The Etching Behaviour and Fluorine-Based-Plasma Resistance of YOF Coatings Deposited by Atmospheric Plasma Spraying
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
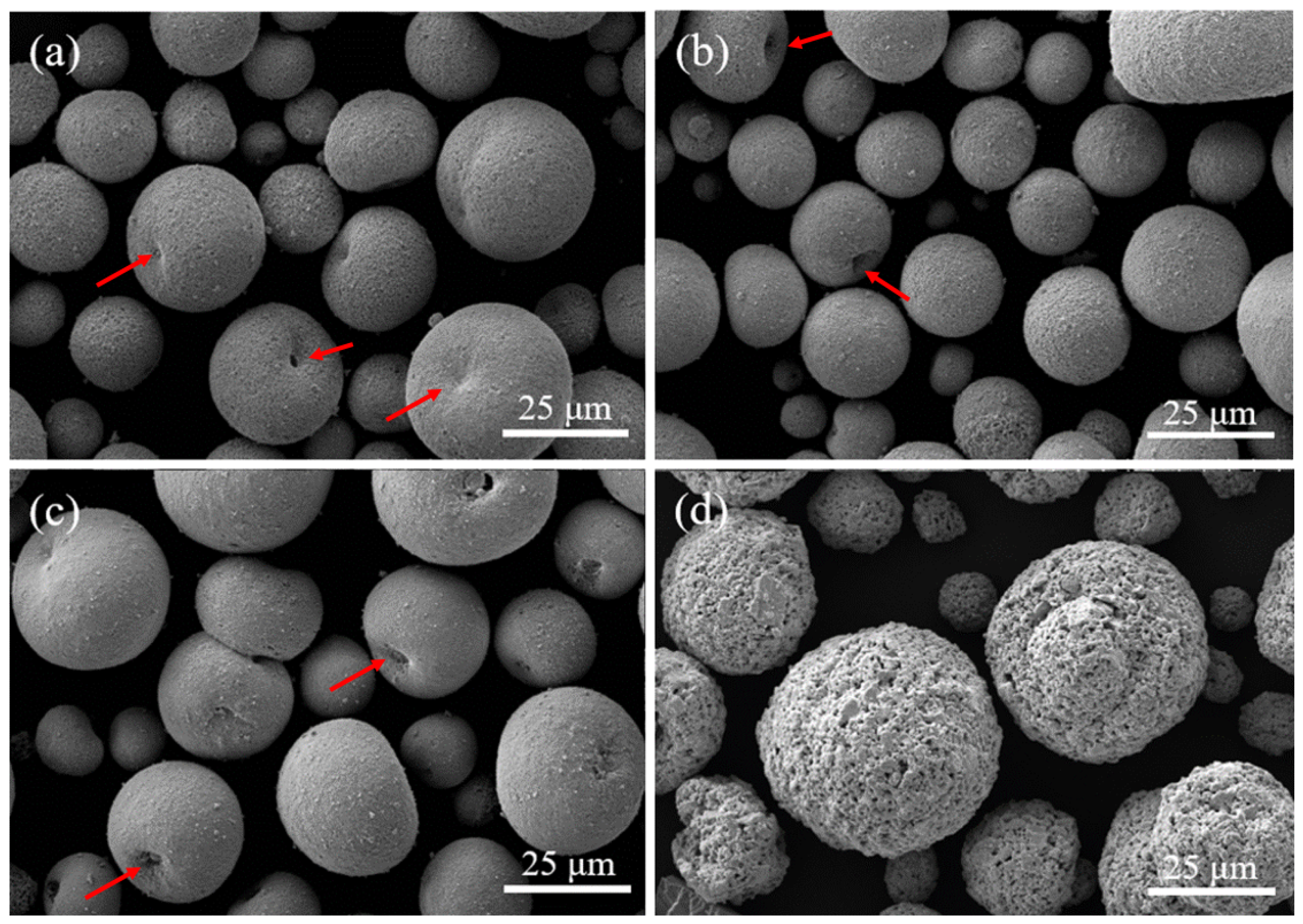
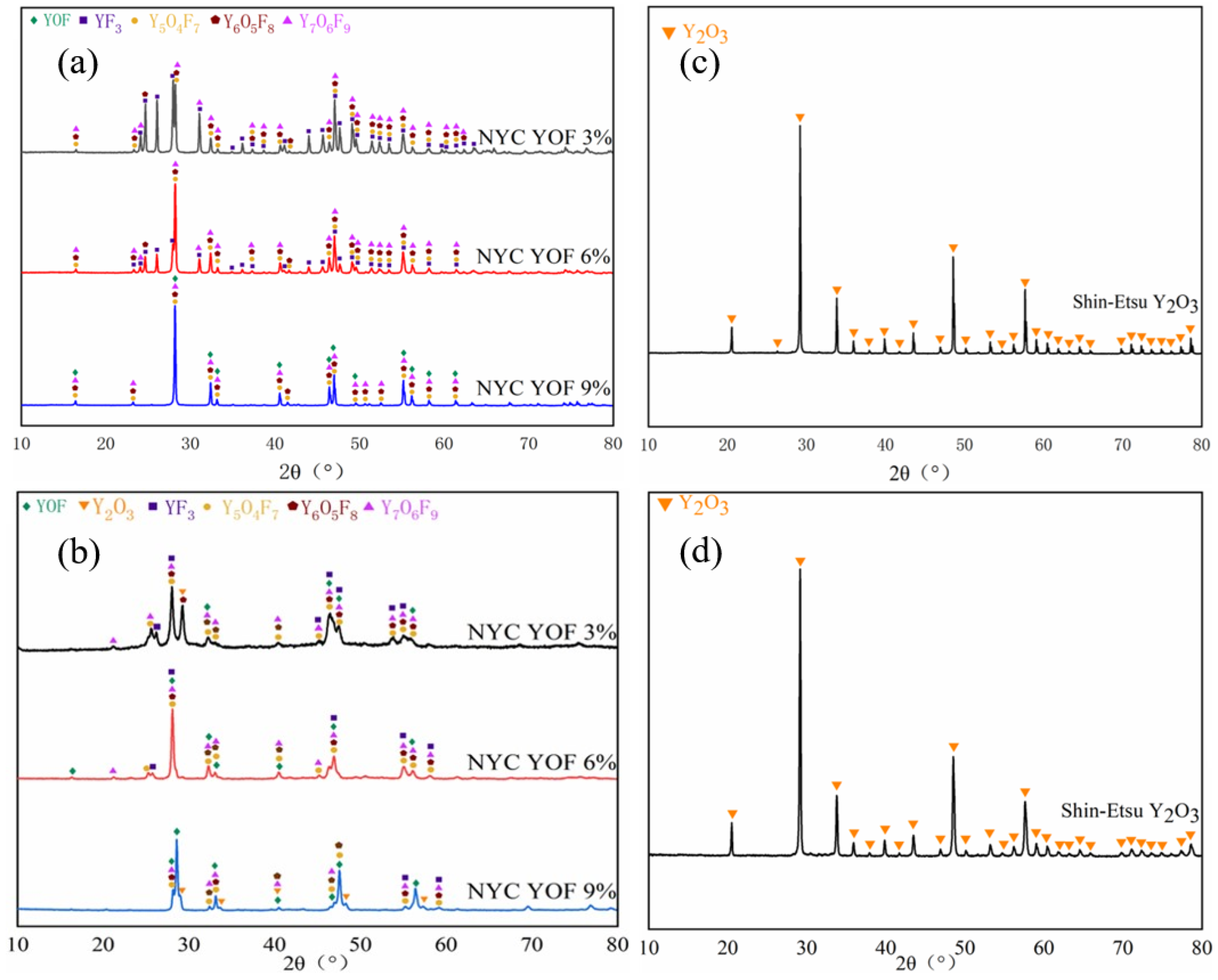
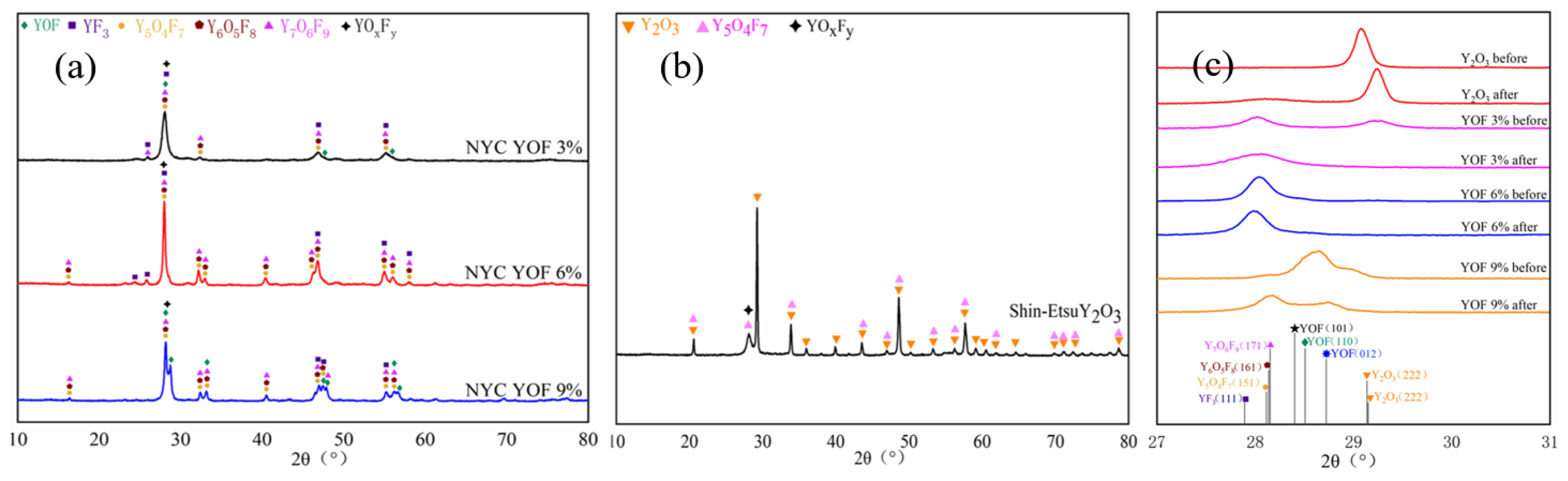
3.1. Characterisation of YOF and Y2O3 Powders, as Well as Atmospheric Plasma Spray Coatings
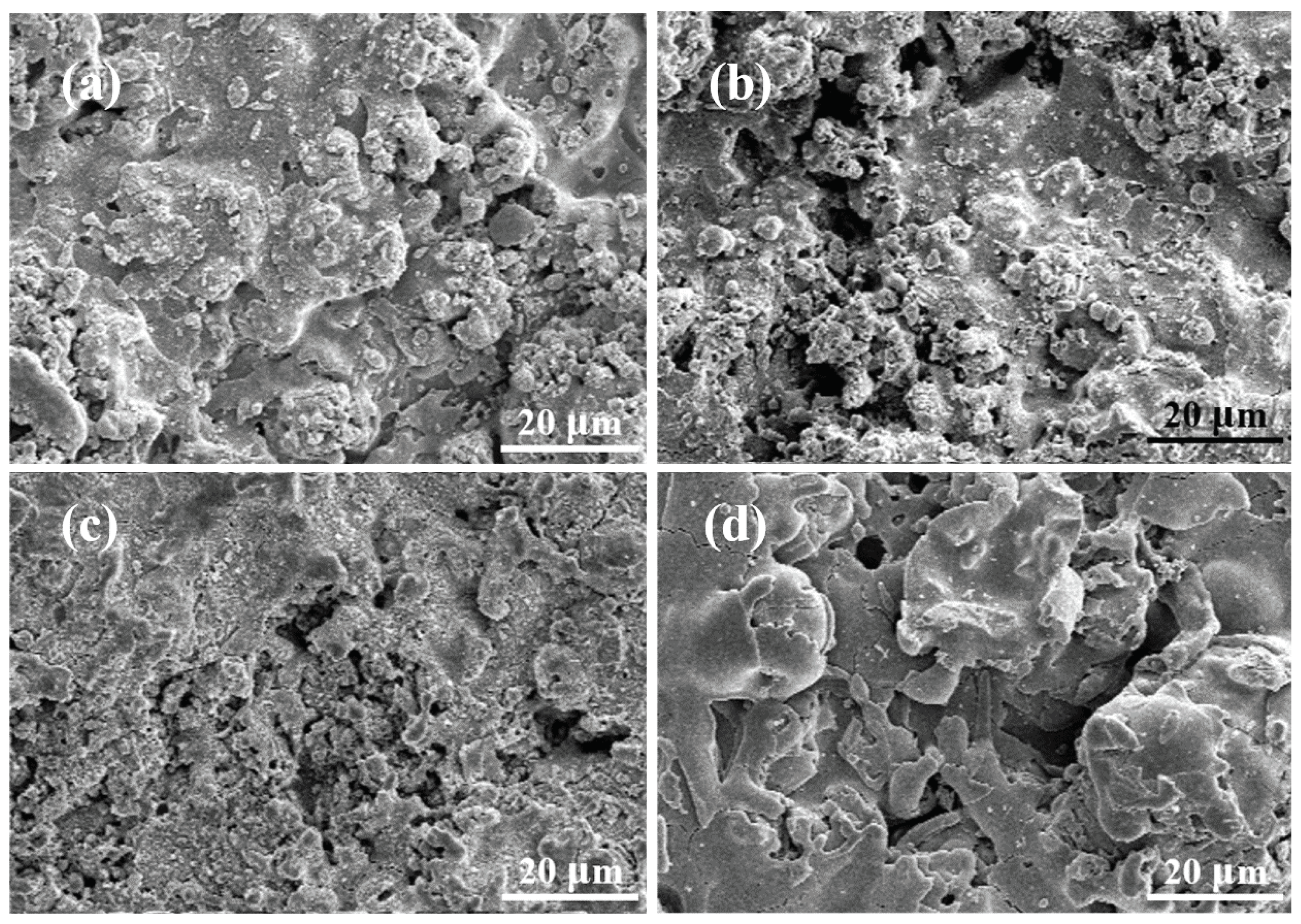
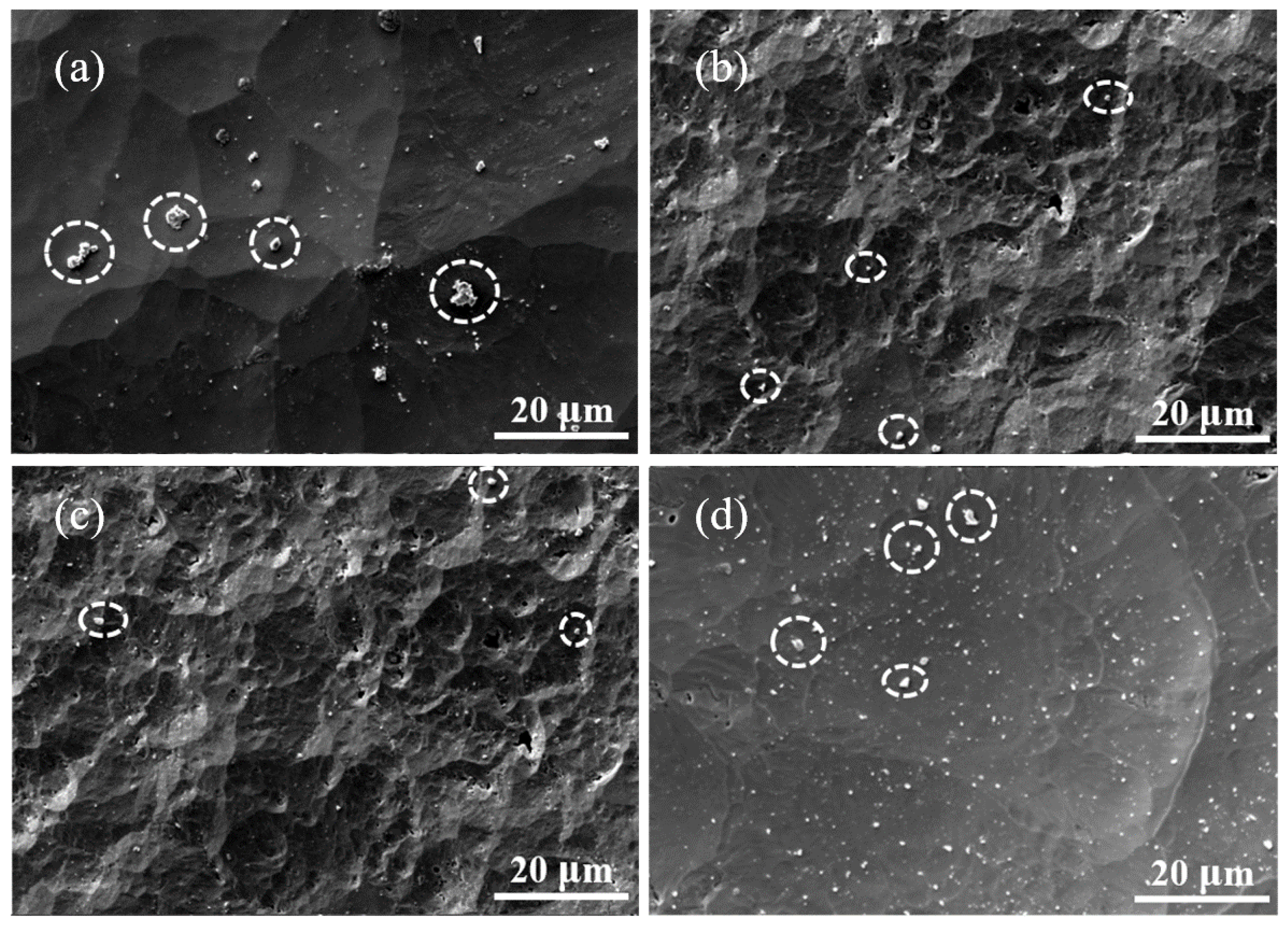
3.2. NF3 Plasma Etching Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moriya, T.; Murakami, T.; Nakayama, H.; Nagaike, H.; Sugawara, E.; Kobayashi, Y.; Shimada, M.; Okuyama, K. Reduction of particle contamination in an actual plasma etching process. In Proceedings of the IEEE International Symposium on Semiconductor Manufacturing, San Jose, CA, USA, 13–15 September 2005; pp. 229–232. [Google Scholar]
- So, J.; Kim, M.; Kwon, H.; Maeng, S.; Choi, E.; Chung, C.W.; Yun, J.Y. Investigation of contamination particles generation and surface chemical reactions on Al2O3, Y2O3, and YF3 coatings in F-based plasma. Appl. Surf. Sci. 2023, 629, 157367. [Google Scholar] [CrossRef]
- Lee, C.G.; Kanarik, K.J.; Gottscho, R.A. The grand challenges of plasma etching: A manufacturing perspective. J. Phys. D Appl. Phys. 2014, 47, 273001. [Google Scholar] [CrossRef]
