Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review
Abstract
1. Introduction
2. Bioactive Glasses
2.1. Bioglass®
2.2. Other Bioactive Glasses
3. Bioactivity of Glasses
- Stage 1: Rapid ion exchange between the glass network modifiers ( and ) with ions (or ) from the solution leads to the hydrolysis of the silica groups and the creation of silanol () groups on the glass surface:The pH of the solution increases due to the consumption of ions.
- Stage 2: The increase in the pH (or concentration) leads to the attack of the glass network, the dissolution of silica, in the form of silicic acid into the solution, and the continuous formation of Si–OH groups on the glass surface:
- Stage 3: Condensation and repolymerization of an amorphous -rich layer (typically 1 or 2 μm thick) occur on the surface of the glass depleted in and by leaching:
- Stage 4: The migration of and ions from the glass through the -rich layer and from the solution leads to the formation of an amorphous calcium phosphate (ACP) layer on the surface of the -rich layer.
- Stage 5: The amorphous calcium phosphate (APC) layer incorporates and from the solution and crystallizes as an HCA layer.
4. Electrophoretic Deposition (EPD)
4.1. Surface Charge Formation
4.2. Suspension Stability
4.3. Deposition Mechanism of Bioactive Glass Coating
5. Current Developments in the Process and Perspectives
5.1. Ionic Substitution
5.2. Composite Coatings
5.2.1. Bioactive Glass and Bioceramics
5.2.2. Bioactive Glass and Polymers
5.2.3. Bioactive Glass and Carbon Nanotubes (CNTs)
5.3. Drug-Loaded Bioactive Glass Coatings
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 July 2024).
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskel. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Gheno, R.; Cepparo, J.M.; Rosca, C.E.; Cotton, A. Musculoskeletal Disorders in the Elderly. J. Clin. Imaging Sci. 2012, 2, 39. [Google Scholar] [CrossRef]
- Li, G.; Thabane, L.; Papaioannou, A.; Ioannidis, G.; Levine, M.A.H.; Adachi, J.D. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet. Disord. 2017, 18, 46. [Google Scholar] [CrossRef]
- Boskey, A.L.; Coleman, R. Aging and Bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Bone Cell Senescence: Mechanisms and Perspectives. J. Bone Miner. Res. 2014, 29, 1311–1321. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Biomaterials Design for Human Body Repair. Designs 2024, 8, 65. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- He, G.; Hagiwara, M. Ti alloy design strategy for biomedical applications. Mater. Sci. Eng. C 2006, 26, 14–19. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Laillé, D.; Héraud, L.; Gordin, D.M.; Castany, P.; Gloriant, T. Design of a novel superelastic Ti-23Hf-3Mo-4Sn biomedical alloy combining low modulus, high strength and large recovery strain. Mater. Lett. 2016, 177, 39–41. [Google Scholar] [CrossRef]
- Sheremetyev, V.; Lukashevich, L.; Kreitcberg, A.; Kudryashova, A.; Tsaturyants, M.; Galkin, G.; Andreev, V.; Prokoshkin, S.; Brailovski, V. Optimization of a thermomechanical treatment of superelastic Ti-Zr-Nb alloys for the production of bar stock for orthopedic implants. J. Alloys Compd. 2022, 928, 167143. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Malikova, P.; Dubinskiy, S.; Korotitskiy, A.; Pustov, Y.; Prokoshkin, S. Martensitic Transformations and Mechanical and Corrosion Properties of Fe-Mn-Si Alloys for Biodegradable Medical Implants. Metall. Mater. Trans. A 2018, 49, 1006–1013. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Kadirov, P.; Dubinskiy, S.; Kazakbiev, A.; Pustov, Y.; Prokoshkin, S. Tunable corrosion behavior of calcium phosphate coated Fe-Mn-Si alloys for bone implant applications. Metall. Mater. Trans. A 2018, 49, 6553–6560. [Google Scholar] [CrossRef]
- Kadirov, P.; Zhukova, Y.; Pustov, Y.; Karavaeva, M.; Sheremetyev, V.; Korotitskiy, A.; Shcherbakova, E.; Baranova, A.; Komarov, V.; Prokoshkin, S. Effect of Plastic Deformation in Various Temperature-Rate Conditions on Structure and Mechanical Properties of Biodegradable Fe-30Mn-5Si Alloy. Metall. Mater. Trans. A 2024, 55, 895–909. [Google Scholar] [CrossRef]
- Koumya, Y.; Ait Salam, Y.; Khadiri, M.E.; Benzakour, J.; Romane, A.; Abouelfida, A.; Benyaich, A. Pitting corrosion behavior of SS-316L in simulated body fluid and electrochemically assisted deposition of hydroxyapatite coating. Chem. Pap. 2021, 75, 2667–2682. [Google Scholar] [CrossRef]
- Trincă, L.C.; Burtan, L.; Mareci, D.; Fernández-Pérez, B.M.; Stoleriu, I.; Stanciu, T.; Stanciu, S.; Solcan, C.; Izquierdo, J.; Souto, R.M. Evaluation of in vitro corrosion resistance and in vivo osseointegration properties of a FeMnSiCa alloy as potential degradable implant biomaterial. Mater. Sci. Eng. C 2021, 118, 111436. [Google Scholar] [CrossRef]
- Sultana, N.; Nishina, Y.; Nizami, M.Z.I. Surface Modifications of Medical Grade Stainless Steel. Coatings 2024, 14, 248. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C 2017, 70, 1057–1070. [Google Scholar] [CrossRef]
- Putrantyo, I.; Anilbhai, N.; Vanjani, R.; De Vega, B. Tantalum as a novel biomaterial for bone implant: A literature review. J. Biomim. Biomater. Biomed. Eng. 2021, 52, 55–65. [Google Scholar] [CrossRef]
- Gao, H.; Yang, J.; Jin, X.; Qu, X.; Zhang, F.; Zhang, D.; Chen, H.; Wei, H.; Zhang, S.; Jia, W.; et al. Porous tantalum scaffolds: Fabrication, structure, properties, and orthopedic applications. Mater. Des. 2021, 210, 110095. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Hydari, M.H.; Mahmoodi, L.; Gazanfari, L.; Mirhaj, M. Electrophoretic deposition of graphene oxide reinforced hydroxyapatite on the tantalum substrate for bone implant applications: In vitro corrosion and bio-tribological behavior. Surf. Coat. Technol. 2021, 424, 127642. [Google Scholar] [CrossRef]
- Rupérez, E.; Manero, J.M.; Riccardi, K.; Li, Y.; Aparicio, C.; Gil, F.J. Development of tantalum scaffold for orthopedic applications produced by space-holder method. Mater. Des. 2015, 83, 112–119. [Google Scholar] [CrossRef]
- Luo, C.; Wang, C.; Wu, X.; Xie, X.; Wang, C.; Zhao, C.; Zou, C.; Lv, F.; Huang, W.; Liao, J. Influence of porous tantalum scaffold pore size on osteogenesis and osteointegration: A comprehensive study based on 3D-printing technology. Mater. Sci. Eng. C 2021, 129, 112382. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pan, S.T.; Qiu, J.X. The Clinical Application of Porous Tantalum and Its New Development for Bone Tissue Engineering. Materials 2021, 14, 2647. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ning, B.; Pei, X. Tantalum and its derivatives in orthopedic and dental implants: Osteogenesis and antibacterial properties. Colloids Surf. B Biointerfaces 2021, 208, 112055. [Google Scholar] [CrossRef]
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Z.; Xiao, H.; Luo, C.; Luo, X.; Lv, F.; Liao, J.; Huang, W. Three-Dimensional, MultiScale, and Interconnected Trabecular Bone Mimic Porous Tantalum Scaffold for Bone Tissue Engineering. ACS Omega 2020, 5, 22520–22528. [Google Scholar] [CrossRef]
- Tchana Nkonta, D.V.; Simescu-Lazar, F.; Drevet, R.; Aaboubi, O.; Fauré, J.; Retraint, D.; Benhayoune, H. Influence of the surface mechanical attrition treatment (SMAT) on the corrosion behavior of Co28Cr6Mo alloy in Ringer’s solution. J. Solid State Electrochem. 2018, 22, 1091–1098. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Kurosu, S.; Yamanaka, K.; Tang, N.; Koizumi, Y.; Chiba, A. Effects of sigma phase and carbide on the wear behavior of CoCrMo alloys in Hanks’ solution. Wear 2014, 310, 51–62. [Google Scholar] [CrossRef]
- Tchana Nkonta, D.V.; Drevet, R.; Fauré, J.; Benhayoune, H. Effect of surface mechanical attrition treatment on the microstructure of cobalt-chromium-molybdenum biomedical alloy. Microsc. Res. Tech. 2021, 84, 238–245. [Google Scholar] [CrossRef] [PubMed]
- AlMangour, B.; Luqman, M.; Grzesiak, D.; Al-Harbi, H.; Ijaz, F. Effect of processing parameters on the microstructure and mechanical properties of Co–Cr–Mo alloy fabricated by selective laser melting. Mater. Sci. Eng. A 2020, 792, 139456. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Jack, T.A.; Feng, R.; Yang, Q. Wear and corrosion characteristics of nano-crystalline tantalum nitride coatings deposited on CoCrMo alloy for hip joint applications. Mater. Charact. 2021, 182, 111516. [Google Scholar] [CrossRef]
- Yamanaka, K.; Mori, M.; Kurosu, S.; Matsumoto, H.; Chiba, A. Ultrafine grain refinement of biomedical Co-29Cr-6Mo alloy during conventional hot-compression deformation. Metall. Mater. Trans. A 2009, 40, 1980–1994. [Google Scholar] [CrossRef]
- İbrahim Coşkun, M.; Karahan, İ.H.; Yücel, Y.; Golden, T.D. Optimization of electrochemical step deposition for bioceramic hydroxyapatite coatings on CoCrMo implants. Surf. Coat. Technol. 2016, 301, 42–53. [Google Scholar] [CrossRef]
