent. 2020, 123, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Thome, G.; Sandgren, R.; Bernardes, S.; Trojan, L.; Warfving, N.; Bellon, B.; Pippenger, B.E. Osseointegration of a novel injection molded 2-piece ceramic dental implant: A study in minipigs. Clin. Oral Investig. 2021, 25, 603–615. [Google Scholar] [CrossRef]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implants Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Nishihara, H.; Haro Adanez, M.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef]
- Song, X.; Ding, Y.; Zhang, J.; Jiang, C.; Liu, Z.; Lin, C.; Zheng, W.; Zeng, Y. Thermophysical and mechanical properties of cubic, tetragonal and monoclinic ZrO2. J. Mater. Res. Technol. 2023, 23, 648–655. [Google Scholar] [CrossRef]
- Zhang, F.; Monzavi, M.; Li, M.; Cokic, S.; Manesh, A.; Nowzari, H.; Vleugels, J.; Van Meerbeek, B. Fracture analysis of one/two-piece clinically failed zirconia dental implants. Dent. Mater. 2022, 38, 1633–1647. [Google Scholar] [CrossRef]
- Pessanha-Andrade, M.; Sordi, M.B.; Henriques, B.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Custom-made root-analogue zirconia implants: A scoping review on mechanical and biological benefits. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2888–2900. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Nakanishi, Y.; Alao, A.R.; Song, X.F.; Abduo, J.; Zhang, Y. A review of engineered zirconia surfaces in biomedical applications. Procedia CIRP 2017, 65, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Leighton, Y.; Miranda, J.; Souza, R.F.D.; Weber, B.; Borie, E. A Novel, Minimally Invasive Method to Retrieve Failed Dental Implants in Elderly Patients. Appl. Sci. 2021, 11, 2766. [Google Scholar] [CrossRef]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20 (Suppl. S4), 32–47. [Google Scholar] [CrossRef] [PubMed]
- Jodha, K.S.; Salazar Marocho, S.M.; Scherrer, S.S.; Griggs, J.A. Fractal analysis at varying locations of clinically failed zirconia dental implants. Dent. Mater. 2020, 36, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Weng, D.; Bachle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef]
- Hempel, U.; Hefti, T.; Kalbacova, M.; Wolf-Brandstetter, C.; Dieter, P.; Schlottig, F. Response of osteoblast-like SAOS-2 cells to zirconia ceramics with different surface topographies. Clin. Oral Implants Res. 2010, 21, 174–181. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Chiou, L.L.; Panariello, B.H.D.; Hamada, Y.; Gregory, R.L.; Blanchard, S.; Duarte, S. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. Biomed. Res. Int. 2023, 2023, 8728499. [Google Scholar] [CrossRef]
- Al-Radha, A.S.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Scarano, A.; Perrotti, V.; Gehrke, P.; Piattelli, A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J. Periodontol. 2006, 77, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Cesar, P.F.; Miranda, R.B.P.; Santos, K.F.; Scherrer, S.S.; Zhang, Y. Recent advances in dental zirconia: 15 years of material and processing evolution. Dent. Mater. 2024, 40, 824–836. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Springer: New York, NY, USA, 2017; pp. 209–234. [Google Scholar]