- Huo, D.; Zheng, Y.; Sun, X.; Li, X.; Liu, S. Preparation of transparent Y2O3 ceramic by slip casting and vacuum sintering. J. Rare Earths 2012, 30, 57–62. [Google Scholar] [CrossRef]
- Ku, C.M.; Cheng, S. Factor design for the oxide etching process to reduce edge particle contamination in capacitively coupled plasma etching equipment. Appl. Sci. 2022, 12, 5684. [Google Scholar] [CrossRef]
- Kitamura, J.; Ibe, H.; Yuasa, F.; Mizuno, H. Plasma sprayed coatings of high-purity ceramics for semiconductor and flat-panel-display production equipment. J. Therm. Spray Technol. 2008, 17, 878–886. [Google Scholar] [CrossRef]
- Hussein, M.A.; Turkot, R.B. Particle control in dielectric etch chamber. IEEE Trans. Semicond. Manuf. 2006, 19, 146–155. [Google Scholar] [CrossRef]
- Lee, H.K.; Baek, K.H.; Shin, K. Resolving critical dimension drift over time in plasma etching through virtual metrology based wafer-to-wafer control. Jpn. J. Appl. Phys. 2017, 56, 066502. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Z.; Qian, X.; Holland, J.; Podlesnik, D. Characteristics and mechanism of etch process sensitivity to chamber surface condition. J. Vac. Sci. Technol. B 2001, 19, 166–171. [Google Scholar] [CrossRef]