- Coşkun, M.I.; Karahan, I.H.; Yücel, Y. Optimized electrodeposition concentrations for hydroxyapatite coatings on CoCrMo biomedical alloys by computational techniques. Electrochim. Acta 2014, 150, 46–54. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Williams, D. Revisiting the definition of biocompatibility. Med. Device Technol. 2003, 14, 10–13. [Google Scholar] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Barrère, F.; Mahmood, T.A.; de Groot, K.; van Blitterswijk, C.A. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R 2008, 59, 38–71. [Google Scholar] [CrossRef]
- Moniruzzaman, M.d.; O’Neal, C.; Bhuiyan, A.; Egan, P.F. Design and Mechanical Testing of 3D Printed Hierarchical Lattices Using Biocompatible Stereolithography. Designs 2020, 4, 22. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Rahandi Lubis, M.A.; Juliadmi, D.; Mardawati, E.; Antov, P.; Kristak, L.; Seng Hua, L. Bio-Based Adhesives for Orthopedic Applications: Sources, Preparation, Characterization, Challenges, and Future Perspectives. Designs 2022, 6, 96. [Google Scholar] [CrossRef]
- Williams, D.F. Biocompatibility pathways and mechanisms for bioactive materials: The bioactivity zone. Bioact. Mater. 2022, 10, 306–322. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Andreucci, C.A.; Fonseca, E.M.M.; Jorge, R.N. Bio-Lubricant Properties Analysis of Drilling an Innovative Design of Bioactive Kinetic Screw into Bone. Designs 2023, 7, 21. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Fareed, M.A.; Zafar, M.S. Bioactive Glass Applications in Different Periodontal Lesions: A Narrative Review. Coatings 2023, 13, 716. [Google Scholar] [CrossRef]
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. Semiconducting glass-ceramics. J. Non-Cryst. Solids 1970, 2, 250–277. [Google Scholar] [CrossRef]
- Hench, L.L.; Clark, A.E.; Schaake, H.F. Effects of microstructure on the radiation stability of amorphous semiconductors. J. Non-Cryst. Solids 1972, 8–10, 837–843. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Paschall, H.A. Histochemical responses at a biomaterial’s interface. J. Biomed. Mater. Res. 1974, 8, 49–64. [Google Scholar] [CrossRef]
- Piotrowski, G.; Hench, L.L.; Allen, W.C.; Miller, G.J. Mechanical studies of the bone bioglass interfacial bond. J. Biomed. Mater. Res. 1975, 9, 47–61. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Nikolaev, A.L.; Kharisov, B.I.; Dorozhkin, S.V.; López, I.; Peña Méndez, Y.; Gómez de la Fuente, I. Enzymatic synthesis of calcium phosphates: A review. Nano-Struct. Nano-Objects 2024, 39, 101214. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef]
- Heimann, R.B. Structural Changes of Hydroxylapatite during Plasma Spraying: Raman and NMR Spectroscopy Results. Coatings 2021, 11, 987. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4)-Based Bioceramics: Preparation, Properties, and Applications. Coatings 2022, 12, 1380. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Bioactive Calcium Phosphate Coatings for Bone Implant Applications: A Review. Coatings 2023, 13, 1091. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Calcium Phosphates and Bioactive Glasses for Bone Implant Applications. Coatings 2023, 13, 1217. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed Osseoconductive Hydroxylapatite Coatings for Endoprosthetic Hip Implants: Phase Composition, Microstructure, Properties, and Biomedical Functions. Coatings 2024, 14, 787. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. There Are over 60Ways to Produce Biocompatible Calcium Orthophosphate (CaPO4) Deposits on Various Substrates. J. Compos. Sci. 2023, 7, 273. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Cañas, E.; Orts, M.J.; Boccaccini, A.R.; Sánchez, E. Microstructural and in vitro characterization of 45S5 bioactive glass coatings deposited by solution precursor plasma spraying (SPPS). Surf. Coat. Technol. 2019, 371, 151–160. [Google Scholar] [CrossRef]
- Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A.; Stiegler, N. High velocity suspension flame sprayed (HVSFS) potassium-based bioactive glass coatings with and without TiO2 bond coat. Surf. Coat. Technol. 2012, 206, 3857–3868. [Google Scholar] [CrossRef]
- Cañas, E.; Orts, M.J.; Sánchez, E.; Bellucci, D.; Cannillo, V. Deposition of bioactive glass coatings based on a novel composition containing strontium and magnesium. J. Eur. Ceram. Soc. 2022, 42, 6213–6221. [Google Scholar] [CrossRef]
- Cañas, E.; Rojas, O.; Orts, M.J.; Ageorges, H.; Sánchez, E. Effect of feedstock and plasma gun on the microstructure and bioactivity of plasma sprayed bioactive glass coatings. Surf. Coat. Technol. 2021, 406, 126704. [Google Scholar] [CrossRef]
- Cannillo, V.; Sola, A. Different approaches to produce coatings with bioactive glasses: Enamelling vs. plasma spraying. J. Eur. Ceram. Soc. 2010, 30, 2031–2039. [Google Scholar] [CrossRef]
- Monsalve, M.; Ageorges, H.; Lopez, E.; Vargas, F.; Bolivar, F. Bioactivity and mechanical properties of plasma-sprayed coatings of bioglass powders. Surf. Coat. Technol. 2013, 220, 60–66. [Google Scholar] [CrossRef]
- Moritz, N.; Vedel, E.; Ylänen, H.; Jokinen, M.; Hupa, M.; Yli-Urpo, A. Characterisation of bioactive glass coatings on titanium substrates produced using a CO2 laser. J. Mater. Sci. Mater. Med. 2004, 15, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Joshi, S.S.; Lu, K.; Ho, Y.H.; Xiang, Y.; Dahotre, N.B.; Du, J. Laser coating of bioactive glasses on bioimplant titanium alloys. Int. J. Appl. Glass Sci. 2019, 10, 307–320. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Bajda, S.; Liu, Y.; Triantaphyllou, A.; Rainforth, W.M.; Glendenning, M. 3D analysis of thermal and stress evolution during laser cladding of bioactive glass coatings. J. Mech. Behav. Biomed. Mater. 2016, 59, 404–417. [Google Scholar] [CrossRef]
- Bajda, S.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Liu, Y.; Rai, A. Laser-directed energy deposition of bioactive glass on Ti-6Al-7Nb titanium alloy substrate with highly refined grain structure. Surf. Coat. Technol. 2024, 485, 130904. [Google Scholar] [CrossRef]
- Bajda, S.; Liu, Y.; Tosi, R.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Dymek, S.; Polyakov, A.V.; Semenova, I.P.; et al. Laser cladding of bioactive glass coating on pure titanium substrate with highly refined grain structure. J. Mech. Behav. Biomed. Mater. 2021, 119, 104519. [Google Scholar] [CrossRef]
- Baino, F.; Montealegre, M.A.; Orlygsson, G.; Novajra, G.; Vitale-Brovarone, C. Bioactive glass coatings fabricated by laser cladding on ceramic acetabular cups: A proof-of-concept study. J. Mater. Sci. 2017, 52, 9115–9128. [Google Scholar] [CrossRef]
- Comesaña, R.; Quintero, F.; Lusquiños, F.; Pascual, M.J.; Boutinguiza, M.; Durán, A.; Pou, J. Laser cladding of bioactive glass coatings. Acta Biomater. 2010, 6, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Berbecaru, C.; Stan, G.E.; Pina, S.; Tulyaganov, D.U.; Ferreira, J.M.F. The bioactivity mechanism of magnetron sputtered bioglass thin films. Appl. Surf. Sci. 2012, 258, 9840–9848. [Google Scholar] [CrossRef]
- Stan, G.E.; Montazerian, M.; Shearer, A.; Stuart, B.W.; Baino, F.; Mauro, J.C.; Ferreira, J.M.F. Critical advances in the field of magnetron sputtered bioactive glass thin-films: An analytical review. Appl. Surf. Sci. 2024, 646, 158760. [Google Scholar] [CrossRef]
- Stuart, B.W.; Gimeno-Fabra, M.; Segal, J.; Ahmed, I.; Grant, D.M. Mechanical, structural and dissolution properties of heat treated thin-film phosphate based glasses. Appl. Surf. Sci. 2017, 416, 605–617. [Google Scholar] [CrossRef]
- Popa, A.C.; Stan, G.E.; Besleaga, C.; Ion, L.; Maraloiu, V.A.; Tulyaganov, D.U.; Ferreira, J.M.F. Submicrometer Hollow Bioglass Cones Deposited by Radio Frequency Magnetron Sputtering: Formation Mechanism, Properties, and Prospective Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 4357–4367. [Google Scholar] [CrossRef]
- Stuart, B.; Gimeno-Fabra, M.; Segal, J.; Ahmed, I.; Grant, D.M. Preferential sputtering in phosphate glass systems for the processing of bioactive coatings. Thin Solid Film. 2015, 589, 534–542. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Galca, A.C.; Aldica, G.; Ferreira, J.M.F. Strong bonding between sputtered bioglass-ceramic films and Ti-substrate implants induced by atomic inter-diffusion post-deposition heat-treatments. Appl. Surf. Sci. 2013, 280, 530–538. [Google Scholar] [CrossRef]
- Stan, G.E.; Morosanu, C.O.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Reumont, G. Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings. Appl. Surf. Sci. 2009, 255, 9132–9138. [Google Scholar] [CrossRef]
- Stan, G.E.; Marcov, D.A.; Pasuk, I.; Miculescu, F.; Pina, S.; Tulyaganov, D.U.; Ferreira, J.M.F. Bioactive glass thin films deposited by magnetron sputtering technique: The role of working pressure. Appl. Surf. Sci. 2010, 256, 7102–7110. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Chirica, I.M.; Stuart, B.W.; Galca, A.C.; Balescu, L.M.; Popescu-Pelin, G.; Grant, D.M.; Ferreira, J.M.F.; Stan, G.E. Phosphate bioglass thin-films: Cross-area uniformity, structure and biological performance tailored by the simple modification of magnetron sputtering gas pressure. Appl. Surf. Sci. 2021, 541, 148640. [Google Scholar] [CrossRef]