- Koller, M.; Steyer, E.; Theisen, K.; Stagnell, S.; Jakse, N.; Payer, M. Two-piece zirconia versus titanium implants after 80 months: Clinical outcomes from a prospective randomized pilot trial. Clin. Oral Implants Res. 2020, 31, 388–396. [Google Scholar] [CrossRef]
- Kim, H.K.; Yoo, K.W.; Kim, S.J.; Jung, C.H. Phase Transformations and Subsurface Changes in Three Dental Zirconia Grades after Sandblasting with Various Al2O3 Particle Sizes. Materials 2021, 14, 5321. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Vijayan, K.; Pramila Bai, B.N.; Biswas, S.K. Phase transformation introduced by mechanical and chemical surface preparations of tetragonal zirconia polycrystals. J. Am. Ceram. Soc. 1993, 76, 533–535. [Google Scholar] [CrossRef]
- Schunemann, F.H.; Galarraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, R.; Zhang, L.; Sun, Y.; Xie, Q. Effects of Different Microstructured Surfaces on the Osseointegration of CAD/CAM Zirconia Dental Implants: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implants 2020, 35, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Jank, S.; Hochgatterer, G. Success Rate of Two-Piece Zirconia Implants: A Retrospective Statistical Analysis. Implant Dent. 2016, 25, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: Six-year results of a prospective cohort study. Clin. Oral Implants Res. 2021, 32, 695–701. [Google Scholar] [CrossRef]

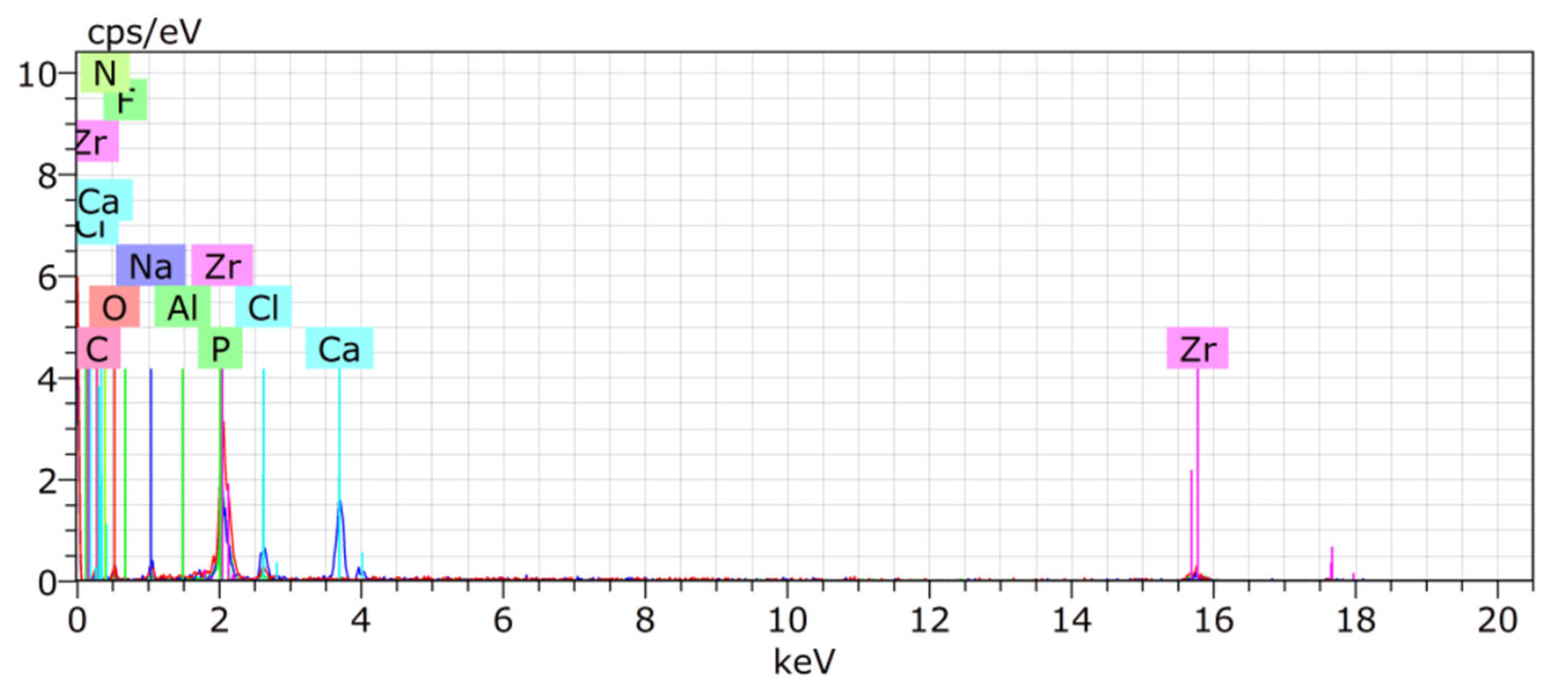
| Zr | O | C | Ca | P | Na | Cl | Al | F | |
|---|---|---|---|---|---|---|---|---|---|
| T11 | 41.55 | 24.03 | 24.98 | 5.55 | 1.47 | 0.2 | - | - | - |
| T12 | 49.29 | 16.32 | 30.37 | - | - | - | - | - | - |
| T21 | 48.09 | 22.34 | 18.3 | 4.44 | 2.38 | - | - | - | - |
| T22 | 46.13 | 19.55 | 18.8 | 1.74 | 2.95 | - | 0.2 | - | - |
| T31 | 53.88 | 12.38 | 21.49 | 17.74 | 4.68 | - | - | - | - |
| T32 | 50.48 | 14.17 | 24.19 | - | - | - | - | 0.01 | 0.01 |
| Mean | 48.23 | 16.95 | 22.63 | 7.97 | 3.34 | 0.2 | 0.2 | 0.01 | 0.01 |
| SD | 4.17 | 4.61 | 4.51 | 7.10 | 1.35 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borie, E.; Rosas, E.; de Souza, R.F.; Dias, F.J. Zirconia Implants: A Brief Review and Surface Analysis of a Lost Implant. Coatings 2024, 14, 995. https://doi.org/10.3390/coatings14080995
Borie E, Rosas E, de Souza RF, Dias FJ. Zirconia Implants: A Brief Review and Surface Analysis of a Lost Implant. Coatings. 2024; 14(8):995. https://doi.org/10.3390/coatings14080995
Chicago/Turabian StyleBorie, Eduardo, Eduardo Rosas, Raphael Freitas de Souza, and Fernando José Dias. 2024. "Zirconia Implants: A Brief Review and Surface Analysis of a Lost Implant" Coatings 14, no. 8: 995. https://doi.org/10.3390/coatings14080995