- Cunge, G.; Joubert, O.; Sadeghi, N. Enhancement of the recombination rate of Br atoms by CF4 addition and resist etching in HBr/Cl2/O2 plasmas. J. Appl. Phys. 2003, 94, 6285–6290. [Google Scholar] [CrossRef]
- Schaepkens, M.; Bosch, R.C.M.; Standaert, T.E.F.M.; Oehrlein, G.S.; Cook, J.M. Influence of reactor wall conditions on etch processes in inductively coupled fluorocarbon plasmas. J. Vac. Sci. Technol. A 1998, 16, 2099–2107. [Google Scholar] [CrossRef]
- Tanikawa, T.; Shinohara, S. Plasma performance in very large helicon device. Thin Solid Films 2006, 506, 559–563. [Google Scholar] [CrossRef]
- Fukumoto, H.; Fujikake, I.; Takao, Y.; Eriguchi, K.; Ono, K. Plasma chemical behaviour of reactants and reaction products during inductively coupled CF4 plasma etching of SiO2. Plasma Sources Sci. Technol. 2009, 18, 045027. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Chen, Y.; Wang, Y. Phase composition, structural, and plasma erosion properties of ceramic coating prepared by suspension plasma spraying. Int. J. Appl. Ceram. Technol. 2018, 15, 1388–1396. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Han, H.N.; Kim, W.; Hwang, N.M. Yttrium oxyfluoride coatings deposited by suspension plasma spraying using coaxial feeding. Coatings 2020, 10, 481. [Google Scholar] [CrossRef]