- Popa, A.C.; Marques, V.M.F.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Films 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Stan, G.E.; Pina, S.; Tulyaganov, D.U.; Ferreira, J.M.F.; Pasuk, I.; Morosanu, C.O. Biomineralization capability of adherent bio-glass films prepared by magnetron sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1047–1055. [Google Scholar] [CrossRef]
- Durán, A.; Conde, A.; Gómez Coedo, A.; Dorado, T.; García, C.; Ceré, S. Sol-gel coatings for protection and bioactivation of metals used in orthopaedic devices. J. Mater. Chem. 2004, 14, 2282–2290. [Google Scholar] [CrossRef]
- Huang, K.; Cai, S.; Xu, G.; Ren, M.; Wang, X.; Zhang, R.; Niu, S.; Zhao, H. Sol-gel derived mesoporous 58S bioactive glass coatings on AZ31 magnesium alloy and in vitro degradation behavior. Surf. Coat. Technol. 2014, 240, 137–144. [Google Scholar] [CrossRef]
- Niu, S.; Cai, S.; Liu, T.; Zhao, H.; Wang, X.; Ren, M.; Huang, K.; Wu, X. 45S5 bioactive glass-ceramic coated magnesium alloy with strong interfacial bonding strength by “superplasticity diffusion bonding”. Mater. Lett. 2015, 141, 96–99. [Google Scholar] [CrossRef]
- Huang, K.; Cai, S.; Xu, G.; Ye, X.; Dou, Y.; Ren, M.; Wang, X. Preparation and characterization of mesoporous 45S5 bioactive glass-ceramic coatings on magnesium alloy for corrosion protection. J. Alloys Compd. 2013, 580, 290–297. [Google Scholar] [CrossRef]
- Omar, S.A.; Ballarre, J.; Castro, Y.; Martinez Campos, E.; Schreiner, W.; Durán, A.; Cere, S.M. 58S and 68S sol-gel glass-like bioactive coatings for enhancing the implant performance of AZ91D magnesium alloy. Surf. Coat. Technol. 2020, 400, 126224. [Google Scholar] [CrossRef]
- Soule, L.D.; Pajares Chomorro, N.; Chuong, K.; Mellott, N.; Hammer, N.; Hankenson, K.D.; Chatzistavrou, X. Sol-Gel-Derived Bioactive and Antibacterial Multi-Component Thin Films by the Spin-Coating Technique. ACS Biomater. Sci. Eng. 2020, 6, 5549–5562. [Google Scholar] [CrossRef]
- Ye, X.; Leeflang, S.; Wu, C.; Chang, J.; Zhou, J.; Huan, Z. Mesoporous Bioactive Glass Functionalized 3D Ti-6Al-4V Scaffolds with Improved Surface Bioactivity. Materials 2017, 10, 1244. [Google Scholar] [CrossRef]
- Farag, M.M.; Ahmed, H.Y.; Al-Rashidy, Z.M. Improving the Corrosion Resistance of Magnesium Alloy by Magnesium Phosphate/Glass Composite Coatings Using Sol-Gel Method. Silicon 2023, 15, 3841–3854. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, S.H.; Chien, C.S.; Kung, J.C.; Shih, C.J. In Vitro Bioactivity and Antibacterial Effects of a Silver-Containing Mesoporous Bioactive Glass Film on the Surface of Titanium Implants. Int. J. Mol. Sci. 2022, 23, 9291. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.; Thomas, B.; Leinenbach, C.; Eifler, D.; Minay, E.J.; Boccaccini, A.R. The electrophoretic deposition of Bioglass® particles on stainless steel and Nitinol substrates. Surf. Coat. Technol. 2006, 200, 4835–4845. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K.; Taherian, M. Electrophoretic deposition of bioactive glass nanopowders on magnesium based alloy for biomedical applications. Ceram. Int. 2014, 40, 7879–7888. [Google Scholar] [CrossRef]
- Fiorilli, S.; Baino, F.; Cauda, V.; Crepaldi, M.; Vitale-Brovarone, C.; Demarchi, D.; Onida, B. Electrophoretic deposition of mesoporous bioactive glass on glass-ceramic foam scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 21. [Google Scholar] [CrossRef] [PubMed]
- Virk, R.S.; Atiq Ur Rehman, M.; Munawar, M.A.; Schubert, D.W.; Goldmann, W.H.; Dusza, J.; Boccaccini, A.R. Curcumin-Containing Orthopedic Implant Coatings Deposited on Poly-Ether-Ether-Ketone/Bioactive Glass/Hexagonal Boron Nitride Layers by Electrophoretic Deposition. Coatings 2019, 9, 572. [Google Scholar] [CrossRef]
- Azzouz, I.; Faure, J.; Khlifi, K.; Cheikh Larbi, A.; Benhayoune, H. Electrophoretic Deposition of 45S5 Bioglass® Coatings on the Ti6Al4V Prosthetic Alloy with Improved Mechanical Properties. Coatings 2020, 10, 1192. [Google Scholar] [CrossRef]
- Ammam, M. Electrophoretic deposition under modulated electric fields: A review. RSC Adv. 2012, 2, 7633–7646. [Google Scholar] [CrossRef]
- Mehdipour, M.; Afshar, A.; Mohebali, M. Electrophoretic deposition of bioactive glass coating on 316L stainless steel and electrochemical behavior study. Appl. Surf. Sci. 2012, 258, 9832–9839. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Comprehensive Review of Bioactive Glass Coatings: State of the Art, Challenges and Future Perspectives. Coatings 2020, 10, 757. [Google Scholar] [CrossRef]
- Maximov, M.; Maximov, O.C.; Craciun, L.; Ficai, D.; Ficai, A.; Andronescu, E. Bioactive Glass-An Extensive Study of the Preparation and Coating Methods. Coatings 2021, 11, 1386. [Google Scholar] [CrossRef]
- Liang, J.; Lu, X.; Zheng, X.; Li, Y.R.; Geng, X.; Sun, K.; Cai, H.; Jia, Q.; Jiang, H.B.; Liu, K. Modification of titanium orthopedic implants with bioactive glass: A systematic review of in vivo and in vitro studies. Front. Bioeng. Biotechnol. 2023, 11, 1269223. [Google Scholar] [CrossRef] [PubMed]
- Hadem, H.; Mitra, A.; Kumar Ojha, A.; Rajasekaran, R.; Satpathy, B.; Das, D.; Mukherjee, S.; Dhara, S.; Das, S.; Das, K. Electrophoretic Deposition of 58S Bioactive Glass-Polymer Composite Coatings on 316L Stainless Steel: An Optimization for Corrosion, Bioactivity, and Cytocompatibility. ACS Appl. Bio Mater. 2024, 7, 2966–2981. [Google Scholar] [CrossRef] [PubMed]
- Joughehdoust, S.; Manafi, S. Synthesis and In Vitro Investigation of Sol-Gel Derived Bioglass-58S Nanopowders. Mater. Sci. 2012, 30, 45–52. [Google Scholar] [CrossRef]
- Cañaveral, S.; Morales, D.; Vargas, A.F. Synthesis and characterization of a 58S bioglass modified with manganese by a sol-gel route. Mater. Lett. 2019, 255, 126575. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of Melt-Derived 45S5 and sol-gel-derived 58S Bioactive Glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef]
- Silver, I.A.; Deas, J.; Erecińska, M. Interactions of bioactive glasses with osteoblasts in vitro: Effects of 45S5 Bioglass®, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials 2001, 22, 175–185. [Google Scholar] [CrossRef]
- Bosetti, M.; Cannas, M. The effect of bioactive glasses on bone marrow stromal cells differentiation. Biomaterials 2005, 26, 3873–3879. [Google Scholar] [CrossRef]
- Hamadouche, M.; Meunier, A.; Greenspan, D.C.; Blanchat, C.; Zhong, J.P.; La Torre, G.P.; Sedel, L. Long-term in vivo bioactivity and degradability of bulk sol-gel bioactive glasses. J. Biomed. Mater. Res. 2001, 54, 560–566. [Google Scholar] [CrossRef]
- Arcos, D.; Greenspan, D.C.; Vallet-Regí, M. A new quantitative method to evaluate the in vitro bioactivity of melt and sol-gel-derived silicate glasses. J. Biomed. Mater. Res. 2003, 65A, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Björkenheim, R.; Strömberg, G.; Ainola, M.; Uppstu, P.; Aalto-Setäläe, L.; Leino, V.M.; Hupa, L.; Pajarinen, J.; Lindfors, N.C. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-stage d induce d membrane technique. Acta Biomater. 2021, 126, 463–476. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Hyvönen, P.; Nyyssönen, M.; Kirjavainen, M.; Kankare, J.; Gullichsen, E.; Salo, J. Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212–218. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, N.A.P.; Geurts, J.; Hulsen, D.J.W.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical Applications of S53P4 Bioactive Glass in Bone Healing and Osteomyelitic Treatment: A Literature Review. BioMed Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef]
- Kolana, K.C.R.; Leu, M.C.; Hilmas, G.E.; Velez, M. Effect of material, process parameters, and simulated body fluids on mechanical properties of 13-93 bioactive glass porous constructs made by selective laser sintering. J. Mech. Behav. Biomed. Mater. 2012, 13, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Sarker, B.; Detsch, R.; Hild, N.; Mohn, D.; Stark, W.J.; Boccaccini, A.R. In vitro reactivity of Sr-containing bioactive glass (type 1393) nanoparticles. J. Non-Cryst. Solids 2014, 387, 41–46. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Trueba, P.; Borie, F.; Alcudia, A.; Begines, B.; Rodriguez-Ortiz, J.A.; Torres, Y. Bioactive Bilayer Glass Coating on Porous Titanium Substrates with Enhanced Biofunctional and Tribomechanical Behavior. Coatings 2022, 12, 245. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Büttner, T.; Miguez Pacheco, V.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Bio Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Midha, S.; Kim, T.B.; van den Bergh, W.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Preconditioned 70S30C bioactive glass foams promote osteogenesis in vivo. Acta Biomaterialia 2013, 9, 9169–9182. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Yua, C.; Deng, H.; Wang, Y.; Zhang, Z.; Qiao, S.; Lu, G.; Zhao, D. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef]