- Cao, Y.C.; Zhao, L.; Luo, J.; Wang, K.; Zhang, B.P.; Yokota, H.; Ito, Y.; Li, J.F. Plasma etching behavior of Y2O3 ceramics: Comparative study with Al2O3. Appl. Surf. Sci. 2016, 366, 304–309. [Google Scholar] [CrossRef]
- Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication, characterization, and fluorine-plasma exposure behavior of dense yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2017, 56, 06HC02. [Google Scholar] [CrossRef]
- Lin, T.K.; Wuu, D.S.; Huang, S.Y.; Wang, W.K. Preparation and Characterization of Sprayed-Yttrium Oxyfluoride Corrosion Protective Coating for Plasma Process Chambers. Coatings 2018, 8, 373. [Google Scholar] [CrossRef]
- Shiba, Y.; Teramoto, A.; Goto, T.; Kishi, Y.; Shirai, Y.; Sugawa, S. Stable yttrium oxyfluoride used in plasma process chamber. J. Vac. Sci. Technol. A 2017, 35, 021405. [Google Scholar] [CrossRef]
- Ma, T.; List, T.; Donnelly, V.M.P. Comparisons of NF3 plasma-cleaned Y2O3, YOF, and YF3 chamber coatings during silicon etching in Cl2 plasmas. J. Vac. Sci. Technol. A 2018, 36, 031305. [Google Scholar] [CrossRef]
- Tahara, R.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fabrication of dense yttrium oxyfluoride ceramics by hot pressing and their mechanical, thermal, and electrical properties. Jpn. J. Appl. Phys. 2018, 57, 06JF04. [Google Scholar] [CrossRef]
- Miyashita, K.; Tsunoura, T.; Yoshida, K.; Yano, T.; Kishi, Y. Fluorine and oxygen plasma exposure behavior of yttrium oxyfluoride ceramics. Jpn. J. Appl. Phys. 2019, 58, SEEC01. [Google Scholar] [CrossRef]
- Lin, T.K.; Wang, W.K.; Huang, S.Y.; Tasi, C.T.; Wuu, D.S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Kim, W.; Hwang, N. Plasma Etching Behavior of YOF Coating Deposited by Suspension Plasma Spraying in Inductively Coupled CHF3/Ar Plasma. Coatings 2020, 10, 1023. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Hwang, N.M. Yttrium Oxyfluoride Coating Deposited with a Y5O4F7/YF3 Suspension by Suspension Plasma Spraying Under Atmospheric Pressure. J. Therm. Spray Technol. 2022, 31, 1–13. [Google Scholar] [CrossRef]
- Kim, C.; Kim, M.J.; Cho, H.; Park, T.E.; Yun, Y.H. Fabrication and plasma resistance of Y2O3 ceramics. Ceram. Int. 2015, 10, 12757–12762. [Google Scholar] [CrossRef]
- Ashizawa, H.; Yoshida, K. Plasma-resistance evaluation of yttrium oxyfluoride coating prepared by aerosol deposition method. Int. J. Appl. Ceram. Technol. 2022, 19, 375–382. [Google Scholar] [CrossRef]