- Thi Hoa, B.; Trong Hoa, H.T.; Anh Tien, N.; Huu Duy Khang, N.; Guseva, E.V.; Tuan, T.H.; Xuan Vuong, B. Green synthesis of bioactive glass 70SiO2-30CaO by hydrothermal method. Mater. Lett. 2020, 274, 128032. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Jones, J.R.; Pryce, R.S.; Hench, L.L. Bioactivity of gel-glass powders in the CaO-SiO2 system: A comparison with ternary (CaO-P2O5-SiO2) and quaternary glasses (SiO2-CaO-P2O5-Na2O). J. Biomed. Mater. Res. 2003, 66A, 110–119. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol-gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Song, X.; Segura-Egea, J.J.; Díaz-Cuenca, A. Sol-Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules 2023, 28, 6967. [Google Scholar] [CrossRef]
- Sakka, S. Birth of the sol-gel method: Early history. J. Sol.-Gel. Sci. Technol. 2022, 102, 478–481. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol-Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Hench, L.L. Bioglass: 10 milestones from concept to commerce. J. Non-Cryst. Solids 2016, 432, 2–8. [Google Scholar] [CrossRef]
- Hench, L.L. The future of bioactive ceramics. J. Mater. Sci. Mater. Med. 2015, 26, 86. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 2, 97–108. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ragel, C.V.; Salinas, A.J. Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.D.; Khurshid, Z.; Zafar, M.S.; Farooq, I.; Khan, R.S.; Najmi, A. Bioactive Glasses and their Applications in Dentistry. J. Pak. Dent. Assoc. 2017, 26, 32–38. [Google Scholar] [CrossRef]
- Oliver, J.N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Aparajita Dash, P.; Mohanty, S.; Kumar Nayak, S. A review on bioactive glass, its modifications and applications in healthcare sectors. J. Non-Cryst. Solids 2023, 614, 122404. [Google Scholar] [CrossRef]
- Brink, M.; Turunen, T.; Happonen, R.P.; Yli-Urpo, A. Compositional dependence of bioactivity of glasses in the system Na2O-K2O-MgO-CaO-B2O3-P2O5-SiO2. J. Biomed. Mater. Res. 1997, 37, 114–121. [Google Scholar] [CrossRef]
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440. [Google Scholar] [CrossRef]
- Al-Noaman, A.; Rawlinson, S.C.F.; Hill, R.G. The role of MgO on thermal properties, structure and bioactivity of bioactive glass coating for dental implants. J. Non-Cryst. Solids 2012, 358, 3019–3027. [Google Scholar] [CrossRef]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Sly, J.J.; Hill, R.G.; Mauro, J.C. Trends and perspectives on the commercialization of bioactive glasses. Acta Biomater. 2023, 160, 14–31. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Dubinskiy, S.; Kazakbiev, A.; Naumenko, V.; Abakumov, M.; Fauré, J.; Benhayoune, H.; Prokoshkin, S. Electrodeposition of cobalt-substituted calcium phosphate coatings on Ti22Nb6Zr alloy for bone implant applications. J. Alloys Compd. 2019, 793, 576–582. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashidy, Z.M.; Farag, M.M.; Abdel Ghany, N.A.; Ibrahim, A.M.; Abdel-Fattah, W.I. Orthopaedic bioactive glass/chitosan composites coated 316L stainless steel by green electrophoretic co-deposition. Surf. Coat. Technol. 2018, 334, 479–490. [Google Scholar] [CrossRef]
- Ning, J.; Yao, A.; Wang, D.; Huang, W.; Fu, H.; Liu, X.; Jiang, X.; Zhang, X. Synthesis and in vitro bioactivity of a borate-based bioglass. Mater. Lett. 2007, 61, 5223–5226. [Google Scholar] [CrossRef]
- Al-Rashidy, Z.M.; Farag, M.M.; Abdel Ghany, N.A.; Ibrahim, A.M.; Abdel-Fattah, W.I. Aqueous electrophoretic deposition and corrosion protection of borate glass coatings on 316 L stainless steel for hard tissue fixation. Surf. Int. 2017, 7, 125–133. [Google Scholar] [CrossRef]
- Peddi, L.; Brow, R.K.; Brown, R.F. Bioactive borate glass coatings for titanium alloys. J. Mater. Sci. Mater. Med. 2008, 19, 3145–3152. [Google Scholar] [CrossRef]
- Peitl, O.; Dutra Zanotto, E.; Hench, L.L. Highly bioactive P2O5-Na2O-CaO-SiO2 glass-cerasmics. J. Non-Cryst. Solids 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Roki, N.; Fenn, M.B. Bioactive glasses: Importance of structure and properties in bone regeneration. J. Mol. Struct. 2014, 1073, 24–30. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Progr. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Munyensanga, P.; Bricha, M.; El Mabrouk, K. Recent Developments of Bioactive Glass Electrophoretically Coated Cobalt-Chromium Metallic Implants. Johns. Matthey Technol. Rev. 2024, 68, 161–180. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of Biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef]
- Azzouz, I.; Khlifi, K.; Faure, J.; Dhiflaoui, H.; Ben Cheikh Larbi, A.; Benhayoune, H. Mechanical behavior and corrosion resistance of sol-gel derived 45S5 bioactive glass coating on Ti6Al4V synthesized by electrophoretic deposition. J. Mech. Behav. Biomed. Mater. 2022, 134, 105352. [Google Scholar] [CrossRef] [PubMed]
- Dusoulier, L. Elaboration de Dépôts d’YBa2Cu3O7-x par Électrophorèse et Projection Plasma, Chapitre 1, La Technique de Dépôt par Électrophorèse. Ph.D. Thesis, Liège University, Liège, Belgium, 2007. [Google Scholar]
- Yang, J.; Wang, E.G. Reaction of water on silica surfaces. Curr. Opin. Solid State Mater. Sci. 2006, 10, 33–39. [Google Scholar] [CrossRef]
- Lowe, B.M.; Skylaris, C.K.; Green, N.G. Acid-base dissociation mechanisms and energetics at the silica-water interface: An activation less process. J. Colloid Interface Sci. 2015, 451, 231–244. [Google Scholar] [CrossRef]
- Image by Mjones1984. Available online: https://commons.wikimedia.org/wiki/File:Diagram_of_zeta_potential_and_slipping_planeV2.svg (accessed on 1 July 2024).
- Behrens, S.H.; Grier, D.G. The Charge of Glass and Silica Surface. J. Chem. Phys. 2001, 115, 6716–6721. [Google Scholar] [CrossRef]
- Van der Biest, O.; Vandeperre, L.J. Electrophoretic Deposition of Materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. EPD kinetics: A review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Sujith, S.V.; Kim, H.; Lee, J. A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals 2022, 12, 165. [Google Scholar] [CrossRef]
- O’Brien, R.W.; White, L.R. Electrophoretic Mobility of a Spherical Colloidal Particle. J. Chem. Soc. Faraday Trans. 2 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Nicholson, P.S. Electrophoretic Deposition (EPD): Mechanisms, Kinetics, and Application to Ceramics. J. Am. Ceram. Soc. 1996, 79, 1987–2002. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Jiang, Z. Electrophoretic Deposition Forming of Sic-TZP Composites in a Nonaqueous Sol Media. J. Am. Ceram. Soc. 1994, 77, 1946–1949. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Vafa, E.; Tayebi, L.; Abbasi, M.; Azizli, M.J.; Bazargan-Lari, R.; Talaiekhozani, A.; Zareshahrabadi, Z.; Vaez, A.; Amani, A.M.; Kamyab, H.; et al. A better roadmap for designing novel bioactive glasses: Effective approaches for the development of innovative revolutionary bioglasses for future biomedical applications. Environ. Sci. Pollut. Res. 2023, 30, 116960–116983. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, C.; Zhang, F.; Feng, X.; Li, J.; Liu, T.; Chen, J.; Zhang, J. Preparation and antibacterial property of silver-containing mesoporous 58S bioactive glass. Mater. Sci. Eng. C 2014, 42, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Ge, K.X.; Lung, C.Y.K.; Chu, C.H. Developing a novel glass ionomer cement with enhanced mechanical and chemical properties. Dent. Mater. 2024, 40, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Vernè, E.; Di Nunzio, S.; Bosetti, M.; Appendino, P.; Vitale Brovarone, C.; Maina, G.; Cannas, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef]
- Shendage, S.S.; Gaikwad, K.; Kachare, K.; Kashte, S.; Chang, J.Y.; Ghule, A.V. In situ silver-doped antibacterial bioactive glass for bone regeneration application. J. Mater. Sci. 2024, 59, 10744–10762. [Google Scholar] [CrossRef]
- Chen, X.; Karpukhina, N.; Brauer, D.S.; Hill, R.G. Novel Highly Degradable Chloride Containing Bioactive Glasses. Biomed. Glasses 2015, 1, 108–118. [Google Scholar] [CrossRef]
- Chen, X.; Karpukhina, N.; Brauer, D.S.; Hill, R.G. High chloride content calcium silicate glasses. Phys. Chem. Chem. Phys. 2017, 19, 7078. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Pedone, A.; Apperley, D.; Hill, R.G.; Karpukhina, N. New Insight into Mixing Fluoride and Chloride in Bioactive Silicate Glasses. Sci. Rep. 2018, 8, 1316. [Google Scholar] [CrossRef]
- Brauer, D.S.; Karpukhina, N.; Law, R.V.; Hill, R.G. Structure of fluoride-containing bioactive glasses. J. Mater. Chem. 2009, 19, 5629–5636. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Menabue, L.; Aina, V.; Morterra, C. Fluoride-containing bioactive glasses: Surface reactivity in simulated body fluids solutions. Acta Biomater. 2009, 5, 3548–3562. [Google Scholar] [CrossRef]
- Gentleman, E.; Stevens, M.M.; Hill, R.G.; Brauer, D.S. Surface properties and ion release from fluoride-containing bioactive glasses promote osteoblast differentiation and mineralization in vitro. Acta Biomater. 2013, 9, 5771–5779. [Google Scholar] [CrossRef] [PubMed]
- Cannillo, V.; Sola, A. Potassium-based composition for a bioactive glass. Ceram. Int. 2009, 35, 3389–3393. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Low Temperature Sintering of Innovative Bioactive Glasses. J. Am. Ceram. Soc. 2012, 95, 1313–1319. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A. A new potassium-based bioactive glass: Sintering behaviour and possible applications for bioceramic scaffolds. Ceram. Int. 2011, 37, 145–157. [Google Scholar] [CrossRef]