- Kim, D.M.; Oh, Y.S.; Kim, S.; Kim, H.T.; Lim, D.S.; Lee, S.M. The erosion behaviors of Y2O3 and YF3 coatings under fluorocarbon plasma. Thin Solid Films 2011, 519, 6698–6702. [Google Scholar] [CrossRef]
- Khan, S.; Han, J.S.; Lee, S.Y.; Cho, S.H. Flame-synthesized Y2O3: Tb3 nanocrystals as spectral converting materials. J. Nanoparticle Res. 2018, 20, 241. [Google Scholar] [CrossRef]
- Khan, S.; Park, B.I.; Han, J.S.; Lee, S.Y.; Cho, S.H. Flame synthesized Y2O3: Tb3–Yb3 phosphors as spectral convertors for solar cells. Res. Chem. Intermed. 2018, 44, 4619–4632. [Google Scholar] [CrossRef]
- Kim, D.M.; Lee, S.H.; Alexander, W.B.; Kim, K.B.; Oh, Y.S.; Lee, S.M. X-ray photoelectron spectroscopy study on the interaction of yttrium–aluminum oxide with fluorine-based plasma. J. Am. Ceram. Soc. 2011, 94, 3455–3459. [Google Scholar] [CrossRef]
- Xia, J.; Liang, W.; Miao, Q.; Depla, D. The effect of energy and momentum transfer during magnetron sputter deposition of yttrium oxide thin films. Appl. Surf. Sci. 2018, 439, 545–551. [Google Scholar] [CrossRef]



| Pressure (mTorr) | TCP RF Power (W) | Bias RF Voltage (V) | Etching Time (s) | NF3 Flow Rate (sccm) | He Flow Rate (sccm) |
|---|---|---|---|---|---|
| 15 | 1540 | 330 | 300 | 200 | 500 |