- de Magalhães Gomes, G.H.; Oliveira Guimarães, G.; Dorion Rodas, A.C.; Barbosa Milesi, M.T.; Nascimento Costa, F.; Rodrigues Pais Alves, M.F.; Santos, C.; Barboza Daguano, J.K.M. In vitro biodegradation, blood, and cytocompatibility studies of a bioactive lithium silicate glass-ceramic. Mater. Chem. Phys. 2024, 314, 128828. [Google Scholar] [CrossRef]
- Khorami, M.; Hesaraki, S.; Behnamghader, A.; Nazarian, H.; Shahrabi, S. In vitro bioactivity and biocompatibility of lithium substituted 45S5 bioglass. Mater. Sci. Eng. C 2011, 31, 1584–1592. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Synthesis and in vitro studies of sol-gel derived lithium substituted 58S bioactive glass. Ceram. Int. 2017, 43, 12835–12843. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Büttner, T.; Maçon, A.L.B.; Jones, J.R.; Fey, T.; de Ligny, D.; Greil, P.; Chevalier, J.; Malchere, A.; Boccaccini, A.R. Development and characterization of lithium-releasing silicate bioactive glasses and their scaffolds for bone repair. J. Non-Cryst. Solids 2016, 432, 65–72. [Google Scholar] [CrossRef]
- Pantulap, U.; Unalan, I.; Zheng, K.; Boccaccini, A.R. Hydroxycarbonate apatite formation, cytotoxicity, and antibacterial properties of rubidium-doped mesoporous bioactive glass nanoparticles. J. Porous Mater. 2024, 31, 685–696. [Google Scholar] [CrossRef]
- Ouyang, S.; Zheng, K.; Huang, Q.; Liu, Y.; Boccaccini, A.R. Synthesis and characterization of rubidium-containing bioactive glass nanoparticles. Mater. Lett. 2020, 273, 127920. [Google Scholar] [CrossRef]
- Majumdar, S.; Tiwari, A.; Mallick, D.; Patel, D.K.; Trigun, S.K.; Krishnamurthy, S. Oral Release Kinetics, Biodistribution, and Excretion of Dopants from Barium-Containing Bioactive Glass in Rats. ACS Omega 2024, 9, 7188–7205. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Vermani, Y.K.; Al-Buriahi, M.S.; Alzahrani, J.S.; Singh, T. Fabrication and characterization of barium based bioactive glasses in terms of physical, structural, mechanical and radiation shielding properties. Ceram. Int. 2021, 47, 21730–21743. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Tripathi, H.; Vyas, V.K.; Jain, S.; Suman, S.K.; Pyare, R.; Singh, S.P. Influence of barium substitution on bioactivity, thermal and physico-mechanical properties of bioactive glass. Mater. Sci. Eng. C 2015, 49, 549–559. [Google Scholar] [CrossRef]
- Majumdar, S.; Hira, S.K.; Tripathi, H.; Kumar, A.S.; Manna, P.P.; Singh, S.P.; Krishnamurthy, S. Synthesis and characterization of barium-doped bioactive glass with potential anti-inflammatory activity. Ceram. Int. 2021, 47, 7143–7158. [Google Scholar] [CrossRef]
- Barrioni, B.R.; Norris, E.; Jones, J.R.; Pereira, M.d.M. The influence of cobalt incorporation and cobalt precursor selection on the structure and bioactivity of sol-gel-derived bioactive glass. J. Sol.-Gel. Sci. Technol. 2018, 88, 309–321. [Google Scholar] [CrossRef]
- Jiménez-Holguín, J.; Lozano, D.; Saiz-Pardo, M.; de Pablo, D.; Ortega, L.; Enciso, S.; Fernández-Tomé, B.; Díaz-Güemes, I.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Osteogenic-angiogenic coupled response of cobalt-containing mesoporous bioactive glasses in vivo. Acta Biomater. 2024, 176, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Montazerian, M.; Verné, E. Cobalt-Doped Bioactive Glasses for Biomedical Applications: A Review. Materials 2023, 16, 4994. [Google Scholar] [CrossRef] [PubMed]
- Azari, Z.; Kermani, F.; Mollazadeh, S.; Alipour, F.; Sadeghi-Avalshahr, A.; Ranjbar-Mohammadi, M.; Jalali Kondori, B.; Mollaei, Z.; Atefe Hosseini, S.; Nazarnezhad, S.; et al. Fabrication and characterization of cobalt- and copper-doped mesoporous borate bioactive glasses for potential applications in tissue engineering. Ceram. Int. 2023, 49, 38773–38788. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Ghodrat, S.; Fiume, E.; Baino, F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater. Sci. Eng. C 2021, 121, 111741. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Verné, E. Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions. Materials 2016, 9, 405. [Google Scholar] [CrossRef]
- Vecchio, G.; Darcos, V.; Le Grill, S.; Brouillet, F.; Coppel, Y.; Duttine, M.; Pugliara, A.; Combes, C.; Soulié, J. Spray-dried ternary bioactive glass microspheres: Direct and indirect structural effects of copper-doping on acellular degradation behavior. Acta Biomater. 2024, 181, 453–468. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Tiama, T.M.; Ibrahim, M.A.; Sharaf, M.H.; Mabied, A.F. Effect of germanium oxide on the structural aspects and bioactivity of bioactive silicate glass. Sci. Rep. 2023, 13, 9582. [Google Scholar] [CrossRef]
- Mokhtari, S.; Krull, E.A.; Sanders, L.M.; Coughlan, A.; Mellott, N.P.; Gong, Y.; Borges, R.; Wren, A.W. Investigating the effect of germanium on the structure of SiO2-ZnO-CaO-SrO-P2O5 glasses and the subsequent influence on glass polyalkenoate cement formation, solubility and bioactivity. Mater. Sci. Eng. C 2019, 103, 109843. [Google Scholar] [CrossRef] [PubMed]
- Silva Pereira, A.F.; da Silva Neto, O.C.; Gomes Dias, T.; Silva Reis, A.; Pedrochi, F.; Steimacher, A.; Barboza, M.J. The role of MgO on physical and bioactive properties of borophosphate glasses for biomedical applications. Ceram. Int. 2024, 50, 17532–17543. [Google Scholar] [CrossRef]
- Watts, S.J.; Hill, R.G.; O’Donnell, M.D.; Law, R.V. Influence of magnesia on the structure and properties of bioactive glasses. J. Non-Cryst. Solids 2010, 356, 517–524. [Google Scholar] [CrossRef]
- Saboori, A.; Rabiee, M.; Moztarzadeh, F.; Sheikhi, M.; Tahriri, M.; Karimi, K. Synthesis, characterization and in vitro bioactivity of sol-gel-derived SiO2-CaO-P2O5-MgO bioglass. Mater. Sci. Eng. C 2009, 29, 335–340. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J.; Román, J.; Gil, M. Effect of magnesium content on the in vitro bioactivity of CaO-MgO-SiO2-P2O5 sol-gel glasses. J. Mater. Chem. 1999, 9, 515–518. [Google Scholar] [CrossRef]
- Abati, M.; Contreras Jaimes, A.T.; Rigamonti, L.; Carrozza, D.; Lusvardi, G.; Brauer, D.S.; Malavasi, G. Assessing Mn as an antioxidant agent in bioactive glasses by quantification of catalase and superoxide dismutase enzymatic mimetic activities. Ceram. Int. 2024, 50, 2574–2587. [Google Scholar] [CrossRef]
- Curcio, M.; Bochicchio, B.; Pepe, A.; Laezza, A.; De Stefanis, A.; Rau, J.V.; Teghil, R.; De Bonis, A. Mn-Doped Glass-Ceramic Bioactive (Mn-BG) Thin Film to Selectively Enhance the Bioactivity of Electrospun Fibrous Polymeric Scaffolds. Coatings 2022, 12, 1427. [Google Scholar] [CrossRef]
- Miola, M.; Vitale Brovarone, C.; Maina, G.; Rossi, F.; Bergandi, L.; Ghigo, D.; Saracino, S.; Maggiora, M.; Canuto, R.A.; Muzio, G.; et al. In vitro study of manganese-doped bioactive glasses for bone regeneration. Mater. Sci. Eng. C 2014, 38, 107–118. [Google Scholar] [CrossRef]
- Nawaz, Q.; Atiq Ur Rehman, M.; Burkovski, A.; Schmidt, J.; Beltrán, A.M.; Shahid, A.; Alber, N.K.; Peukert, W.; Boccaccini, A.R. Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2018, 29, 64. [Google Scholar] [CrossRef]
- Boukhris, I.; Alalawi, A.; Al-Buriahi, M.S.; Kebaili, I.; Sayyed, M.I. Radiation attenuation properties of bioactive glasses doped with NiO. Ceram. Int. 2020, 46, 19880–19889. [Google Scholar] [CrossRef]
- Vyas, V.K.; Kumar, A.S.; Singh, S.P.; Pyare, R. Effect of nickel oxide substitution on bioactivity and mechanical properties of bioactive glass. Bull. Mater. Sci. 2016, 39, 1355–1361. [Google Scholar] [CrossRef]
- Vyas, V.K.; Kumar, A.S.; Singh, S.P.; Pyare, R. Destructive and non-destructive behavior of nickel oxide doped bioactive glass and glass-ceramic. J. Aust. Ceram. Soc. 2017, 53, 939–951. [Google Scholar] [CrossRef]
- Silva, A.V.; Gomes, D.d.S.; Victor, R.d.S.; Santana, L.N.d.L.; Neves, G.A.; Menezes, R.R. Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration. Materials 2023, 16, 7654. [Google Scholar] [CrossRef]
- Rohilla, R.; Dahiya, M.S.; Agarwal, A.; Khasa, S. Influence of Sr2+ ions on structural, optical and bioactive behaviour of phosphoborate glass system. J. Mol. Struct. 2023, 1291, 136095. [Google Scholar] [CrossRef]
- Ryu, J.H.; Mangal, U.; Lee, M.J.; Seo, J.Y.; Jeong, I.J.; Park, J.Y.; Na, J.Y.; Lee, K.J.; Yu, H.S.; Cha, J.K.; et al. Effect of strontium substitution on functional activity of phosphate-based glass. Biomater. Sci. 2023, 11, 6299. [Google Scholar] [CrossRef]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Synthesis, characterization, and in vitro apatite formation of strontium-doped sol-gel-derived bioactive glass nanoparticles for bone regeneration applications. Ceram. Int. 2023, 49, 23020–23034. [Google Scholar] [CrossRef]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef]
- Aina, V.; Perardi, A.; Bergandi, L.; Malavasi, G.; Menabue, L.; Morterra, C.; Ghigo, D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. Biol. Interact. 2007, 167, 207–218. [Google Scholar] [CrossRef]