| Atomic Percentage (%) | Y2O3 | YOF 3% | YOF 6% | YOF 9% | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-Etching | Post-Etching | Pre-Etching | Post-Etching | Pre-Etching | Post-Etching | Pre-Etching | Post-Etching | |
| Y | 43.4 | 34.7 | 29.5 | 37.1 | 32.0 | 36.9 | 38.5 | 38.1 |
| O | 53.5 | 25.4 | 12.3 | 15.5 | 18.0 | 17.6 | 34.3 | 15.1 |
| F | 0 | 38.4 | 57.2 | 47.1 | 49.6 | 45.1 | 26.4 | 46.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Wang, Y.; Ang, K.; Xu, J.; Meng, H.; Chen, H.; Wei, Y.; Shi, Y.; Wang, L. The Etching Behaviour and Fluorine-Based-Plasma Resistance of YOF Coatings Deposited by Atmospheric Plasma Spraying. Coatings 2024, 14, 1091. https://doi.org/10.3390/coatings14091091
Tang Z, Wang Y, Ang K, Xu J, Meng H, Chen H, Wei Y, Shi Y, Wang L. The Etching Behaviour and Fluorine-Based-Plasma Resistance of YOF Coatings Deposited by Atmospheric Plasma Spraying. Coatings. 2024; 14(9):1091. https://doi.org/10.3390/coatings14091091
Chicago/Turabian StyleTang, Zaifeng, Yuwei Wang, Kaiqu Ang, Jin Xu, Hua Meng, Hongli Chen, Yuxuan Wei, Ying Shi, and Linjun Wang. 2024. "The Etching Behaviour and Fluorine-Based-Plasma Resistance of YOF Coatings Deposited by Atmospheric Plasma Spraying" Coatings 14, no. 9: 1091. https://doi.org/10.3390/coatings14091091