- Paramita, P.; Ramachandran, M.; Narashiman, S.; Nagarajan, S.; Kumar Sukumar, D.; Chung, T.W.; Ambigapathi, M. Sol–gel based synthesis and biological properties of zinc integrated nano bioglass ceramics for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2021, 32, 5. [Google Scholar] [CrossRef]
- Oki, A.; Parveen, B.; Hossain, S.; Adeniji, S.; Donahue, H. Preparation and in vitro bioactivity of zinc containing sol-gel-derived bioglass materials. J. Biomed. Mater. Res. A 2004, 69, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol-gel derived CaO-P2O5-SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Clavijo-Mejía, G.A.; Michálek, M.; Youssef, L.; Kaňková, H.; Galusek, D.; Boccaccini, A.R. Bioactivity of radiopaque 45S5 bioactive glass with progressive additions of Bi2O3: A dissolution study under static conditions. Ceram. Int. 2024, 50, 27216–27226. [Google Scholar] [CrossRef]
- Wang, L.; Long, N.J.; Li, L.; Lu, Y.; Li, M.; Cao, J.; Zhang, Y.; Zhang, Q.; Xu, S.; Yang, Z.; et al. Multi-functional bismuth-doped bioglasses: Combining bioactivity and photothermal response for bone tumor treatment and tissue repair. Light Sci. Appl. 2018, 7, 1. [Google Scholar] [CrossRef]
- Du, J.; Ding, H.; Fu, S.; Li, D.; Yu, B. Bismuth-coated 80S15C bioactive glass scaffolds for photothermal antitumor therapy and bone regeneration. Front. Bioeng. Biotechnol. 2023, 10, 1098923. [Google Scholar] [CrossRef]
- Meng, X.; Wang, W.D.; Li, S.R.; Sun, Z.J.; Zhang, L. Harnessing cerium-based biomaterials for the treatment of bone diseases. Acta Biomater. 2024, 183, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Chromčíková, M.; Svoboda, R.; Hruška, B.; Pecušová, B.; Nowicka, A. Thermo-kinetic and structural characterization of Ce-doped glasses based on Bioglass 45S5. Mater. Chem. Phys. 2023, 304, 127833. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and characterization of cerium-doped glasses and in vitro evaluation of bioactivity. J. Non-Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Nicolini, V.; Malavasi, G.; Menabue, L.; Lusvardi, G.; Benedetti, F.; Valeri, S.; Luches, P. Cerium-doped bioactive 45S5 glasses: Spectroscopic, redox, bioactivity and biocatalytic properties. J. Mater. Sci. 2017, 52, 8845–8857. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zhou, Y.; Zhang, Y. Luminescence biomonitoring and antibacterial properties of Er3+-doped SiO2-CaO-P2O5 bioactive glass microspheres. Ceram. Int. 2023, 49, 22924–22931. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, X.; Zhou, Y. Erbium-ytterbium containing upconversion mesoporous bioactive glass microspheres for tissue engineering: Luminescence monitoring of biomineralization and drug release. Acta Biomater. 2023, 168, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Son, S.A.; Park, J.K.; Yoon, S.Y. Influence of glass composition on the network structure and mineralization of europium containing mesoporous bioactive glass nanoparticles. Mater. Chem. Phys. 2024, 317, 129179. [Google Scholar] [CrossRef]
- Baranowska, A.; Leśniak, M.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Dorosz, D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials 2020, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xia, L.; Han, P.; Mao, L.; Wang, J.; Zhai, D.; Fang, B.; Chang, J.; Xiao, Y. Europium-Containing Mesoporous Bioactive Glass Scaffolds for Stimulating in Vitro and in Vivo Osteogenesis. ACS Appl. Mater. Interfaces 2016, 8, 11342–11354. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Z.; Sun, H.; Beltrán, A.M.; Nawaz, Q.; Sui, B.; Boccaccini, A.R.; Zheng, K. Unlocking the potential of iron-containing mesoporous bioactive glasses: Orchestrating osteogenic differentiation in bone marrow mesenchymal stem cells and osteoblasts. Colloids Surf. A Physicochem. Eng. Asp. 2024, 694, 134188. [Google Scholar] [CrossRef]
- Nitu; Fopase, R.; Mohan Pandey, L.; Seal, P.; Prasad Borah, J.; Srinivasan, A. Assessment of sol-gel derived iron oxide substituted 45S5 bioglass-ceramics for biomedical applications. J. Mater. Chem. B 2023, 11, 7502. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Ahmed, I.; Blaker, J.J.; Bismarck, A.; Boccaccini, A.R.; Lewis, M.P.; Nazhat, S.N.; Knowles, J.C. Effect of iron on the surface, degradation and ion release properties of phosphate-based glass fibres. Acta Biomater. 2005, 1, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Kurtuldu, F.; Mutlu, N.; Friedrich, R.P.; Beltrán, A.M.; Liverani, L.; Detsch, R.; Alexiou, C.; Galusek, D.; Boccaccini, A.R. Gallium-containing mesoporous nanoparticles influence in-vitro osteogenic and osteoclastic activity. Biomater. Adv. 2024, 162, 213922. [Google Scholar] [CrossRef] [PubMed]
- Maha Lakshmi, A.; Prasad, A.; Murimadugula, S.; Venkateswara Rao, P.; Madaboosi, N.; Özcan, M.; Kumari, K.; Syam Prasad, P. Efficacy of Ga3+ ions on structural, biological and antimicrobial activity of mesoporous lithium silicate bioactive glasses for tissue engineering. Microporous Mesoporous Mater. 2024, 373, 113132. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Vasudevaraj Naveen, S.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71. [Google Scholar] [CrossRef]
- Franchini, M.; Lusvardi, G.; Malavasi, G.; Menabue, L. Gallium-containing phospho-silicate glasses: Synthesis and in vitro bioactivity. Mater. Sci. Eng. C 2012, 32, 1401–1406. [Google Scholar] [CrossRef]
- Piagentini Delpino, G.; Borges, R.; Zambanini, T.; Sousa Joca, J.F.; Gaubeur, I.; Santos de Souza, A.C.; Marchi, J. Sol-gel-derived 58S bioactive glass containing holmium aiming brachytherapy applications: A dissolution, bioactivity, and cytotoxicity study. Mater. Sci. Eng. C 2021, 119, 111595. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Marchi, J.; Borges, R.; Verné, E. Holmium-doped 58S glass-derived foam-like scaffolds. Mater. Lett. 2023, 341, 134256. [Google Scholar] [CrossRef]
- Ashemary, A.Z.; Haidary, S.M.; Muhammed, Y.; Çardakli, İ.S. Preparation and characterization of mesoporous Lanthanum-doped bioactive glass nanoparticles. Digest J. Nanomater. Biostruct. 2023, 18, 681–688. [Google Scholar] [CrossRef]
- Jodati, H.; Güner, B.; Evis, Z.; Keskin, D.; Tezcaner, A. Synthesis and characterization of magnesium-lanthanum dual doped bioactive glasses. Ceram. Int. 2020, 46, 10503–10511. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Miranda Salvado, I.M.; Ferreira, J.M.F.; Pullar, R.C. The effects of Cu2+ and La3+ doping on the sintering ability of sol-gel derived high silica bioglasses. Ceram. Int. 2019, 45, 10269–10278. [Google Scholar] [CrossRef]
- Orgaz, F.; Dzika, A.; Szycht, O.; Amat, D.; Barba, F.; Becerra, J.; Santos-Ruiz, L. Surface nitridation improves bone cell response to melt-derived bioactive silicate/borosilicate glass composite scaffolds. Acta Biomater. 2016, 29, 424–434. [Google Scholar] [CrossRef]
- Bachar, A.; Mercier, C.; Tricoteaux, A.; Leriche, A.; Follet, C.; Hampshire, S. Bioactive oxynitride glasses: Synthesis, structure and properties. J. Eur. Ceram. Soc. 2016, 36, 2869–2881. [Google Scholar] [CrossRef]
- Kushan Akin, S.R.; Dolekcekic, E.; Webster, T.J. Effect of nitrogen on the antibacterial behavior of oxynitride glasses. Ceram. Int. 2021, 47, 18213–18217. [Google Scholar] [CrossRef]
- Ershad, M.; Vyas, V.K.; Prasad, S.; Ali, A.; Pyare, R. Effect of Sm2O3 substitution on mechanical and biological properties of 45S5 bioactive glass. J. Aust. Ceram. Soc. 2018, 54, 621–630. [Google Scholar] [CrossRef]
- Deliormanli, A.M.; ALMisned, G.; Tekin, H.O. Synthesis and characterization of Nb5+ and Sm3+-doped 13-93 bioactive glass particles with improved photon transmission properties for advanced biomedical and dental applications. Ceram. Int. 2024, 50, 31211–31224. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lin, C.; Zhong, W. Sol-gel derived terbium-containing mesoporous bioactive glasses nanospheres: In vitro hydroxyapatite formation and drug delivery. Colloids Surf. B 2017, 160, 406–415. [Google Scholar] [CrossRef]
- Deliormanli, A.M.; Issa, S.A.M.; Al-Buriahi, M.S.; Rahman, B.; Zakaly, H.M.H.; Tekin, H.O. Erbium (III)- and Terbium (III)-containing silicate-based bioactive glass powders: Physical, structural and nuclear radiation shielding characteristics. Appl. Phys. A Mater. Sci. Process. 2021, 127, 463. [Google Scholar] [CrossRef]
- Hadush Tesfay, A.; Chou, Y.J.; Tan, C.Y.; Bakare, F.F.; Tsou, N.T.; Huang, E.W.; Shih, S.J. Control of Dopant Distribution in Yttrium-Doped Bioactive Glass for Selective Internal Radiotherapy Applications Using Spray Pyrolysis. Materials 2019, 12, 986. [Google Scholar] [CrossRef]
- Cacaina, D.; Ylänen, H.; Simon, S.; Hupa, M. The behaviour of selected yttrium containing bioactive glass microspheres in simulated body environments. J. Mater. Sci. Mater. Med. 2008, 19, 1225–1233. [Google Scholar] [CrossRef]
- Christie, J.K.; Tilocca, A. Integrating biological activity into radioisotope vectors: Molecular dynamics models of yttrium-doped bioactive glasses. J. Mater. Chem. 2012, 22, 12023–12031. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; Faria, P.C.; Delpino, G.P.; Pereira, I.S.; Marques, M.M.; Marchi, J. Dissolution, bioactivity behavior, and cytotoxicity of rare earth containing bioactive glasses (RE = Gd, Yb). Int. J. Appl. Ceram. Technol. 2019, 16, 2028–2039. [Google Scholar] [CrossRef]
- Chen, D.; Liang, Z.; Su, Z.; Huang, J.; Pi, Y.; Ouyang, Y.; Luo, T.; Guo, L. Selenium-Doped Mesoporous Bioactive Glass Regulates Macrophage Metabolism and Polarization by Scavenging ROS and Promotes Bone Regeneration In Vivo. ACS Appl. Mater. Interfaces 2023, 15, 34378–34396. [Google Scholar] [CrossRef]
- Hu, M.; Fang, J.; Zhang, Y.; Wang, X.; Zhong, W.; Zhou, Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: Selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020, 579, 654–666. [Google Scholar] [CrossRef]
- Karakuzu-İkizler, B.; Terzioğlu, P.; Oduncu-Tekerek, B.S.; Yücel, S. Effect of selenium incorporation on the structure and in vitro bioactivity of 45S5 bioglass. J. Aust. Ceram. Soc. 2020, 56, 697–709. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Zhang, W.; Zhang, X. Construction of tellurium-doped mesoporous bioactive glass nanoparticles for bone cancer therapy by promoting ROS-mediated apoptosis and antibacterial activity. J. Colloid Interface Sci. 2022, 610, 719–730. [Google Scholar] [CrossRef]
- Miola, M.; Massera, J.; Cochis, A.; Kumar, A.; Rimondini, L.; Vernè, E. Tellurium: A new active element for innovative multifunctional bioactive glasses. Mater. Sci. Eng. C 2021, 123, 111957. [Google Scholar] [CrossRef]
- Moghanian, A.; Zohourfazeli, M.; Mahdi Tajer, M.H. The effect of zirconium content on in vitro bioactivity, biological behavior and antibacterial activity of sol-gel derived 58S bioactive glass. J. Non-Cryst. Solids 2020, 546, 120262. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glass Modified with Zirconium Incorporation for Dental Implant Applications: Fabrication, Structural, Electrical, and Biological Analysis. Int. J. Mol. Sci. 2023, 24, 10571. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Wu, C.; Fang, Y.; Yang, J.; Wang, S. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous Mesoporous Mater. 2011, 143, 311–319. [Google Scholar] [CrossRef]
- de Souza, L.P.L.; Lopes, J.H.; Ferreira, F.V.; Martin, R.A.; Bertran, C.A.; Camilli, J.A. Evaluation of effectiveness of 45S5 bioglass doped with niobium for repairing critical-sized bone defect in in vitro and in vivo models. J. Biomed. Mater. Res. 2020, 108A, 446–457. [Google Scholar] [CrossRef]
- de Souza Balbinot, G.; Branco Leitune, V.C.; da Cunha Bahlis, E.A.; Ponzoni, D.; Visioli, F.; Mezzomo Collares, F. Niobium-containing bioactive glasses modulate alkaline phosphatase activity during bone repair. J. Biomed. Mater. Res. 2023, 111, 1224–1231. [Google Scholar] [CrossRef]
- Hammami, I.; Gavinho, S.R.; Pádua, A.S.; Lança, M.d.C.; Borges, J.P.; Silva, J.C.; Sá-Nogueira, I.; Jakka, S.K.; Graça, M.P.F. Extensive Investigation on the Effect of Niobium Insertion on the Physical and Biological Properties of 45S5 Bioactive Glass for Dental Implant. Int. J. Mol. Sci. 2023, 24, 5244. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.H.; Souza, L.P.; Domingues, J.A.; Ferreira, F.V.; de Alencar Hausen, M.; Camilli, J.A.; Martin, R.A.; de Rezende Duek, E.A.; Mazali, I.O.; Bertran, C.A. In vitro and in vivo osteogenic potential of niobium-doped 45S5 bioactive glass: A comparative study. J. Biomed. Mater. Res. 2020, 108B, 1372–1387. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Azeem, P.A.; Manavathi, B.; Adhikari, A.; Padala, C. In-vitro biomineralization, mechanical properties and drug release efficacy of tantalum containing borophosphate bioactive glasses. J. Drug Deliv. Sci. Technol. 2023, 84, 104436. [Google Scholar] [CrossRef]
- Mendonca, A.; Rahman, M.S.; Alhalawani, A.; Rodriguez, O.; Gallant, R.C.; Ni, H.; Clarkin, O.M.; Towler, M.R. The effect of tantalum incorporation on the physical and chemical properties of ternary silicon-calcium-phosphorous mesoporous bioactive glasses. J. Biomed. Mater. Res. B Part B 2019, 107B, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Alhalawani, A.M.F.; Towler, M.R. A novel tantalum-containing bioglass. Part I. Structure and solubility. Mater. Sci. Eng. C 2017, 72, 202–211. [Google Scholar] [CrossRef]
- Alhalawani, A.M.F.; Mehrvar, C.; Stone, W.; Waldman, S.D.; Towler, M.R. A novel tantalum-containing bioglass. Part II. Development of a bioadhesive for sternal fixation and repair. Mater. Sci. Eng. C 2017, 71, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Cong, D.; Zhang, Z.; Xu, M.; Wang, J.; Pu, X.; Huang, Z.; Liao, X.; Yin, G. Vanadium-Doped Mesoporous Bioactive Glass Promotes Osteogenic Differentiation of rBMSCs via the WNT/β-Catenin Signaling Pathway. ACS Appl. Bio Mater. 2023, 6, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Wei, Y.; Xu, N.; Li, J.; Pu, X.; Wang, J.; Huang, Z.; Liao, X.; Yin, G. Ion release behavior of vanadium-doped mesoporous bioactive glass particles and the effect of the released ions on osteogenic differentiation of BMSCs via the FAK/MAPK signaling pathway. J. Mater. Chem. B 2021, 9, 7848. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Pu, X.; Wang, J.; Huang, Z.; Yin, G. Effects of incorporated vanadium and its chemical states on morphology and mesostructure of mesoporous bioactive glass particles. Microporous Mesoporous Mater. 2021, 319, 111061. [Google Scholar] [CrossRef]
- Ponta, O.; Ciceo-Lucacel, R.; Vulpoi, A.; Radu, T.; Simon, S. Molybdenum effect on the structure of SiO2-CaO-P2O5 bioactive xerogels and on their interface processes with simulated biofluids. J. Biomed. Mater. Res. Part A 2014, 102A, 3177–3185. [Google Scholar] [CrossRef]
- Ciceo Lucacel, R.; Ponta, O.; Licarete, E.; Radu, T.; Simon, V. Synthesis, structure, bioactivity and biocompatibility of melt-derived P2O5-CaO-B2O3-K2O-MoO3 glasses. J. Non-Cryst. Solids 2016, 439, 67–73. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Dhiflaoui, H.; Ben Salem, S.; Salah, M.; Dabaki, Y.; Chayoukhi, S.; Gassoumi, B.; Hajjaji, A.; Ben Cheikh Larbi, A.; Amlouk, M.; Benhayoune, H. Influence of TiO2 on the Microstructure, Mechanical Properties and Corrosion Resistance of Hydroxyapatite HaP + TiO2 Nanocomposites Deposited Using Spray Pyrolysis. Coatings 2023, 13, 1283. [Google Scholar] [CrossRef]
- Elalmis, Y.B.; Ikizler, B.K.; Depren, S.K.; Yucel, S.; Aydin, I. Investigation of alumina doped 45S5 glass as a bioactive filler for experimental dental composites. Int. J. Appl. Glass Sci. 2021, 12, 313–327. [Google Scholar] [CrossRef]
- Pawlik, J.; Widziołek, M.; Cholewa-Kowalska, K.; Łączka, M.; Osyczka, A.M. New sol-gel bioactive glass and titania composites with enhanced physico-chemical and biological properties. J. Biomed. Mater. Res. Part A 2014, 102A, 2383–2394. [Google Scholar] [CrossRef]
- Radice, S.; Kern, P.; Bürki, G.; Michler, J.; Textor, M. Electrophoretic deposition of zirconia-Bioglass® composite coatings for biomedical implants. J. Biomed. Mater. Res. 2007, 82A, 436–444. [Google Scholar] [CrossRef]
- Laybidi, F.H.; Bahrami, A. Antibacterial properties of ZnO-containing bioactive glass coatings for biomedical applications. Mater. Lett. 2024, 365, 136433. [Google Scholar] [CrossRef]
- Marin, E.; Adachi, T.; Boschetto, F.; Zanocco, M.; Rondinella, A.; Zhu, W.; Bock, R.; McEntirec, B.; Bal, S.B.; Pezzotti, G. Biological response of human osteosarcoma cells to Si3N4-doped Bioglasses. Mater. Des. 2018, 159, 79–89. [Google Scholar] [CrossRef]
- Farnoush, H.; Muhaffel, F.; Cimenoglu, H. Fabrication and characterization of nano-HA-45S5 bioglass composite coatings on calcium-phosphate containing micro-arc oxidized CP-Ti substrates. Appl. Surf. Sci. 2015, 324, 765–774. [Google Scholar] [CrossRef]
- Soares, V.O.; Daguano, J.K.M.B.; Lombello, C.B.; Bianchin, O.S.; Gonçalves, L.M.G.; Zanotto, E.D. New sintered wollastonite glass-ceramic for biomedical applications. Ceram. Int. 2018, 44, 20019–20027. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Kuo, H.C.; Tuan, W.H.; Shih, S.J.; Naito, M.; Lai, P.L. Manipulation of the degradation behavior of calcium sulfate by the addition of bioglass. Prog. Biomater. 2019, 8, 115–125. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, F.; Zhu, J.; Peng, Y.; Tian, X.; Chen, X. Fabrication of a Novel Calcium Carbonate Composite Ceramic as Bone Substitute. J. Am. Ceram. Soc. 2015, 98, 223–228. [Google Scholar] [CrossRef]
- Batool, S.A.; Wadood, A.; Hussain, S.W.; Yasir, M.; Ur Rehman, M.A. A Brief Insight to the Electrophoretic Deposition of PEEK-, Chitosan-, Gelatin-, and Zein-Based Composite Coatings for Biomedical Applications: Recent Developments and Challenges. Surfaces 2021, 4, 205–239. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic deposition of PEEK/bioactive glass composite coatings on stainless steel for orthopedic applications: An optimization for in vitro bioactivity and adhesion strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Manzur, J.; Akhtar, M.; Aizaz, A.; Ahmad, K.; Yasir, M.; Minhas, B.Z.; Avcu, E.; Ur Rehman, M.A. Electrophoretic Deposition, Microstructure, and Selected Properties of Poly(lactic-co-glycolic) Acid-Based Antibacterial Coatings on Mg Substrate. ACS Omega 2023, 8, 18074–18089. [Google Scholar] [CrossRef]
- Sadeghinia, Z.; Emadi, R.; Shamoradi, F. A study of the electrophoretic deposition of polycaprolactone-chitosan-bioglass nanocomposite coating on stainless steel (316L) substrates. J. Bioact. Compat. Pol. 2022, 37, 53–71. [Google Scholar] [CrossRef]
- Chen, Q.; Cabanas-Polo, S.; Goudouri, O.M.; Boccaccini, A.R. Electrophoretic co-deposition of polyvinyl alcohol (PVA) reinforced alginate-Bioglass® composite coating on stainless steel: Mechanical properties and in-vitro bioactivity assessment. Mater. Sci. Eng. C 2014, 40, 55–64. [Google Scholar] [CrossRef]
- Rouein, Z.; Jafari, H.; Pishbin, F.; Mohammadi, R.; Simchi, A. Biodegradation behavior of polymethyl methacrylate-bioactive glass 45S5 composite coated magnesium in simulated body fluid. Trans. Nonferrous Met. Soc. China 2022, 32, 2216–2228. [Google Scholar] [CrossRef]
- Clifford, A.; Luo, D.; Zhitomirsky, I. Colloidal strategies for electrophoretic deposition of organic-inorganic composites for biomedical applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 219–225. [Google Scholar] [CrossRef]
- Deen, I.; Singh Selopal, G.; Wang, Z.M.; Rosei, F. Electrophoretic deposition of collagen/chitosan films with copper-doped phosphate glasses for orthopaedic implants. J. Colloid Interface Sci. 2022, 607, 869–880. [Google Scholar] [CrossRef]
- Sikkema, R.; Keohan, B.; Zhitomirsky, I. Hyaluronic-Acid-Based Organic-Inorganic Composites for Biomedical Applications. Materials 2021, 14, 4982. [Google Scholar] [CrossRef]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass® composite coatings on stainless steel deposited by direct current and alternating current electrophoretic deposition. Surf. Coat. Technol. 2012, 233, 49–56. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic deposition of chondroitin sulfate-chitosan/bioactive glass composite coatings with multilayer design. Surf. Coat. Technol. 2017, 315, 417–425. [Google Scholar] [CrossRef]
- Ramos Rivera, L.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact. Mater. 2021, 6, 1479–1490. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Ur Rehman, M.A.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Progr. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Meng, D.; Narayan Rath, S.; Mordan, N.; Salih, V.; Kneser, U.; Boccaccini, A.R. In vitro evaluation of 45S5 Bioglass®-derived glass-ceramic scaffolds coated with carbon nanotubes. J. Biomed. Mater. Res. Part A 2011, 99A, 435–444. [Google Scholar] [CrossRef]
- Meng, D.; Ioannou, J.; Boccaccini, A.R. Bioglass®-based scaffolds with carbon nanotube coating for bone tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 2139–2144. [Google Scholar] [CrossRef]
- Charlotte Schausten, M.; Meng, D.; Telle, R.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes and bioactive glass particles for bioactive composite coatings. Ceram. Int. 2010, 36, 307–312. [Google Scholar] [CrossRef]
- Sun, F.; Sask, K.N.; Brash, J.L.; Zhitomirsky, I. Surface modifications of Nitinol for biomedical applications. Colloids Surf. B Biointerfaces 2008, 67, 132–139. [Google Scholar] [CrossRef]
- Pishbin, F.; Mouriño, V.; Flor, S.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition of Gentamicin-Loaded Bioactive Glass/Chitosan Composite Coatings for Orthopaedic Implants. ACS Appl. Mater. Interfaces 2014, 6, 8796–8806. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Aghajani, H.; Farrokhi-Rad, M. Vancomycin loaded-mesoporous bioglass/hydroxyapatite/chitosan coatings by electrophoretic deposition. Ceram. Int. 2022, 48, 20176–20186. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Mariotti, C.E.; Conti, B.; Boccaccini, A.R. Electrophoretic deposition of ferulic acid loaded bioactive glass/chitosan as antibacterial and bioactive composite coatings. Surf. Coat. Technol. 2021, 405, 126657. [Google Scholar] [CrossRef]
- Radda’a, N.S.; Goldmann, W.H.; Detsch, R.; Roether, J.A.; Cordero-Arias, L.; Virtanen, S.; Moskalewicz, T.; Boccaccini, A.R. Electrophoretic deposition of tetracycline hydrochloride loaded halloysite nanotubes chitosan/bioactive glass composite coatings for orthopedic implants. Surf. Coat. Technol. 2017, 327, 146–157. [Google Scholar] [CrossRef]
- Moses, J.C.; Mandal, B.B. Mesoporous Silk-Bioactive Glass Nanocomposites as Drug Eluting Multifunctional Conformal Coatings for Improving Osseointegration and Bactericidal Properties of Metal Implants. ACS Appl. Mater. Interfaces 2022, 14, 14961–14980. [Google Scholar] [CrossRef]
- Patel, K.D.; El-Fiqi, A.; Lee, H.Y.; Singh, R.K.; Kim, D.A.; Lee, H.H.; Kim, H.W. Chitosan-nanobioactive glass electrophoretic coatings with bone regenerative and drug delivering potential. J. Mater. Chem. 2012, 22, 24945. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, Q.; Goldmann, W.H.; Maqbool, M.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of lawsone loaded bioactive glass (BG)/chitosan composite on polyetheretherketone (PEEK)/BG layers as antibacterial and bioactive coating. J. Biomed. Mater. Res. Part A 2018, 106A, 3111–3122. [Google Scholar] [CrossRef]
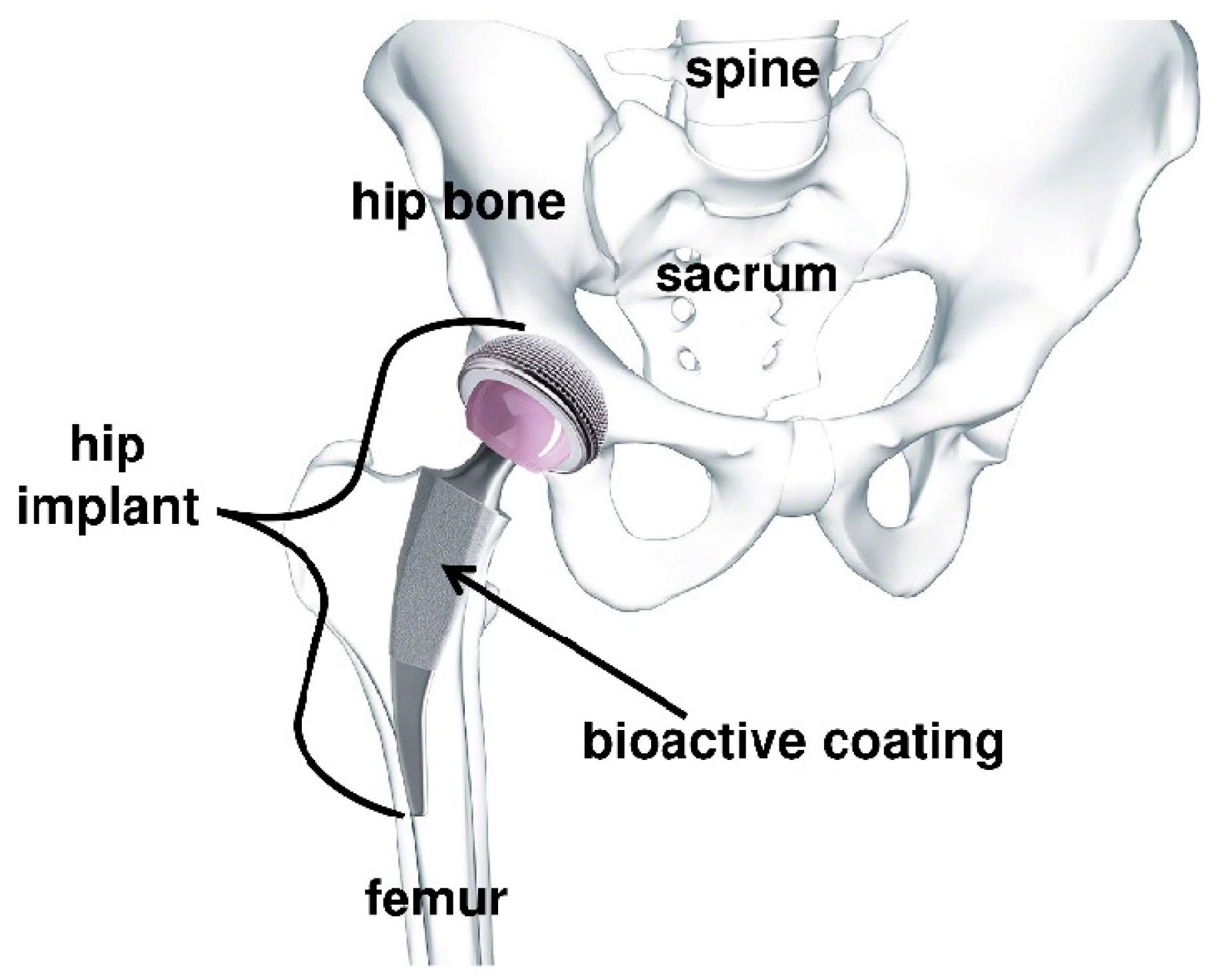
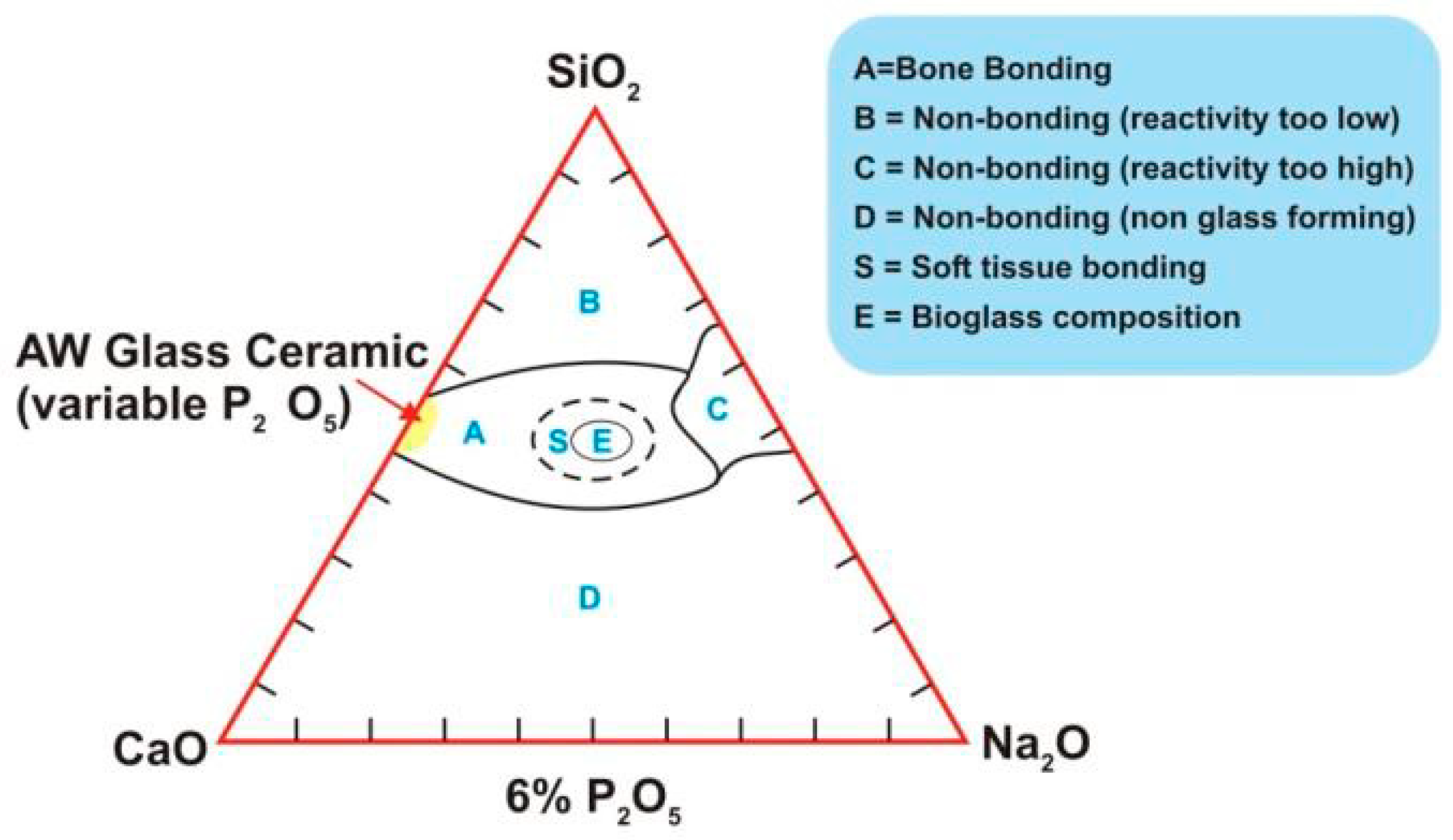
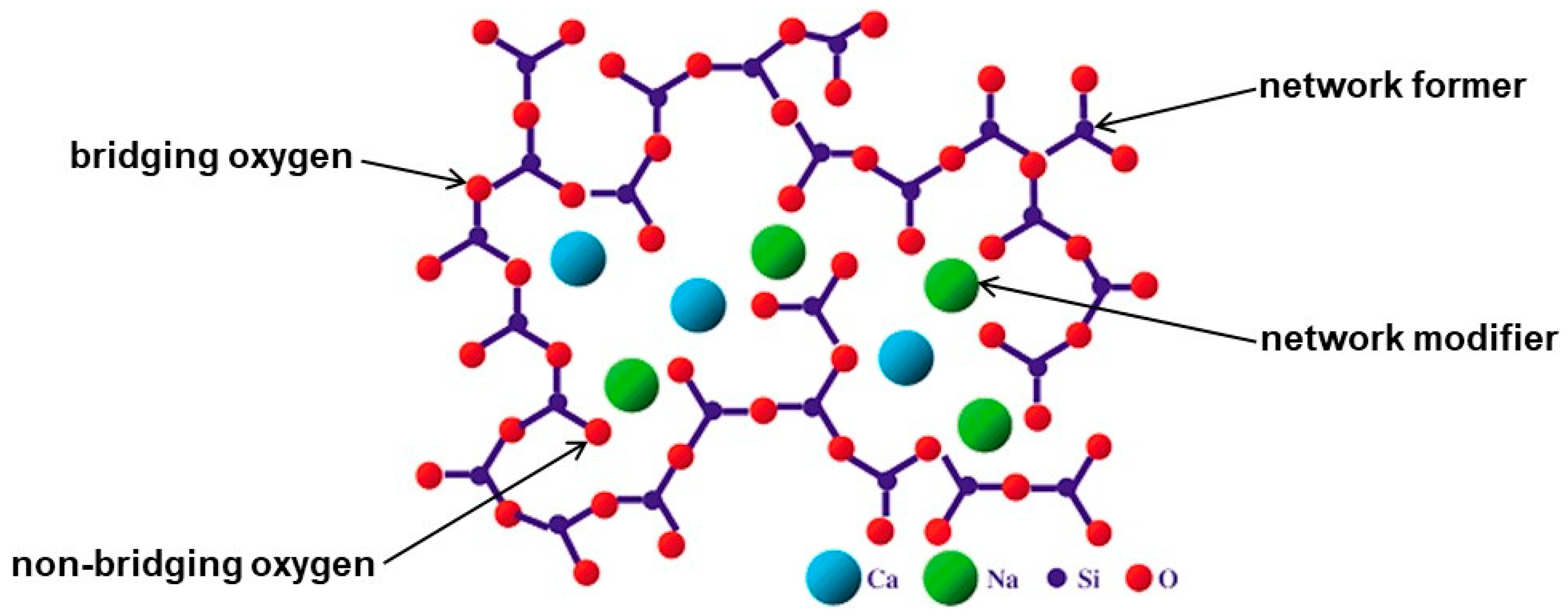
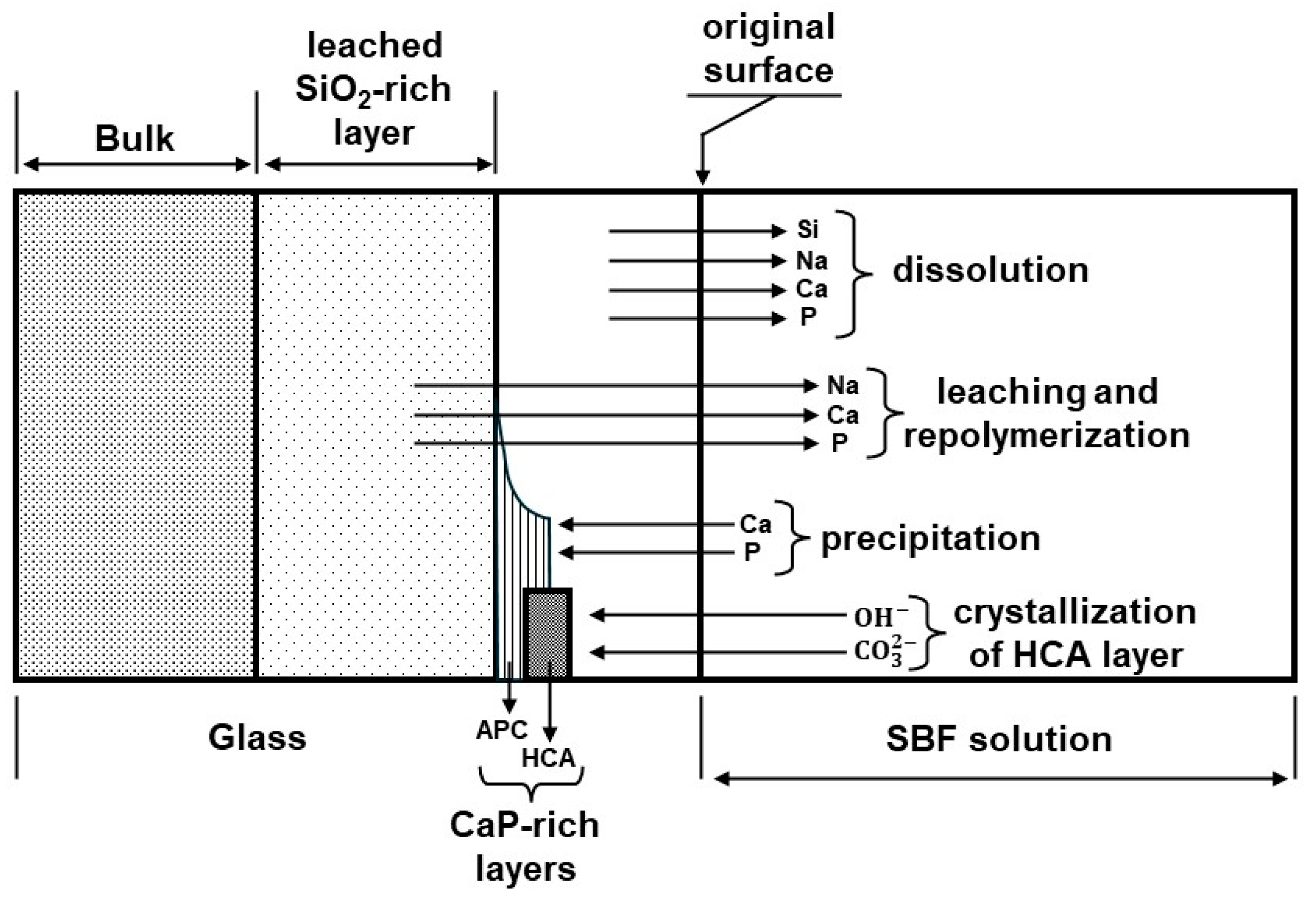
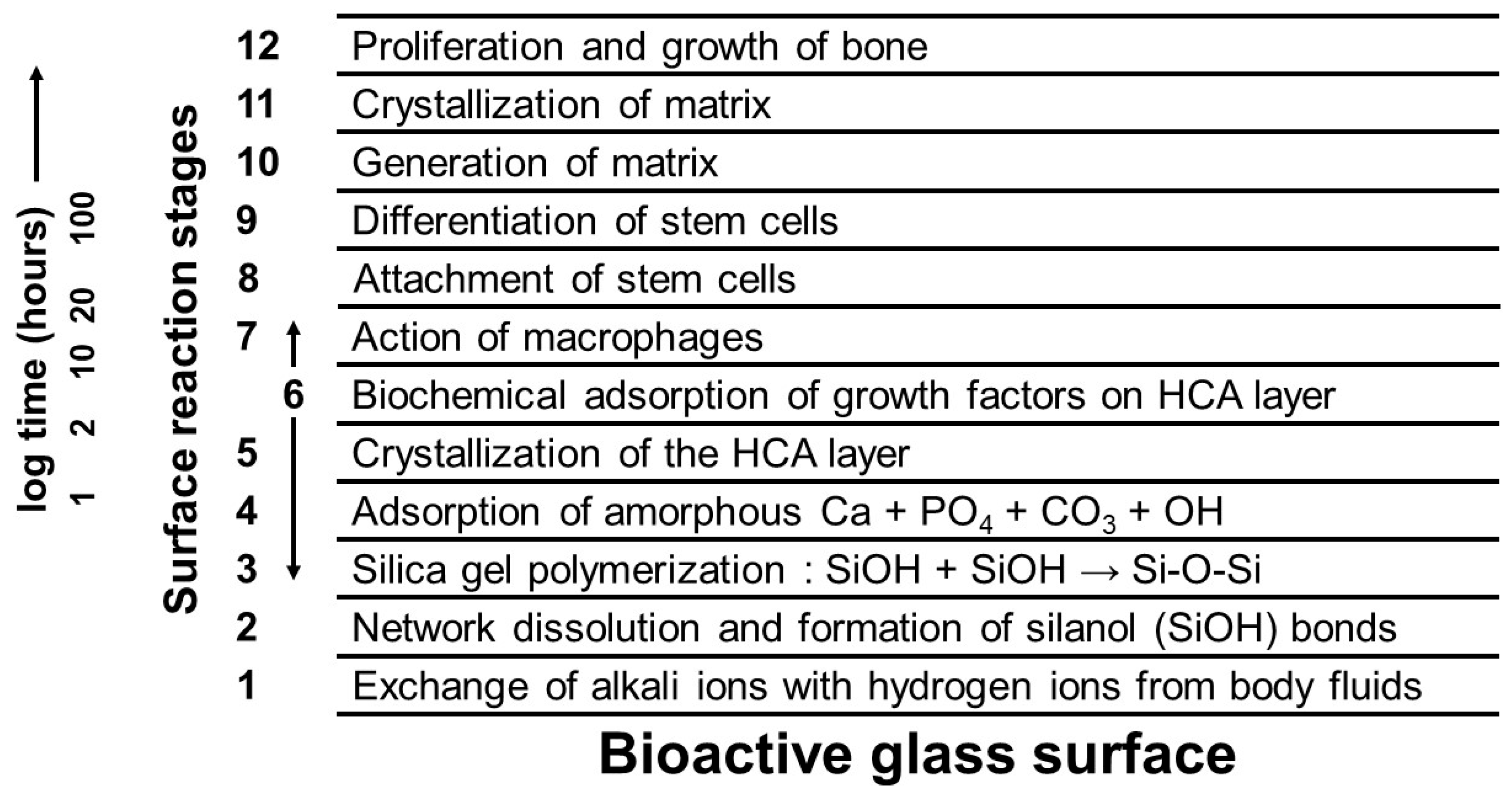
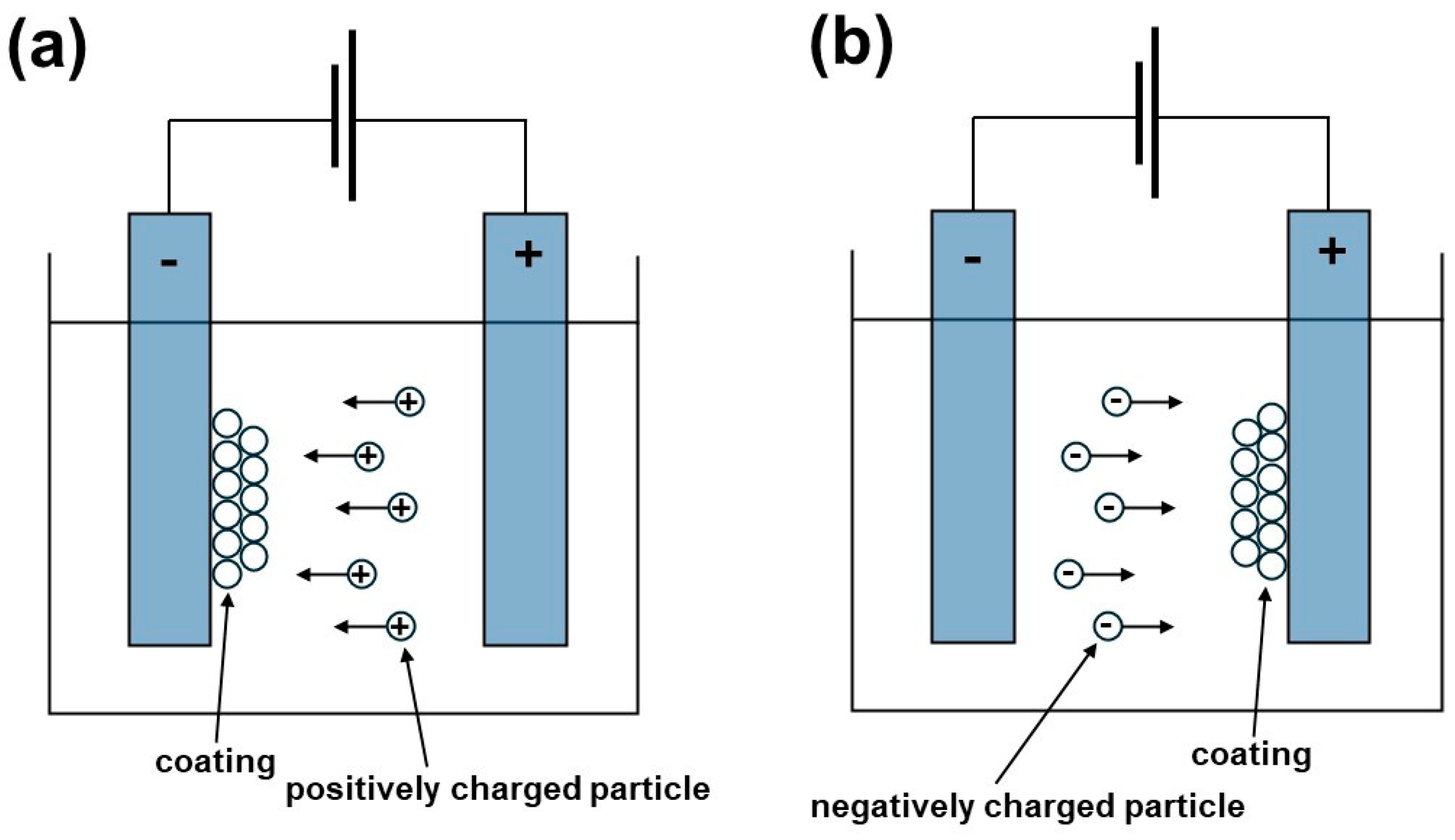
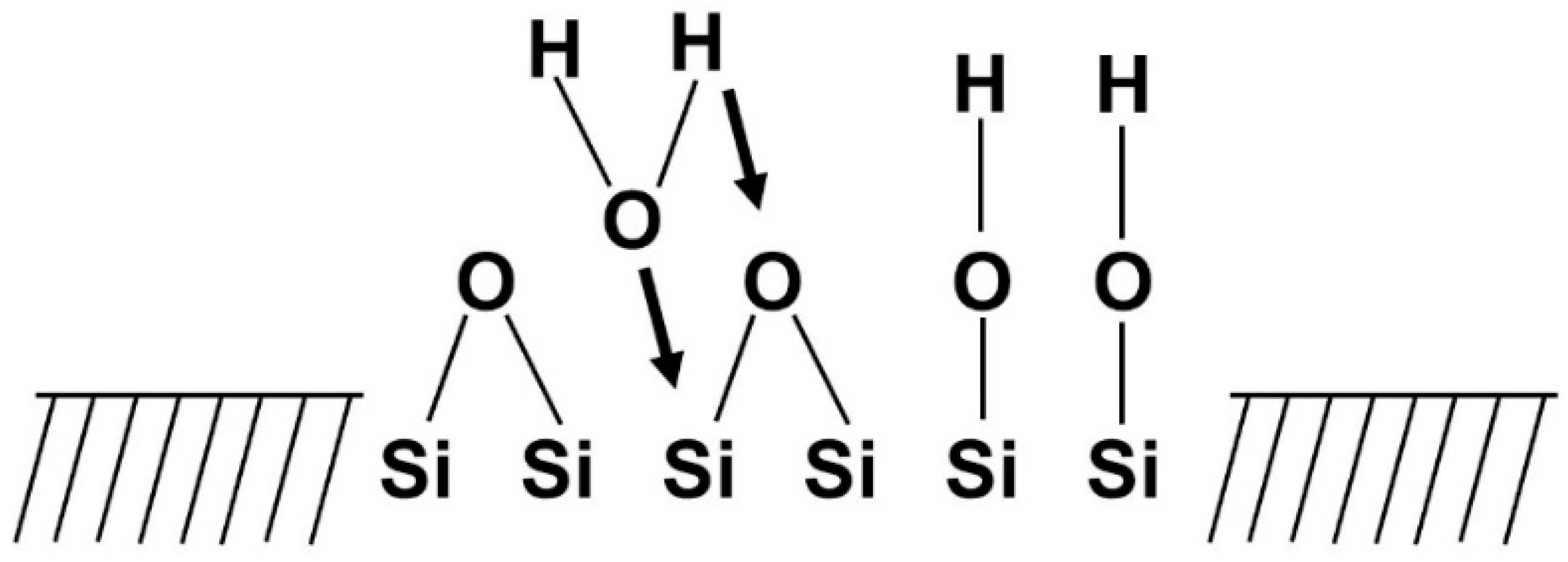
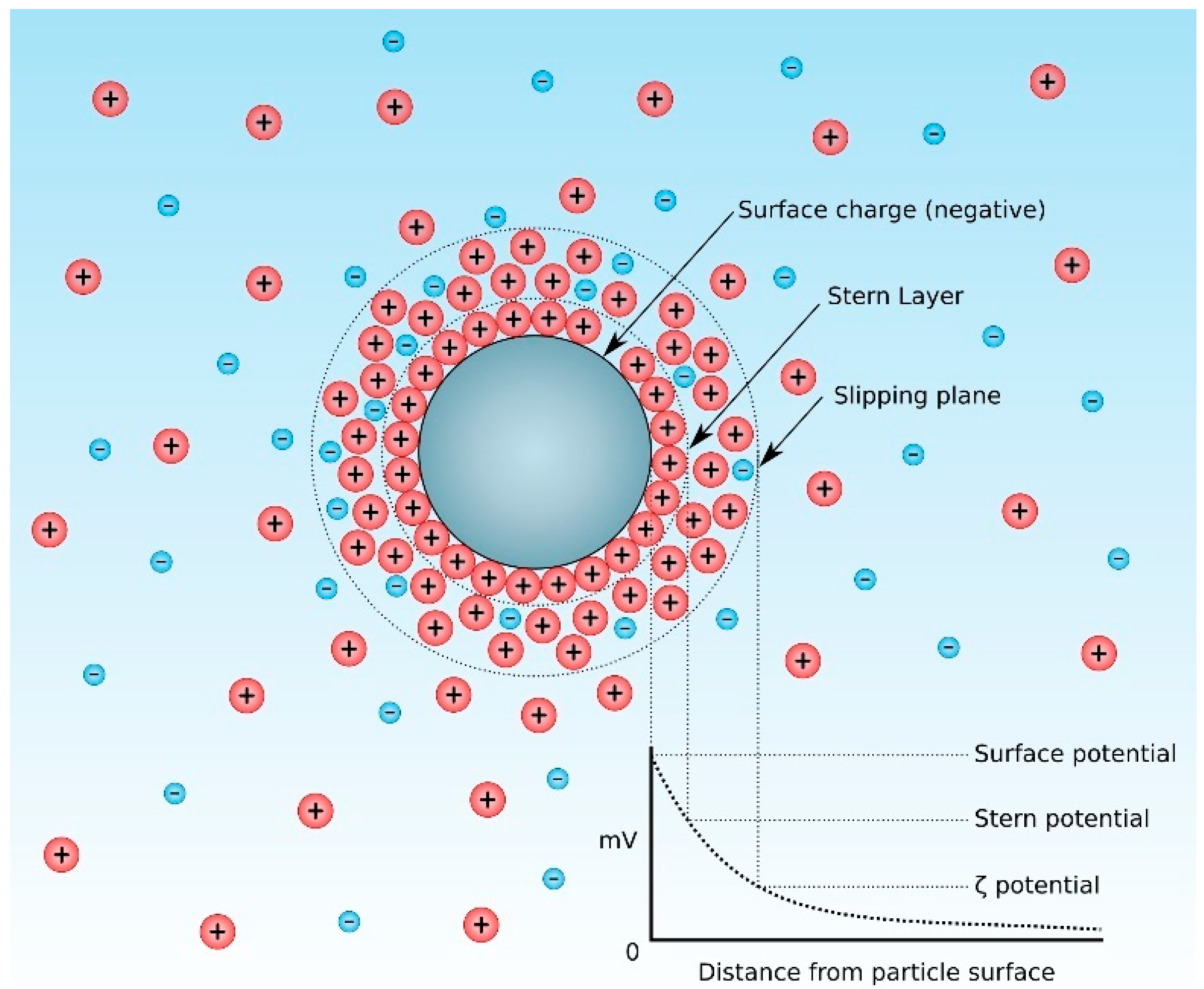
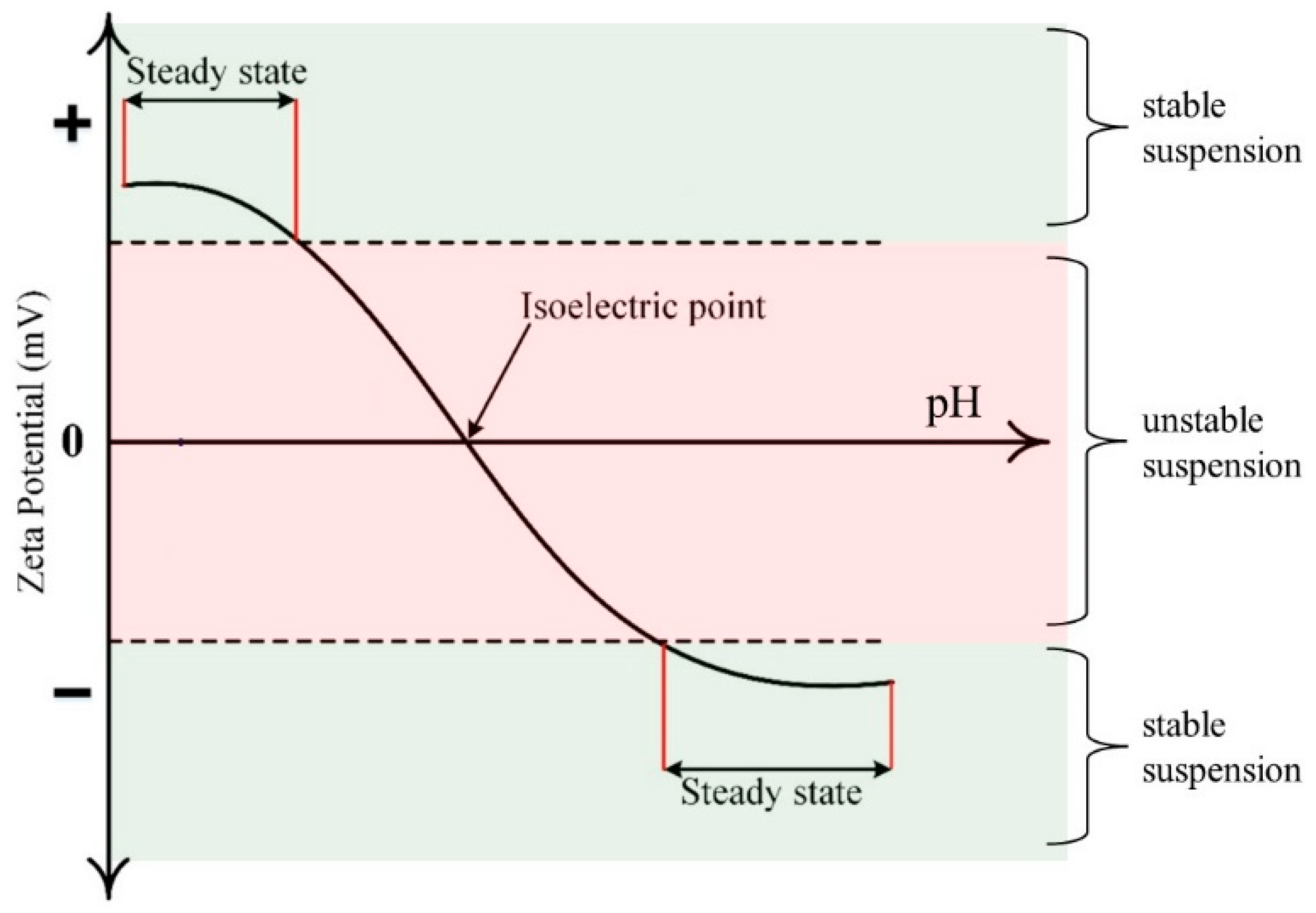

| SiO2 | CaO | Na2O | P2O5 | K2O | MgO | B2O3 | Reference | |
|---|---|---|---|---|---|---|---|---|
| 45S5 Bioglass® | 45 wt.% | 24.5 wt.% | 24.5 wt.% | 6 wt.% | - | - | - | [53,54,55,56,57,58,59] |
| 58S | 58 wt.% | 33 wt.% | - | 9 wt.% | - | - | - | [114,115,116,117] |
| 77S | 77 wt.% | 14 wt.% | – | 9 wt.% | - | - | - | [118,119,120,121] |
| S53P4 | 53 wt.% | 20 wt.% | 23 wt.% | 4 wt.% | - | - | - | [122,123,124] |
| 13-93 | 53 wt.% | 20 wt.% | 6 wt.% | 4 wt.% | 12 wt.% | 5 wt.% | - | [125,126,127] |
| 13-93B1 | 34 wt.% | 20 wt.% | 6 wt.% | 4 wt.% | 12 wt.% | 5 wt.% | 19 wt.% | [128,129] |
| 13-93B3 | - | 20 wt.% | 6 wt.% | 4 wt.% | 12 wt.% | 5 wt.% | 53 wt.% | [129,130] |
| 70S30C | 71 wt.% | 29 wt.% | - | - | - | - | - | [131,132,133,134] |
| Ions | Biological/Physical Effect | References |
|---|---|---|
| Monovalent Ions | ||
| Antibacterial activity | [193,194,195,196] | |
| Osteogenesis | [197,198,199] | |
| Osteogenesis | [200,201,202] | |
| Osteogenesis | [203,204,205] | |
| Antibacterial/osteogenesis | [206,207,208,209] | |
| Antibacterial/drug carrier/osteogenesis | [210,211] | |
| Divalent Ions | ||
| Osteogenesis/radiation shielding | [212,213,214,215] | |
| Angiogenesis | [216,217,218,219] | |
| Antibacterial activity | [220,221,222,223] | |
| Osteogenesis/radiation shielding | [224,225] | |
| Osteogenesis | [226,227,228,229] | |
| Antibacterial/osteogenesis | [230,231,232,233] | |
| Drug carrier/osteogenesis/radiation shielding | [234,235,236] | |
| Osteogenesis | [237,238,239,240,241] | |
| Antibacterial/anti-inflammatory/osteogenesis | [242,243,244,245] | |
| Trivalent Ions | ||
| Antibacterial/anticancer/osteogenesis | [246,247,248] | |
| Osteogenesis/antibacterial | [249,250,251,252] | |
| Osteogenesis/photoluminescence | [253,254] | |
| Drug carrier/photoluminescence | [255,256,257] | |
| Antibacterial/anticancer/osteogenesis | [258,259,260,261] | |
| Antibacterial/anticancer/osteogenesis | [262,263,264,265] | |
| Brachytherapy/osteogenesis | [266,267] | |
| Osteogenesis | [268,269,270] | |
| Osteogenesis | [271,272,273] | |
| Drug carrier/osteogenesis/photoluminescence | [274,275] | |
| Osteogenesis/photoluminescence | [276,277] | |
| Anticancer/osteogenesis | [278,279,280] | |
| Osteogenesis/photoluminescence | [254,281] | |
| Tetravalent Ions | ||
| Anticancer/drug carrier/osteogenesis | [282,283,284] | |
| Antibacterial/anticancer/osteogenesis | [285,286] | |
| Antibacterial/osteogenesis | [287,288,289] | |
| Pentavalent Ions | ||
| Osteogenesis | [290,291,292,293] | |
| Antibacterial/osteogenesis | [294,295,296,297] | |
| Osteogenesis/photoluminescence/radiation shielding | [298,299,300] | |
| Hexavalent Ions | ||
| Drug carrier/osteogenesis | [301,302] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drevet, R.; Fauré, J.; Benhayoune, H. Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review. Coatings 2024, 14, 1084. https://doi.org/10.3390/coatings14091084
Drevet R, Fauré J, Benhayoune H. Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review. Coatings. 2024; 14(9):1084. https://doi.org/10.3390/coatings14091084
Chicago/Turabian StyleDrevet, Richard, Joël Fauré, and Hicham Benhayoune. 2024. "Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review" Coatings 14, no. 9: 1084. https://doi.org/10.3390/coatings14091084
APA StyleDrevet, R., Fauré, J., & Benhayoune, H. (2024). Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review. Coatings, 14(9), 1084. https://doi.org/10.3390/coatings14091084








